Abstract
Objective
Gene × diet interaction plays an important role in atherosclerosis, an inflammatory disorder. Leukotrienes are the most potent inflammatory mediators, and genetic variants encoding leukotriene genes have been implicated in atherosclerosis. This study tests nutrigenetic interaction of a previously defined leukotriene haplotype on carotid artery hypertrophy and atherosclerosis in American Indians.
Methods
This study included 3,402 American Indians participating in the Strong Heart Family Study (SHFS). Carotid artery measurements, including intima-media thickness (IMT), vascular mass, and plaque, were assessed using ultrasound. Eleven tagSNPs in the leukotriene A4 hydrolase (LTA4H) gene were genotyped in all subjects. Main haplotype effect and haplotype × diet interaction were examined by generalized estimating equation, adjusting for known risk factors.
Results
There was no significant main effect of haplotype or diet on any of the carotid artery measures. However, a previously defined LTA4H haplotype, called HapE, significantly interacted with dietary intake of n-3 and n-6 fatty acids on both IMT (P HapE x n3 = 0.018, P HapE x n6 = 0.040) and vascular mass (P HapE x n3 = 0.012, P HapE x n6 = 0.018), but not plaque. The direction of this nutrigenetic interaction on IMT was consistent with that reported in a recent study of Caucasian twins.
Conclusion
Dietary intake of polyunsaturated fatty acids significantly modifies the effect of a leukotriene haplotype on carotid artery hypertrophy but not atherosclerosis in American Indians, independent of established cardiovascular risk factors. Replication of nutrigenetic interaction in two distinct ethnic groups suggests the robustness and generalizability of our findings to diverse populations.
Keywords: leukotriene haplotype, gene-diet interaction, carotid atherosclerosis, American Indians
Introduction
Atherosclerosis is an inflammatory disease resulting from the complex interaction between gene and environment. Leukotrienes are the most potent inflammatory mediators that have been implicated in atherosclerotic cardiovascular disease (ACVD).1 Metabolism and biosynthesis of leukotrienes are regulated by several rate-limiting enzymes, such as arachidonate 5-lipoxygenase (ALOX5), arachidonate 5-lipoxygenase activating protein (ALOX5AP) and leukotriene A4 hydrolase (LTA4H).2 Genetic polymorphisms in genes encoding leukotriene enzymes have been associated with ACVD. For example, two haplotypes in the ALOX5AP gene3, 4 and one haplotype in the LTA4H gene (designated HapK)5 have been associated with myocardial infarction (MI) or stroke, probably through increasing leukotriene production and resulting vascular inflammation. These associations were replicated in some3, 6–9 but not all studies.10 The lack of replication for genetic associations could be attributed to the different genetic background of study participants across different studies. However, failure to account for gene × environment interaction may also explain at least part of the discrepancies.11, 12 In support of this, dietary intake of omega-3 (n-3) and omega-6 (n-6) fatty acids significantly modified the association between a ALOX5 promoter variant and carotid intima-media thickness (IMT) in a population of predominantly non-Hispanic Whites and Hispanics.13 Nutrigenetic interaction was also observed in another study, in which ALOX5 variants increased the risk of MI in presence of high arachidonic acid (AA) intake but decreased MI risk in low AA intake.14 However, little research has been done to examine whether dietary factors similarly interact with genetic variants in other key leukotriene enzymes (e.g., LTA4H) on subclinical atherosclerosis. In addition, few studies have investigated gene × diet interaction on carotid artery measures other than IMT.
Omega-3 (n-3) and omega-6 (n-6) fatty acids are the two major classes of polyunsaturated fatty acids (PUFA) in habitual dietary intake, and both are required for normal cell function.15 Eicosapentaenoic acid (EPA; C20: 5, n-3) and docosahexaenoic acid (DHA; C22:6, n-3) are two primary n-3 fatty acids, whereas linoleic acid (LA, C18: 2, n-6) and arachidonic acid (AA; C20:4, n-6) belong to the class of n-6 fatty acids. Dietary intake of n-3 or n-6 fatty acids was found to reduce CVD risk in some populations,16, 17 probably by mediating vascular inflammation18, 19 through leukotriene enzymatic pathways.20
In a recent study of Caucasian twins, we identified a significant gene × diet interaction between a LTA4H haplotype (named HapE) and dietary intake of n-3 and n-6 fatty acids on carotid IMT.21 The current analysis evaluates nutrigenetic interaction of HapE on carotid artery hypertrophy (common carotid IMT and arterial mass) and atherosclerosis (plaque presence and plaque score) in American Indians participating in the Strong Heart Family Study (SHFS), a population that might have distinct genetic and/or habitual dietary intake compared to other ethnic groups. Previous research has shown that these vascular measures are heritable in this population,22 thus investigation of gene × diet interactions for these quantitative traits is particularly useful.
Subjects and Methods
Study population
The SHFS is a family-based prospective study of genetic factors for CVD and its risk factors in American Indians. Detailed descriptions of the SHFS protocols have been described previously.22, 23 In brief, a total of 3,665 tribal members from 94 families residing in Arizona (AZ), North and South Dakota (DK) and Oklahoma (OK) were examined in 2001–2003. Each participant received a standardized personal interview and physical examination. Personal interview used a standard questionnaire and was administered by trained study personnel to collect data on demographic characteristics, medical history and lifestyle risk factors including smoking, alcohol consumption, diet and physical activity. The physical examination included anthropometric and blood pressure measurements and an examination of the heart and lungs. The SHFS protocol was approved by the Institutional Review Boards from the Indian Health Service and the participating centers. All participants have given informed consent for genetic study of CVD, diabetes and associated risk factors.
Carotid ultrasound measurements
All study participants underwent carotid ultrasonography using Acuson Sequoia machines equipped with 7 MHz vascular probes on the day of the study visit. Detailed protocol of ultrasound scanning has been described previously.24–26 Briefly, the extracranial segments of the left and right carotid arteries were extensively scanned using a high-frequency two-dimensional ultrasound probe. The simultaneous ECG was used to time carotid artery measurements at end diastole. IMT of the far wall of the distal common carotid artery was measured at end diastole on multiple cycles of M-mode images. Minimum (end-diastolic) and maximum (peak-systolic) diameters were measured by continuous tracing of the lumen-intima interfaces of the near and far walls. Wall thickness and diameter measurements of the left and right common carotid arteries were averaged, and the averaged values were used in statistical analyses. Wall thickness and diameter measurements were never measured at the level of a plaque. Carotid arteries were also scanned to identify atherosclerotic plaque, defined as the presence of wall thickness >50% of the surrounding wall. Plaque score, a measure of the extent of atherosclerosis, was calculated by the number of left and right segments (common carotid, bulb, internal carotid, external carotid) containing plaque, thus plaque score ranged from 0 to 8. Carotid cross-sectional area, a measure of vascular volume or mass, was calculated as previously described.25 All ultrasound measurements were performed by trained research sonographers and interpreted by a single highly experienced cardiologist (MJR) who was blinded to clinical data.
Dietary assessments
Dietary intakes were assessed using a Block Food Frequency Questionnaire (FFQ).27–30 This interviewer-administered Block FFQ ascertained consumptions of 119 food items, including major traditional foods and foods commonly available in SHS communities. It measures habitual dietary intake over the past 12 months. The Block FFQ is one of the most widely used food questionnaires, and its reliability and validity had been extensively evaluated.27, 29, 31 Average daily energy and macronutrient intakes were calculated using the Nutrition Data System for Research Database (Block Dietary Systems Version 4.06, Berkley, CA).
Risk factor measurements
Weight and height were used to calculate body mass index (BMI) as weight in kilograms divided by height in meters squared. Cigarette smoking was classified into current smoker (any number of cigarettes) versus never or former smoker. Participants were categorized into current drinkers, former drinkers and never drinkers based on their history of alcohol consumption. Hypertension was defined as blood pressure levels of 140/90 mm Hg or higher or use of antihypertensive medications. Diabetes was defined as a fasting glucose ≥126 mg/dL, or current treatment with hypoglycemic agents. Fasting plasma glucose, insulin, and lipids were measured by standard laboratory methods as reported elsewhere.32, 33
Tagging SNPs selection and genotyping
Eleven tagSNPs in the LTA4H gene were genotyped in all SHFS participants. TagSNPs were chosen using the computer program Haploview 4.2 34 with an r2 threshold of 0.80 for linkage disequilibrium. Due to lack of information for American Indians in publicly available genetic databases, we used Asians as the reference group in tagSNPs selection. This choice was based on previous studies demonstrating the similarity of genetic background between Native Americans and Asians.35–37 The following criteria were also considered: minor allele frequency (MAF>5%), SNP location (i.e., coding region) and Illumina design scores (quantifying how likely a SNP can be genotyped). SNPs that could not be tagged (i.e., singletons) were included as long as their design score was greater than 0.15. All genotyping was done at the Texas Biomedical Research Institute using the Illumina VeraCode technology (Illumina, Inc., San Diego, CA). The average genotyping call rates were over 98% for all SNPs and sample success rate was over 96%. Information for the studied SNPs is shown in Table 1. For the current investigation, we excluded participants with missing genotype (n=52) or dietary data (n = 211). Subjects who were excluded did not differ from those included in terms of baseline characteristics. A total of 3,402 individuals comprised the study population of the current analysis.
Table 1.
Genotyped tagSNPs in LTA4H gene
| SNP | Variation | MAF |
|---|---|---|
| rs2247570 | A/G | 0.10 |
| rs2540475 | C/T | 0.11 |
| rs2540482 | A/G | 0.48 |
| rs2660845 | A/G | 0.47 |
| rs2660880 | A/G | 0.01 |
| rs2660898 | A/C | 0.25 |
| rs2660899 | C/A | 0.41 |
| rs6538697 | A/G | 0.12 |
| rs1978331 | A/G | 0.23 |
| rs17677715 | A/G | 0.07 |
| rs61937881 | C/T | 0.09 |
Statistical analyses
Hardy-Weinberg equilibrium (HWE) was tested by a chi-square test with one degree of freedom (df). Log-transformed IMT and vascular mass were used in the statistical analyses. In a recent study, we defined a 6-SNP haplotype (called HapE) that significantly modifies the effect of dietary intake of n-3 and n-6 fatty acids on carotid IMT in a Caucasian twin sample.21 This haplotype spans rs61937881 (C), rs1978331 (G), rs17677715 (T), rs2660899 (G), rs2540482 (T) and rs2660845 (A). Here we employed the same algorithm38 to infer HapE using LTA4H genotype data in all study participants. Subjects carrying at least one copy of HapE were defined as HapE carriers, and those not carrying this haplotype were defined as noncarriers. A binary variable for carrier status of HapE was used in statistical analyses.
To examine whether dietary intake modifies the association between HapE and subclinical measures of atherosclerosis, we constructed multivariate generalized estimating equation (GEE) models to test for the interaction of HapE (carrier vs. noncarrier) with dietary intake of n-3 or n-6 fatty acids (in grams as a continuous variable) by assessing the statistical significance of the interaction term in a GEE model that also included the main effects. GEE was used here to account for the correlation between family members. The following coronary risk factors were adjusted in the model: age, sex, study center, BMI, systolic blood pressure, diabetes, smoking, alcohol consumption, total cholesterol, triglyceride, estimated glomerular filtration rate (eGFR), saturated fat and total daily energy intake (Kcal). Given the significant correlation between EPA and DHA (γ= 0.40, p<0.0001), and between LA and AA (γ= 0.83, p<0.0001), we summed dietary EPA and DHA as the total intake of n-3 fatty acids and summed dietary LA and AA as the total intake of n-6 fatty acids in the statistical analyses. Additionally, because the relationship between dietary intake of n-3 fatty acids and inflammation may depend on the intake of n-6 fatty acids or vice versa,39 the interactions for n-3 or n-6 fatty acids with genetic haplotypes were adjusted for one another. Multiple testing was controlled using the Benjamin-Hochberg false discovery rate (FDR) method,40 and an FDR-adjusted p value (q value) threshold of 0.10 was used to determine statistical significance.
Subgroup analysis
Given the high prevalence of diabetes in American Indians, we conducted secondary analysis by excluding diabetic participants to examine its potential impact on our results. Similarly, we conducted subgroup analysis to examine the potential influence of obesity on our results by either excluding participants with obesity or by comparing participants of the lowest quartile with that of the highest quartile of BMI. As participants may have changed their diet after a diagnosis of disease, we performed additional analyses to test for interaction between diet and disease status, such as diabetes and hypertension. Although EPA and DHA are highly correlated, they might have differential effect on inflammation or atherosclerosis.41, 42 Similarly, LA and AA may also affect carotid IMT differently. We therefore conducted sensitivity analyses to examine whether HapE × diet interaction was primarily driven by a specific nutrient. This was done by testing the interaction of HapE with each individual nutrient in n-3 fatty acids (i.e., EPA or DHA) or n-6 fatty acids (i.e., LA or AA), separately, adjusting for the same covariates listed above. Additional adjustment for physical activity level did not change the results.
Results
All SNPs were in Hardy-Weinberg equilibrium. The prevalence of HapE was 24.3% in American Indians participating in the SHFS. The distribution of major cardiovascular risk factors by HapE carrier status is shown in Table 2. There was no significant difference in all of the listed risk factors between HapE carriers and non-carriers.
Table 2.
Distribution of major cardiovascular risk factors by HapE status in American Indians
| Variables | HapE (−)
|
HapE (+)
|
P* |
|---|---|---|---|
| Mean ± SD or % | Mean ± SD or % | ||
| Age (years) | 39.7±16.9 | 40.7±17.2 | 0.23 |
| Female sex (%) | 59.9 | 60.9 | 0.60 |
| Current smoker (%) | 33.6 | 33.5 | 0.10 |
| Current drinker (%) | 57.8 | 57.6 | 0.86 |
| Type 2 diabetes (%) | 22.6 | 23.8 | 0.66 |
| Hypertension (%) | 33.6 | 33.5 | 0.94 |
| Dietary intake of n-3 fatty acids (g/d) | 0.26±0.47 | 0.21±0.27 | 0.05 |
| Dietary intake of n-6 fatty acids (g/d) | 23.1±19.5 | 21.5±16.4 | 0.10 |
| Dietary intake of saturated fat (g/d) | 37.3±30.9 | 34.5±26.6 | 0.16 |
| Total daily energy intake (Kcal) | 2731.8±2125.0 | 2554.1±1804.6 | 0.15 |
| Total cholesterol(mg/dL) | 181.0±37.9 | 179.6±35.1 | 0.41 |
| Total triglyceride (mg/dL) | 169.6±182.5 | 162.9±128.2 | 0.13 |
| HDL (mg/dL) | 50.8±14.6 | 50.7±14.5 | 0.84 |
| LDL (mg/dL) | 98.2±29.6 | 97.8±28.9 | 0.90 |
| SBP (mmHg) | 122.7±17.0 | 122.5±17.6 | 0.08 |
| DBP(mmHg) | 76.3±11.1 | 75.8±11.3 | 0.07 |
| BMI (kg/m2)) | 32.2±7.9 | 32.4±7.9 | 0.29 |
| eGFR (ml/min/1.73 m2) | 100.2±27.8 | 100.0±30.8 | 0.53 |
| Carotid IMT (μm) | 661.86±160.08 | 671.05±161.90 | 0.97 |
| Vascular mass (mm2) | 13.51±4.35 | 13.65±4.55 | 0.71 |
| Plaque (%) | 29.12 | 30.44 | 0.46 |
| Plaque score | 0.24±0.40 | 0.25±0.41 | 0.89 |
Adjusted for age, sex, study center and family relatedness whenever appropriate by GEE
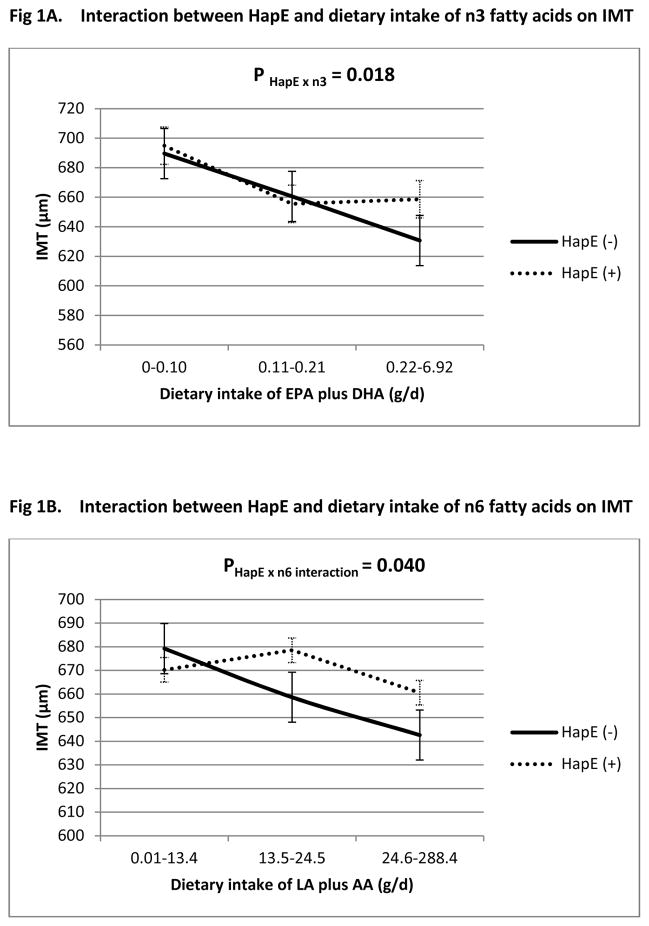
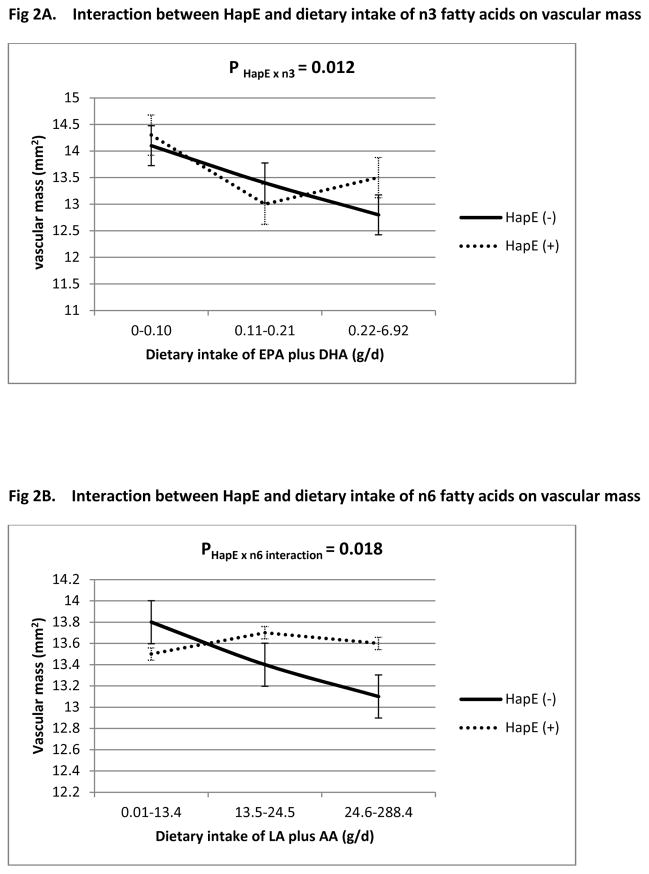
Multivariate GEE analysis indicated that, after adjusting for traditional risk factors, we did not detect a significant main effect of either haplotype or diet on any of the four vascular measures. However, we identified significant gene × diet interactions of HapE with dietary intake of both n-3 (P HapE × n3 = 0.018) and n-6 fatty acids (P HapE × n6 =0.040) on carotid IMT (Table 3). The direction of this nutrigenetic interaction is consistent with that identified previously in the Caucasian twins.21 Specifically, with increased consumption of dietary intake for n-3 or n-6 fatty acids, IMT decreases among both HapE carries and non-carriers, but the degree of this decrease differs according to HapE carrier status. This finding did not change after normalization of n-3 and n-6 PUFAs to the total caloric intake (g/Kcal). Significant HapE × diet interactions were also observed for vascular mass (P HapE × n3 = 0.012, P HapE × n6 = 0.018, Table 4). However, no significant interaction was observed for presence of atherosclerosis (plaque: P HapE × n3 = 0.312, P HapE × n6 = 0.350) or extent of atherosclerosis (plaque score: P HapE × n3 = 0.649, P HapE × n6 = 0.196). Figures 1 and 2 plot the nutrigenetic interactions for IMT and vascular mass, respectively. Sensitivity analyses demonstrated that, excluding participants with diabetes or obesity did not change our results (online supplementary Tables 1 and 2). There was no interaction of dietary intake of n-3 or n-6 fatty acids with diabetes or hypertension or obesity on any of the vascular measures. Both EPA and DHA significantly modified the effect of HapE on IMT (P HapE × EPA = 0.033, P HapE × DHA = 0.026) and vascular mass (P HapE × EPA =0.052, P HapE × DHA =0.018), suggesting that the observed gene × diet interactions on arterial hypertrophy are unlikely to be driven by a specific component in n-3 fatty acids. In contrast, only LA significantly interacted with HapE on IMT (P HapE × LA =0.042) and vascular mass (P HapE × LA =0.026), but not AA (P HapE x AA = 0.115 for IMT, P HapE x AA = 0.068 for vascular mass), indicating that the interaction of HapE with n-6 fatty acids could possibly be driven by LA. However, due to the results obtained by using the sum of EPA and DHA, or the sum of LA and AA, are similar to that by using a specific fatty acid, we chose to report results from the sum of fatty acids.
Table 3.
HapE × diet interaction on carotid IMT by multivariate GEE analysis in American Indians (total N=3402)
| Variables | HapE × n-3 PUFA
|
HapE × n-6 PUFA
|
||
|---|---|---|---|---|
| Coefficient | Multivariate-P* | Coefficient | Multivariate-P* | |
| HapE | 0.99 | 0.54 | 0.99 | 0.40 |
| n-3 fatty acids | 1.05 | 0.02 | 1.00 | 0.62 |
| n-6 fatty acids | 1.00 | 0.59 | 1.00 | 0.96 |
| n-3 × HapE | 1.05 | 0.018 | - | - |
| n-6 × HapE | - | - | 1.01 | 0.04 |
| Age | 1.01 | <0.0001 | 1.01 | <0.0001 |
| Female sex | 0.95 | <0.0001 | 0.95 | <0.0001 |
| Study center | 1.01 | 0.17 | 1.01 | 0.17 |
| BMI | 1.00 | 0.0005 | 1.00 | <0.0001 |
| SBP | 1.00 | <0.0001 | 1.00 | <0.0001 |
| Smoking | 1.02 | 0.02 | 1.02 | 0.02 |
| Alcohol use | 0.98 | 0.02 | 0.97 | 0.01 |
| Total cholesterol | 1.00 | 0.15 | 1.00 | 0.18 |
| Triglyceride | 1.00 | 0.08 | 1.00 | 0.07 |
| eGFR | 1.00 | 0.29 | 1.01 | 0.25 |
| Daily energy intake | 1.00 | 0.59 | 1.00 | 0.50 |
| Diabetes | 1.06 | <0.0001 | 1.06 | <0.0001 |
Adjusted for age, sex, study center, BMI, systolic blood pressure, diabetes, smoking, alcohol consumption, total cholesterol, triglyceride, eGFR, saturated fat and total daily energy intake. The interactions of HapE with n-3 or n-6 fatty acids were adjusted for one another.
Table 4.
HapE × diet interaction on vascular mass by multivariate GEE analysis in American Indians (total N=3402)
| Variables | HapE × n-3 PUFA
|
HapE × n-6 PUFA
|
||
|---|---|---|---|---|
| Coefficient | Multivariate-P* | Coefficient | Multivariate-P* | |
| HapE | 0.99 | 0.41 | 0.98 | 0.22 |
| n-3 fatty acids | 1.07 | 0.007 | 1.01 | 0.45 |
| n-6 fatty acids | 1.00 | 0.08 | 1.00 | 0.26 |
| n-3 × HapE | 1.07 | 0.013 | - | - |
| n-6 × HapE | - | - | 1.00 | 0.02 |
| Age | 1.01 | <0.0001 | 1.01 | <0.0001 |
| Female sex | 0.87 | <0.0001 | 0.87 | <0.0001 |
| Study center | 1.00 | 0.84 | 1.00 | 0.85 |
| BMI | 1.01 | <0.0001 | 1.01 | <0.0001 |
| SBP | 1.00 | <0.0001 | 1.00 | <0.0001 |
| Smoking | 1.03 | 0.009 | 1.03 | 0.01 |
| Alcohol use | -0.021 | 0.29 | 0.99 | 0.28 |
| Total cholesterol | 1.00 | 0.59 | 1.00 | 0.62 |
| Triglyceride | 1.00 | 0.30 | 1.00 | 0.29 |
| eGFR | 1.02 | 0.05 | 1.02 | 0.05 |
| Daily energy intake | 1.00 | 0.05 | 1.00 | 0.04 |
| Diabetes | 1.08 | <0.0001 | 1.08 | <0.0001 |
Adjusted for age, sex, study center, BMI, systolic blood pressure, diabetes, smoking, alcohol consumption, total cholesterol, triglyceride, eGFR, saturated fat and total daily energy intake. The interactions of HapE with n-3 or n-6 fatty acids were adjusted for one another.
Figure 1.
Interaction between HapE and dietary intake of n-3 (Fig. 1A) and n-6 (Fig. 1B) fatty acids on IMT. All interactions adjusted for age, sex, study center, BMI, systolic blood pressure, smoking, alcohol consumption, total cholesterol, triglyceride, eGFR, saturated fat and total daily energy intake. The interactions shown in Fig1 and 1B were adjusted for each other.
Figure 2.
Interaction between HapE and dietary intake of n-3 (Fig. 2A) and n-6 (Fig. 2B) fatty acids on vascular mass. All interactions adjusted for age, sex, study center, BMI, systolic blood pressure, smoking, alcohol consumption, total cholesterol, triglyceride, eGFR, saturated fat and total daily energy intake. The interactions shown in Fig2 and 2B were adjusted for each other.
Discussion
In a large population of American Indians, this study investigates the interaction of a leukotriene haplotype, designated HapE, with dietary intake of n-3 and n-6 fatty acids on four vascular measures (IMT, arterial mass, plaque and plaque score), independent of established cardiovascular risk factors. We observed significant evidence of nutrigenetic interaction on arterial hypertrophy (IMT and arterial mass), but not atherosclerosis (presence or extent of atherosclerosis). In a recent study, we reported interaction of this haplotype with dietary n-3 and n-6 fatty acids on carotid IMT in a sample of Caucasian twins.21 The current analysis not only confirms this nutrigenetic interaction on IMT with same direction, but also identifies interaction of this leukotriene haplotype with diet on arterial mass. These findings suggest a potential important role of diet in mediating the effect of leukotriene variants on carotid arterial hypertrophy. Gene × diet interaction is notoriously known for being difficult to replicate. American Indians may have different genetic makeup and dietary habits from other ethnic groups. Validation of nutrigenetic interaction in two distinct populations with consistent direction suggests the robustness of this interaction on atherosclerosis susceptibility. The observed haplotype × diet interaction persisted after adjusting for known cardiovascular risk factors, indicating that the interactive effects may affect arterial hypertrophy through pathways beyond established risk factors.
Although carotid IMT, arterial mass and plaque are highly correlated with each other, they may represent different manifestations of carotid artery morphology or pathological processes with distinctive biological determinants. For example, previous studies demonstrated that carotid IMT and plaque exhibited different patterns of association with risk factors43, 44 including genetic factors.22, 45, 46 In agreement with this, among American Indians participating in the SHFS, different measures of carotid artery structure exhibited different heritability estimates22 and prognostic value in predicting cardiovascular events,24 supporting the potential different biological pathways that might be implicated in these quantitative traits. It is thus plausible that HapE × diet interaction could affect some traits (e.g., IMT and vascular mass) but not others (e.g., atherosclerosis). The differential nutrigenetic interactions on carotid artery hypertrophy but not plaque formation further highlights the necessity in analyzing these quantitative and qualitative measures separately in future research.
Omega-3 and omega-6 are two polyunsaturated fatty acids essential for normal cell function. They are precursors of potent lipid mediator signaling molecules that have important roles in arterial wall inflammation and coronary atherosclerosis.19, 47 However, subjects with similar dietary habits or sharing a similar dietary pattern may have different risk of ACVD. The interindividual variability in response to diet could likely attribute to the different genetic background between individuals. In the current analysis of American Indians and a previous study of Caucasian twins, we demonstrated that habitual dietary consumption of n-3 and n-6 fatty acids significantly modifies the effect of HapE on carotid IMT and/or vascular mass. Our results reveal a novel biological pathway through which dietary n-3 and n-6 PUFAs affect leukotriene genetic variants, which have been implicated in vascular inflammation and atherosclerotic CVD.3–5, 13 Given that LTA4H and ALOX5 are involved in the same leukotriene pathway, our findings corroborate previous studies13, 14 demonstrating a role of gene × diet interaction in carotid atherosclerosis. The observation that a higher dietary intake of n-3 fatty acids is associated with smaller carotid IMT regardless of HapE carrier status supports the beneficial effect of n-3 fatty acids on human health,17, 39 though the cardioprotective effect of n-6 fatty acids remains controversial.48
LTA4H is a key enzyme involved in leukotriene A4 metabolism. Genetic variants in LTA4H may influence leukotriene production through regulating LTA4H expression, thereby contributing to arterial inflammation and atherosclerosis. A risk haplotype in LTA4H (called HapK) was previously associated with myocardial infarction in European Caucasians,5 but HapE defined in our study has distinct SNP configurations in comparison with HapK.21 For example, among the four SNPs shared by these two haplotypes (rs1978331, rs17677715, rs2540482 and rs2660845), susceptible alleles in three of four shared by them (rs1978331, rs2540482 and rs2660845) are different (i.e., alternative alleles). In addition, none of the haplotypes constructed using these three shared SNPs significantly interact with diet on either IMT or vascular mass. Thus, HapE identified in our study should represent a novel haplotype related to leukotriene production and resulting arterial inflammation. The differences between HapE and HapK have been reported elsewhere.21, 49
In an earlier study of Caucasian twins, we reported that HapE significantly protects against early atherosclerosis as measured by carotid IMT.21 However, such a beneficial main haplotypic effect was not observed in the current analysis of American Indians. This discrepancy is biologically plausible due to one or more of the following reasons. First, carotid IMT was measured differently in two populations. In the Strong Heart Family Study (SHFS), we measured carotid IMT in regions free of plaque, whereas in our previous twin study, carotid artery wall thickness was measured regardless of the absence or presence of plaque. This phenotypic heterogeneity could possibly contribute to the differential effect of HapE in two studies; Second, it is well appreciated that the effect of a genetic variant on phenotypic expression of a trait could be influenced by many factors, such as neighborhood genes (e.g., linkage disequilibrium, gene x gene interaction, etc) and/or environmental factors (e.g., gene x environment interaction, epigenetics). American Indians may be genetically and behaviorally distinct from Caucasians, and thus it is reasonable that HapE may affect IMT differently in two different populations. This further highlights the importance of investigating population-specific biological mechanisms, including gene x gene and gene x environmental interactions, in dissecting the genetic etiology of human complex traits.
Our findings should be interpreted in light of several limitations. First, we used FFQ to assess habitual intake of fatty acids, which may result in measurement errors. Second, the observed haplotype × diet interactions on IMT and vascular mass are statistical but not necessarily biological interactions. Third, due to lack of functional analysis, we cannot determine whether dietary intake of n-3 and n-6 fatty acids influences disease risk through changing leukotriene production or other biological pathways. Fourth, although we were able to control many known coronary risk factors, we cannot completely exclude the possibility of confounding by other unknown or unmeasured factors. In addition, though we cannot preclude recall bias as an explanation for our findings, recall bias seems unlikely because we used subclinical vascular measures of CVD. Finally, though our results are derived from a sample of American Indians with high rates of obesity and diabetes, and generalization to other ethnic groups with different risk profiles awaits further investigation, replication of the nutrigenetic on IMT in Caucasian twins suggests the robustness of this haplotype × diet interaction on carotid artery hypertrophy.
In summary, in a large well-characterized population of American Indians, we identified significant evidence for interactions of a leukotriene haplotype with diet on carotid IMT and vascular mass, but not discrete atherosclerotic plaque, independent of traditional coronary risk factors. The nutrigenetic interaction on IMT is in the same direction as that identified in a Caucasian population. Confirmation of gene ×diet interactions in two populations with distinct genetic background and lifestyle factors suggests that our findings are quite robust and may be generalizable to other ethnic groups. Our results provide useful information for CVD prevention and lifestyle intervention tailored to each individual patient.
Supplementary Material
Highlights.
Genetic variants in genes encoding leukotrienes have been associated with atherosclerosis, but it is unclear whether this association is modified by dietary intake of competing leukotriene substrates.
A recent study reported significant interaction of a leukotriene haplotype (i.e. HapE) with diet on carotid IMT in Caucasian twins.
Dietary intake of PUFAs significantly modifies the association of HapE with carotid artery hypertrophy in American Indians who may have distinct genetic background and dietary habits.
Acknowledgments
This study was supported by NIH grants K01AG034259, R01DK091369, R21HL092363 and cooperative agreement grants U01HL65520, U01HL41642, U01HL41652, U01HL41654, and U01HL65521, and Research Facilities Improvement Program Grant Number C06 RR013556. The authors would also like to thank the Strong Heart Study participants, Indian Health Service facilities, and participating tribal communities for their extraordinary cooperation and involvement, which has contributed to the success of the Strong Heart Study. Without their contributions this research would not have been possible.
Footnotes
Author contributions: J. Z. conceived the study, conducted data analyses and wrote the manuscript. S.C. and K.H. collected genotype data. M.J.R., R.B.D., F.Y., Y. Z., L.G.B., E.T.L., and B.V.H. contributed to discussions and edited/reviewed the manuscript. J.Z. takes full responsibility for the article financially and responsibility for its originality.
Conflict of Interest: No potential conflicts of interest relevant to this article were reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jala VR, Haribabu B. Leukotrienes and atherosclerosis: New roles for old mediators. Trends Immunol. 2004;25:315–322. doi: 10.1016/j.it.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Murphy RC, Gijon MA. Biosynthesis and metabolism of leukotrienes. The Biochemical Journal. 2007;405:379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- 3.Helgadottir A, Gretarsdottir S, St Clair D, Manolescu A, Cheung J, Thorleifsson G, et al. Association between the gene encoding 5-lipoxygenase-activating protein and stroke replicated in a scottish population. American Journal of Human Genetics. 2005;76:505–509. doi: 10.1086/428066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, et al. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36:233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 5.Helgadottir A, Manolescu A, Helgason A, Thorleifsson G, Thorsteinsdottir U, Gudbjartsson DF, et al. A variant of the gene encoding leukotriene a4 hydrolase confers ethnicity-specific risk of myocardial infarction. Nat Genet. 2006;38:68–74. doi: 10.1038/ng1692. [DOI] [PubMed] [Google Scholar]
- 6.Kajimoto K, Shioji K, Ishida C, Iwanaga Y, Kokubo Y, Tomoike H, et al. Validation of the association between the gene encoding 5-lipoxygenase-activating protein and myocardial infarction in a japanese population. Circ J. 2005;69:1029–1034. doi: 10.1253/circj.69.1029. [DOI] [PubMed] [Google Scholar]
- 7.Kaushal R, Pal P, Alwell K, Haverbusch M, Flaherty M, Moomaw C, et al. Association of alox5ap with ischemic stroke: A population-based case-control study. Human Genetics. 2007;121:601–607. doi: 10.1007/s00439-007-0338-y. [DOI] [PubMed] [Google Scholar]
- 8.Lohmussaar E, Gschwendtner A, Mueller JC, Org T, Wichmann E, Hamann G, et al. Alox5ap gene and the pde4d gene in a central european population of stroke patients. Stroke. 2005;36:731–736. doi: 10.1161/01.STR.0000157587.59821.87. [DOI] [PubMed] [Google Scholar]
- 9.Crosslin DR, Shah SH, Nelson SC, Haynes CS, Connelly JJ, Gadson S, et al. Genetic effects in the leukotriene biosynthesis pathway and association with atherosclerosis. Human Genetics. 2009;125:217–229. doi: 10.1007/s00439-008-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zintzaras E, Rodopoulou P, Sakellaridis N. Variants of the arachidonate 5-lipoxygenase-activating protein (alox5ap) gene and risk of stroke: A huge gene-disease association review and meta-analysis. American Journal of Epidemiology. 2009;169:523–532. doi: 10.1093/aje/kwn368. [DOI] [PubMed] [Google Scholar]
- 11.Dimitriou ME, Dedoussis GVZ. Gene–diet interactions in cardiovascular disease. Current Nutrition Reports. 2012;1:153–160. [Google Scholar]
- 12.Qi L. Gene-diet interactions in complex disease: Current findings and relevance for public health. Current Nutrition Reports. 2012;1:222–227. doi: 10.1007/s13668-012-0029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwyer JH, Allayee H, Dwyer KM, Fan J, Wu H, Mar R, et al. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 14.Allayee H, Baylin A, Hartiala J, Wijesuriya H, Mehrabian M, Lusis AJ, et al. Nutrigenetic association of the 5-lipoxygenase gene with myocardial infarction. The American Journal of Clinical Nutrition. 2008;88:934–940. doi: 10.1093/ajcn/88.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spector AA. Essentiality of fatty acids. Lipids. 1999;34 (Suppl):S1–3. doi: 10.1007/BF02562220. [DOI] [PubMed] [Google Scholar]
- 16.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 17.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, et al. Omega-6 fatty acids and risk for cardiovascular disease: A science advisory from the american heart association nutrition subcommittee of the council on nutrition, physical activity, and metabolism; council on cardiovascular nursing; and council on epidemiology and prevention. Circulation. 2009;119:902–907. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 18.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. American Journal of Clinical Nutrition. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 19.Sacks FM, Campos H. Polyunsaturated fatty acids, inflammation, and cardiovascular disease: Time to widen our view of the mechanisms. Journal of Clinical Endocrinology and Metabolism. 2006;91:398–400. doi: 10.1210/jc.2005-2459. [DOI] [PubMed] [Google Scholar]
- 20.Henderson WR., Jr The role of leukotrienes in inflammation. Ann Intern Med. 1994;121:684–697. doi: 10.7326/0003-4819-121-9-199411010-00010. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Goldberg J, Vaccarino V. Leukotriene a4 hydrolase haplotype, diet and atherosclerosis: A twin study. Atherosclerosis. 2013;226:238–244. doi: 10.1016/j.atherosclerosis.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.North KE, MacCluer JW, Devereux RB, Howard BV, Welty TK, Best LG, et al. Heritability of carotid artery structure and function: The strong heart family study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22:1698–1703. doi: 10.1161/01.atv.0000032656.91352.5e. [DOI] [PubMed] [Google Scholar]
- 23.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, et al. The strong heart study. A study of cardiovascular disease in american indians: Design and methods. American Journal of Epidemiology. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 24.Roman MJ, Kizer JR, Best LG, Lee ET, Howard BV, Shara NM, et al. Vascular biomarkers in the prediction of clinical cardiovascular disease: The strong heart study. Hypertension. 2012;59:29–35. doi: 10.1161/HYPERTENSIONAHA.111.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roman MJ, Pickering TG, Schwartz JE, Pini R, Devereux RB. Relation of arterial structure and function to left ventricular geometric patterns in hypertensive adults. Journal of the American College of Cardiology. 1996;28:751–756. doi: 10.1016/0735-1097(96)00225-2. [DOI] [PubMed] [Google Scholar]
- 26.Roman MJ, Saba PS, Pini R, Spitzer M, Pickering TG, Rosen S, et al. Parallel cardiac and vascular adaptation in hypertension. Circulation. 1992;86:1909–1918. doi: 10.1161/01.cir.86.6.1909. [DOI] [PubMed] [Google Scholar]
- 27.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. Journal of the American Dietetic Association. 1992;92:686–693. [PubMed] [Google Scholar]
- 28.Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the block98 food-frequency questionnaire in a sample of canadian women. Public Health Nutr. 2006;9:84–93. doi: 10.1079/phn2005763. [DOI] [PubMed] [Google Scholar]
- 29.Caan BJ, Slattery ML, Potter J, Quesenberry CP, Jr, Coates AO, Schaffer DM. Comparison of the block and the willett self-administered semiquantitative food frequency questionnaires with an interviewer-administered dietary history. American Journal of Epidemiology. 1998;148:1137–1147. doi: 10.1093/oxfordjournals.aje.a009598. [DOI] [PubMed] [Google Scholar]
- 30.Fretts AM, Howard BV, McKnight B, Duncan GE, Beresford SA, Mete M, et al. Associations of processed meat and unprocessed red meat intake with incident diabetes: The strong heart family study. American Journal of Clinical Nutrition. 2012;95:752–758. doi: 10.3945/ajcn.111.029942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the block, willett, and national cancer institute food frequency questionnaires: The eating at america’s table study. American Journal of Epidemiology. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 32.Vaccarino V, Brennan ML, Miller AH, Bremner JD, Ritchie JC, Lindau F, et al. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: A twin study. Biol Psychiatry. 2008;64:476–483. doi: 10.1016/j.biopsych.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee ET, Cowan LD, Welty TK, Sievers M, Howard WJ, Oopik A, et al. All-cause mortality and cardiovascular disease mortality in three american indian populations, aged 45–74 years, 1984–1988. The strong heart study. American Journal of Epidemiology. 1998;147:995–1008. doi: 10.1093/oxfordjournals.aje.a009406. [DOI] [PubMed] [Google Scholar]
- 34.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of ld and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 35.Fagundes NJ, Kanitz R, Eckert R, Valls AC, Bogo MR, Salzano FM, et al. Mitochondrial population genomics supports a single pre-clovis origin with a coastal route for the peopling of the americas. American Journal of Human Genetics. 2008;82:583–592. doi: 10.1016/j.ajhg.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dulik MC, Zhadanov SI, Osipova LP, Askapuli A, Gau L, Gokcumen O, et al. Mitochondrial DNA and y chromosome variation provides evidence for a recent common ancestry between native americans and indigenous altaians. American Journal of Human Genetics. 2012;90:229–246. doi: 10.1016/j.ajhg.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Battilana J, Cardoso-Silva L, Barrantes R, Hill K, Hurtado AM, Salzano FM, et al. Molecular variability of the 16p13.3 region in amerindians and its anthropological significance. Annals of Human Genetics. 2007;71:64–76. doi: 10.1111/j.1469-1809.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- 38.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among us men and women. Circulation. 2003;108:155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 40.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 41.Sekikawa A, Kadowaki T, El-Saed A, Okamura T, Sutton-Tyrrell K, Nakamura Y, et al. Differential association of docosahexaenoic and eicosapentaenoic acids with carotid intima-media thickness. Stroke. 2011;42:2538–2543. doi: 10.1161/STROKEAHA.110.613042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindqvist HM, Sandberg AS, Fagerberg B, Hulthe J. Plasma phospholipid epa and dha in relation to atherosclerosis in 61-year-old men. Atherosclerosis. 2009;205:574–578. doi: 10.1016/j.atherosclerosis.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 43.Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: The british regional heart study. Stroke. 1999;30:841–850. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- 44.Al-Shali K, House AA, Hanley AJ, Khan HM, Harris SB, Mamakeesick M, et al. Differences between carotid wall morphological phenotypes measured by ultrasound in one, two and three dimensions. Atherosclerosis. 2005;178:319–325. doi: 10.1016/j.atherosclerosis.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Moskau S, Golla A, Grothe C, Boes M, Pohl C, Klockgether T. Heritability of carotid artery atherosclerotic lesions: An ultrasound study in 154 families. Stroke. 2005;36:5–8. doi: 10.1161/01.STR.0000149936.33498.83. [DOI] [PubMed] [Google Scholar]
- 46.Sayed-Tabatabaei FA, van Rijn MJ, Schut AF, Aulchenko YS, Croes EA, Zillikens MC, et al. Heritability of the function and structure of the arterial wall: Findings of the erasmus rucphen family (erf) study. Stroke. 2005;36:2351–2356. doi: 10.1161/01.STR.0000185719.66735.dd. [DOI] [PubMed] [Google Scholar]
- 47.Deckelbaum RJ. N-6 and n-3 fatty acids and atherosclerosis: Ratios or amounts? Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:2325–2326. doi: 10.1161/ATVBAHA.110.214353. [DOI] [PubMed] [Google Scholar]
- 48.Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated fatty acids. Journal of Nutrition and Metabolism. 2012;2012:539426. doi: 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao J, Quyyumi AA, Patel R, Zafari AM, Veledar E, Onufrak S, et al. Sex-specific association of depression and a haplotype in leukotriene a4 hydrolase gene. Psychosom Med. 2009;71:691–696. doi: 10.1097/PSY.0b013e3181b05c57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.