Abstract
Conversion of persistent atrial fibrillation (AF) to sinus rhythm is frequently seen during the 3-day in-hospital loading period required during dofetilide initiation, but it is not known whether pharmacologic conversion (PC) without the need for electrical cardioversion (EC) is a predictor of long-term maintenance of sinus rhythm during continued therapy with dofetilide. We sought to test the hypothesis that PC predicts durable maintenance of sinus rhythm and determine additional predictors of long-term maintenance of sinus rhythm on dofetilide. We retrospectively reviewed all elective inpatient admissions for dofetilide loading from 2003 to 2011 at the University of Virginia. A multivariate Cox proportional hazards model was used to assess predictors of maintenance of sinus rhythm after in-hospital dofetilide loading. In all, 101 patients with a current duration of AF lasting for a median of 1.86 months (interquartile range 0.47 to 6.03) were included in the analysis. Forty-seven patients were in the PC group, whereas 54 patients were in the EC group. Patients in the PC group remained longer in sinus rhythm compared with the patients in the EC group (log-rank p =0.032). The seventy-fifth percentile for the current episode duration in the PC group was 5.77 months, indicating that even long-standing persistent AF frequently converted pharmacologically. Hypertension and a longer duration of the current AF episode were also predictors of recurrence in the multivariate model. In conclusion, PC during in-hospital dofetilide loading is an important predictor of durable response even in long-standing persistent patients, which has important public health implications for choice of therapy.
Dofetilide is a class III antiarrhythmic drug first approved by the Food and Drug Administration in 2000 for the cardioversion of patients in atrial fibrillation (AF) or atrial flutter and subsequent maintenance of sinus rhythm.1-3 Spontaneous conversion of persistent AF to sinus rhythm is frequently seen during the 3-day in-hospital loading period required during dofetilide initiation,4 but it is controversial whether pharmacologic conversion (PC) is a predictor of long-term maintenance of sinus rhythm during continued therapy with dofetilide.5 In addition, there are only limited data on patient characteristics that might predict conversion and/or long-term efficacy. In this retrospective review of our patient population with persistent AF treated with dofetilide, we sought to determine whether patients with PC to sinus rhythm after initiation of dofetilide without the need for electrical cardioversion (EC) have a more durable response to therapy compared with patients who require EC after dofetilide initiation to achieve normal sinus rhythm.
Methods
We retrospectively reviewed all elective inpatient admissions for dofetilide loading from 2003 to 2011 at the University of Virginia. Patients who were not in AF at the time of admission or began taking dofetilide immediately after undergoing pulmonary vein isolation procedure were excluded from the analysis. Before administration of the first dofetilide dose, renal function was evaluated and the estimated glomerular filtration rate calculated. The initial dose was selected according to package insert guidelines.6 Patients were admitted to the hospital for monitoring on day 1 and given the first dose in the evening. If AF persisted after 4 doses of dofetilide, patients were electrically cardioverted. All patients were monitored on an inpatient basis for 6 doses, as per guidelines. Renal function was assessed each day, and corrected QT intervals were assessed 2 hours after each dose. Guidelines for dosage adjustment during drug loading were followed. For those patients who pharmacologically converted, we calculated the mean number of doses to cardioversion. Guideline-based definitions for AF were used.7
After discharge from the initial hospitalization, patients underwent regular clinical follow-up with monitoring done per physician preference. Because patients had persistent AF before commencement of therapy, continuation of dofetilide without the need for additional cardioversion was considered treatment success, even if brief self-terminating recurrences might have occurred.
Two patients were treated with dofetilide on 2 separate occasions because of drug interruptions. If they were treated twice, the most recent treatment was included in the analysis. Echocardiographic data were obtained from a 2-dimensional echocardiography performed before dofetilide therapy or immediately after. We also recorded whether patients were in AF or normal rhythm at the time of the echocardiogram. Medical history and previous therapies were recorded from the medical record. Providers defined patients as having hypertension, obesity, and hyperlipidemia.
A univariate Cox regression analysis was performed to determine predictors of dofetilide success. A multivariate Cox analysis was then performed to determine predictors of acute failure. The continuous variables assessed were heart rate, corrected QT interval during AF, time between initial diagnosis of AF and initiation of dofetilide, and duration of the current persistent episode of AF. Categorical variables were recognized risk factors for AF such as hypertension,8 heart failure,9,10 obesity,11 type 2 diabetes mellitus,12 degree of left atrial enlargement13 (none, mild, moderate, or severe enlargement14), historical use of amiodarone or class IC drugs, previous ablation, gender, and final dofetilide dose. Finally, a multivariate logistic analysis was performed to identify predictors of the need for EC.
Statistical analysis was performed using SAS, version 9.3 (The SAS Institute, Cary, North Carolina). The Institutional Review Board for Human Subjects Research at the University of Virginia Health System approved this study.
Results
There were 101 patients who met entry criteria for the study (patient characteristics listed in Table 1). One patient received 2 doses of dofetilide, but because of QT prolongation, the medication was discontinued. This patient was not included in the analysis. No patient had torsades de pointes during loading. The mean age of the total population was 61 years. The group was 40% men with type 2 diabetes mellitus in 9%, hypertension in 63%, hyperlipidemia in 30%, and obesity in 17%; 23% of patients had used amiodarone previously, and 3% had previous use of flecainide or propafenone. Catheter ablation for AF had been attempted in 10% of patients.
Table 1.
Patient characteristics
| Variable | All, n = 101 (%) | PC Group, n = 47 (%) | EC Group, n = 54 (%) | p Value |
|---|---|---|---|---|
| Age, yrs, mean ± SD | 61.4 ± 11.5 | 61.3 ± 9.9 | 61.6 ± 12.9 | 0.89 |
| Women | 41 (40.6) | 18 (38.3) | 23 (42.6) | 0.66 |
| Time since AF Dx (yrs), median (IQR) | 3.82 (1.33–7.38) | 4.36 (1.33–7.29) | 3.47 (1.27–7.83) | 0.96 |
| Duration current AF (mo), median (IQR) | 1.86 (0.47–6.03) | 1.87 (0.45–5.77) | 1.37 (0.47–7.35) | 0.82 |
| Dofetilide dose (μg BID) | ||||
| 125 | 3 (3.0) | 1 (2.1) | 2 (3.7) | 0.71 |
| 250 | 45 (44.5) | 21 (44.7) | 24 (44.4) | |
| 500 | 53 (52.5) | 25 (53.1) | 28 (51.9) | |
| QTc, BL (ms), mean ± SD | 442 ± 33.4 | 439.4 ± 40.0 | 443.8 ± 26.7 | 0.53 |
| HR, BL (beats/min), mean ± SD | 92.9 ± 22.4 | 95.0 ± 24.2 | 91.1 ± 21.0 | 0.39 |
| Previous class IC use | 22 (21.8) | 14 (29.8) | 8 (14.8) | 0.07 |
| Previous amiodarone | 23 (22.8) | 11 (23.4) | 12 (22.2) | 0.89 |
| Previous AF ablation | 10 (9.9) | 7 (14.9) | 3 (5.6) | 0.12 |
| LA size | ||||
| Normal | 13 (12.9) | 6 (12.8) | 7 (13.0) | 0.35 |
| Mildly enlarged | 20 (19.8) | 16 (34.0) | 14 (25.9) | |
| Moderately enlarged | 20 (19.8) | 11 (23.4) | 9 (16.7) | |
| Severely enlarged | 20 (19.8) | 6 (12.8) | 14 (26.0) | |
| Not available | 18 (17.8) | 8 (17.0) | 10 (18.5) | |
| Diabetes mellitus | 9 (8.9) | 6 (12.8) | 3 (5.6) | 0.24 |
| Hypertension | 64 (63.4) | 28 (59.6) | 36 (66.7) | 0.46 |
| Hyperlipidemia | 30 (29.7) | 15 (31.9) | 15 (27.8) | 0.65 |
| Heart failure | 20 (19.8) | 8 (17.0) | 8 (17.0) | 0.51 |
| Obesity | 17 (16.8) | 8 (17.0) | 8 (17.0) | 0.96 |
Chi-square tests were used to test for differences between categorical variables. t Tests were used to test for differences between normally distributed continuous variables (HR, QTc, and age). Wilcoxon tests were used to test for differences between continuous variables that were not normally distributed (time since AF diagnosis and duration of current AF). LA sizes were defined by American Society of Echocardiography criteria.14
BID = twice daily; BL = baseline; Dx = diagnosis; IQR = interquartile range; LA = left atrium.
In the total cohort, the mean ejection fraction was 48% by echocardiography. Mean left atrial size was 4.7 cm. AF had been initially diagnosed a median of 3.82 years (interquartile range 1.33 to 7.38) before dofetilide initiation, and the current episode had begun a median of 1.86 months (interquartile range 0.47 to 6.03) before drug initiation: 47 (46%) of the total cohort of 101 patients converted pharmacologically after initiation of dofetilide (PC group), whereas 54 (54%) underwent planned EC after initiation of dofetilide (EC group) to restore sinus rhythm. Those who had successful PC did so after 2.5 doses. In each group, 8 patients had dose reductions because of QT prolongation, and the average dose did not differ between the groups. There were no significant differences between patients in the EC group and those in the PC group, although there was a trend for greater previous use of class IC drugs and a greater number of patients with previous AF ablation in the PC group. Previous amiodarone use did not differ significantly between these groups.
Patients in the PC group were more likely to remain longer in sinus rhythm compared with the patients in the EC group (Table 2 and Figure 1) based on Kaplan-Meier analysis. The interquartile range upper bound (seventy-fifth percentile) of duration of the current episode of AF was >5 months in the PC group, indicating that a number of patients with longer durations of persistent AF converted with dofetilide; 2.7 years after loading with dofetilide, 40% of patients remained in sinus rhythm. Patients were followed until dofetilide was stopped, and the longest follow-up period was 6.3 years. The mean times to recurrence in the PC and EC groups were 521 and 397 days, respectively.
Table 2.
Predictors of recurrence of AF
| Covariate | HR (95% CI) | p Value |
|---|---|---|
| Electrical cardioversion | 1.72 (1.04–2.84) | 0.0354 |
| Time since first AF diagnosis (yrs) | 0.98 (0.92–1.04) | 0.501 |
| Hypertension | 1.75 (1.04–2.95) | 0.036 |
| Duration of current AF episode (yrs) | 1.06 (1.01–1.12) | 0.018 |
| Amiodarone | 1.16 (0.66–2.05) | 0.602 |
| Class IC | 1.26 (0.72–2.20) | 0.416 |
| Previous AF ablation | 1.12 (0.53–2.36) | 0.764 |
| Age | 1.000 (0.980–1.021) | 0.989 |
| Gender | 0.96 (0.58–1.58) | 0.867 |
| Final dofetilide dose | 1.000 (0.998–1.002) | 0.982 |
| Baseline QTc | 1.000 (0.993–1.007) | 0.972 |
| Type 2 diabetes mellitus | 0.62 (0.19–1.98) | 0.419 |
| Dyslipidemia | 1.10 (0.65–1.87) | 0.717 |
| Obesity | 1.22 (0.64–2.35) | 0.542 |
Figure 1.
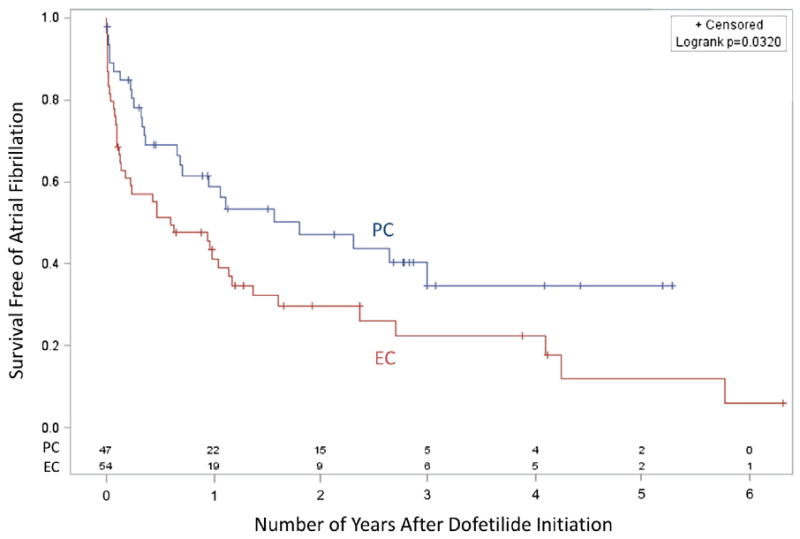
Kaplan-Meier survival curves based on the need for EC to restore sinus rhythm. Survival free of AF is shown based on whether patients were in the PC or EC groups.
In a univariate analysis, the need for EC during dofetilide loading (hazard ratio [HR] 1.72, 95% confidence interval [CI] 1.04 to 2.84) and the duration of the current AF episode (HR 1.06, 95% CI 1.01 to 1.12) were predictors of recurrence (Table 2), although the total time since AF diagnosis was not predictive. In a multivariate analysis (Table 3), the need for EC during loading (HR 1.66, 95% CI 1.002 to 2.77), the duration of the current AF episode in months (HR 1.08, 95% CI 1.02 to 1.13), and hypertension (HR 1.89, 95% CI 1.10 to 3.23) were independent predictors of drug failure.
Table 3.
Multivariate predictors of dofetilide failure
| Covariate | HR (95% CI) | p Value |
|---|---|---|
| Electrical cardioversion | 1.66 (1.002–2.77) | 0.049 |
| Duration of current AF episode (yrs) | 1.08 (1.02–1.13) | 0.004 |
| Hypertension | 1.89 (1.10–3.23) | 0.020 |
We then analyzed the data to determine whether certain recognized AF risk factors15 influenced whether patients converted to sinus rhythm without EC or required EC (PC vs EC). In the multivariate analysis, none of the variables included in the model were independently associated with whether patients required EC to achieve sinus rhythm during the dofetilide load. With the number of patients analyzed, some important associations may not be detected. Of note, antihypertensives used concurrently (β blockers, angiotensin- converting enzyme inhibitors) did not alter the efficacy of dofetilide for cardioversion.
Discussion
The key finding of the present study was that PC during in-hospital dofetilide initiation did, in fact, predict a more durable drug effect for maintenance of sinus rhythm. The high rate of PC in patients with persistent AF and even long- standing persistent AF was also an important observation with high clinical relevance.
Previous studies have indicated that dofetilide can be effective for restoring sinus rhythm in patients with persistent AF, with 1 study demonstrating that conversion can occur despite a duration of AF of 199 days.16 Our study, like others,16,17 demonstrates higher conversion rates than those noted in the European and Australian Multicenter Evaluative Research on Atrial Fibrillation Dofetilide (EMERALD) and Symptomatic Atrial Fibrillation Investigative Research on Dofetilide (SAFIRE-D)1 studies. Although our conversion rates were similar to those in the studies by Guanzon and Crouch17 and Prystowsky et al,16 these other studies included fewer patients than the present study and did not have a pure cohort of patients with persistent AF.
The present data indicate that conversion to sinus rhythm without the need for EC independently influences long-term maintenance of sinus rhythm. A likely reason for the difference between our conclusions and those of another small series5 is that there were significant differences in the arrhythmia characteristics of the patients in the studies. For example, the previous study included a number of patients with paroxysmal AF (33%),5 whereas the present study had predominantly patients with persistent AF and many with long-standing AF. This difference in efficacy may be related to the atrial substrate that is different in patients with paroxysmal AF versus patients with persistent AF. This observation also highlights the clinical importance of identifying patients who would be appropriate candidates for dofetilide therapy.
The present findings indicate that patients who have had persistent AF >5 months frequently convert pharmacologically to sinus rhythm with dofetilide therapy, even in a population of patients who have used other antiarrhythmic agents, including amiodarone. Furthermore, patients using dofetilide for maintenance of sinus rhythm did so for long periods of time, up to 6 years with a median duration of maintenance of sinus rhythm of 2.5 years. These results compare favorably with the results of the Canadian Trial of Atrial Fibrillation Investigators,18 in which amiodarone was used to assist with maintenance of sinus rhythm. Our results indicate a similar drug failure rate at 90 days; however, our population had longer durations of persistent AF and often long-standing persistent AF. Our results compare very favorably with this study of amiodarone,18 which had lower percentages of patients with persistent AF.
Considering the expense of inpatient therapy for patients embarking on dofetilide therapy, the question of whether PC predicts a more durable response to therapy than EC has significant public health importance. The present study suggests that conversion to sinus rhythm during dofetilide loading without the need for EC is a powerful and independent predictor of durability of sinus rhythm in these patients. In fact, in a univariate model to predict dofetilide failure, the need for EC and the duration of the current episode of AF were the only predictors of failure. Using a stepwise algorithm for model selection in a multivariate Cox analysis, hypertension was identified as an additional predictor, consistent with the previous data demonstrating that hypertension is a predominant risk factor for AF.8 Based on our findings, providers may now gain additional insight into the durability of dofetilide therapy based on whether patients require EC to achieve sinus rhythm, which should facilitate planning for subsequent steps in management and provide patients with realistic expectations regarding longterm efficacy of dofetilide therapy.
Using a secondary multivariate logistic analysis with the need for EC as the outcome of interest, we found no significant predictors of the need for EC. Interestingly, a previous study showed that failure to convert to sinus rhythm without EC during dofetilide loading was associated with larger left atrium diameter, longer duration of AF, and use of lower dosages of dofetilide.19 Our data did not confirm these associations.
This is a retrospective chart review of patients who underwent elective inpatient loading with dofetilide for persistent AF. One limitation is the documentation of persistence of AF. Although charts were reviewed, continuous monitoring was not performed clinically, so the exact nature of each patient’s AF could not be characterized beyond the medical record. Furthermore, the exact duration of AF could not be documented beyond the medical record.
Acknowledgments
This work was supported by National Institutes of Health K23 Grant HL094761, Bethesda, Maryland (to Dr. Bilchick).
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Singh S, Zoble RG, Yellen L, Brodsky MA, Feld GK, Berk M, Billing CB. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the Symptomatic Atrial Fibrillation Investigative Research on Dofetilide (SAFIRE-D) study. Circulation. 2000;102:2385–2390. doi: 10.1161/01.cir.102.19.2385. [DOI] [PubMed] [Google Scholar]
- 2.Roukoz H, Saliba W. Dofetilide: a new class III antiarrhythmic agent. Expert Rev Cardiovasc Ther. 2006;5:9–19. doi: 10.1586/14779072.5.1.9. [DOI] [PubMed] [Google Scholar]
- 3.Lenz TL, Hilleman DE. Dofetilide: a new antiarrhythmic agent approved for conversion and/or maintenance of atrial fibrillation/atrial flutter. Drugs Today (Barc) 2000;36:759–771. doi: 10.1358/dot.2000.36.11.601530. [DOI] [PubMed] [Google Scholar]
- 4.Bianconi L, Castro A, Dinelli M, Alboni P, Pappalardo A, Richiardi E, Santini M. Comparison of intravenously administered dofetilide versus amiodarone in the acute termination of atrial fibrillation and flutter. A multicentre, randomized, double-blind, placebo-controlled study. Euro Heart J. 2000;21:1265–1273. doi: 10.1053/euhj.1999.2039. [DOI] [PubMed] [Google Scholar]
- 5.Banchs J, Wolbrette D, Samii S, Penny-Peterson E, Patel P, Young S, Gonzalez M, Naccarelli G. Efficacy and safety of dofetilide in patients with atrial fibrillation and atrial flutter. J Interv Card Electrophysiol. 2008;23:111–115. doi: 10.1007/s10840-008-9290-6. [DOI] [PubMed] [Google Scholar]
- 6.USPI Tikosyn PDF Document. [January 15, 2013]; Available at: http://lp.ncdownloader.com/eb2/?q=USPI%20TIKOSYN%20PDF.
- 7.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey J-Y, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc J-J, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL ACC/AHA Task Force Members, ESC Committee for Practice Guidelines. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:700–752. [Google Scholar]
- 8.Verdecchia P, Reboldi G, Gattobigio R, Bentivoglio M, Borgioni C, Angeli F, Carluccio E, Sardone MG, Porcellati C. Atrial fibrillation in hypertension. Hypertension. 2003;41:218–223. doi: 10.1161/01.hyp.0000052830.02773.e4. [DOI] [PubMed] [Google Scholar]
- 9.Heist EK, Ruskin JN. Atrial fibrillation and congestive heart failure: risk factors, mechanisms, and treatment. Prog Cardiovasc Dis. 2006;48:256–269. doi: 10.1016/j.pcad.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Torp-Pedersen C, Møller M, Bloch-Thomsen PE, Køber L, Sandøe E, Egstrup K, Agner E, Carlsen J, Videbæk J, Jr, Marchant B, Camm AJ. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. New Engl JMed. 1999;341:857–865. doi: 10.1056/NEJM199909163411201. [DOI] [PubMed] [Google Scholar]
- 11.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. AmJ Med. 2005;118:489–495. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56–62. doi: 10.1016/j.amjcard.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry WL, Morganroth J, Pearlman AS, Clark CE, Redwood DR, Itscoitz SB, Epstein SE. Relation between echocardiographically determined left atrial size and atrial fibrillation. Circulation. 1976;53:273–279. doi: 10.1161/01.cir.53.2.273. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Schoonderwoerd BA, Smit MD, Pen L, Van Gelder IC. New risk factors for atrial fibrillation: causes of ‘not-so-lone atrial fibrillation’. Europace. 2008;10:668–673. doi: 10.1093/europace/eun124. [DOI] [PubMed] [Google Scholar]
- 16.Prystowsky EN, Freeland S, Branyas NA, Rardon DP, Fogel RI, Padanilam BJ, Rippy JS. Clinical experience with dofetilide in the treatment of patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14:S287–S290. doi: 10.1046/j.1540-8167.2003.90402.x. [DOI] [PubMed] [Google Scholar]
- 17.Guanzon AV, Crouch MA. Phase IV trial evaluating the effectiveness and safety of dofetilide. Ann Pharmacother. 2004;38:1142–1147. doi: 10.1345/aph.1D465. [DOI] [PubMed] [Google Scholar]
- 18.Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, Kus T, Lambert J, Dubuc M, Gagne’ P, Nattel S, Thibault B. Amiodarone to prevent recurrence of atrial fibrillation. New Engl J Med. 2000;342:913–920. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 19.Cotiga D, Arshad A, Aziz E, Joshi S, Koneru JN, Steinberg JS. Acute conversion of persistent atrial fibrillation during dofetilide initiation. Pacing Clin Electrophysiol. 2007;30:1527–1530. doi: 10.1111/j.1540-8159.2007.00902.x. [DOI] [PubMed] [Google Scholar]


