Abstract
The relationship between diabetes and pancreatic cancer is complex. Diabetes or impaired glucose tolerance is present in more than 2/3rd of pancreatic cancer patients. Epidemiological studies have consistently shown a modest increase in the risk of pancreatic cancer in type 2 diabetes, with an inverse relationship to duration of disease. Additionally, recent studies suggest that anti-diabetic medications may modulate the risk of pancreatic cancer in type 2 diabetes. Subjects >50 years of age with new onset diabetes are at higher risk of having pancreatic cancer. However, to screen new-onset diabetes for pancreatic cancer, additional markers are needed that can distinguish pancreatic cancer-associated diabetes from type 2 diabetes.
Keywords: Diabetes mellitus, Pancreatic cancer, Glucose intolerance, Hyperinsulinemia
In the United States, pancreatic cancer is the tenth most common cancer diagnosis; tenth most common cancer diagnosis but the fourth most common cause of death due to cancer. Only 10% to 20% of patients are resectable at the time of presentation.1 The American Cancer Society estimates that in, 2012, about 43920 people will be diagnosed with pancreatic cancer in the United States, and about 37,390 people will be die from it this year.2 The overall five-year survival rate in all patients is only 5% 3 and that has not significantly changed over the past five decades.
Diabetes mellitus comprises a large group of metabolic disorders characterized by elevated blood glucose levels due to decreased insulin production, insulin resistance or both. The American Diabetes Association classifies it as: 1) type 1 diabetes; 2) type 2 diabetes; 3) other specific types (almost 50 causes are listed); and 4) gestational diabetes.4 Type 1 diabetes is due to auto-immune destruction of insulin producing beta cells. Type 2 diabetes mellitus is likely a heterogeneous disorder which is not due to autoimmune disorder or other listed causes of diabetes; it accounts for 90–95% of all diabetes in the population. The prevalence of type 2 diabetes in the general population increases with age.
The relationship between diabetes mellitus and pancreatic cancer has been known for more than 125 years. Numerous studies have examined the relationship between the two diseases. They suggest that type 2 diabetes is a modest risk factor for the development of pancreatic cancer. More recent studies suggest that medications used to treat type 2 diabetes may independently modify the risk of cancer in diabetes. There is also strong clinical, epidemiological and experimental evidence to show that pancreatic cancer causes diabetes. This would suggest that diabetes caused by pancreatic cancer is distinct from type 2 diabetes. More importantly, new-onset diabetes may be a clue to the early diagnosis of the cancer. Here we review the complex relationship between the two conditions.
Prevalence of diabetes in pancreatic cancer
Numerous studies have reported the prevalence of diabetes in cancer in general and pancreatic cancer in particular. In studying prevalence, researches have taken a number of different approaches in defining the presence of diabetes in cases of controls. These include physician-recorded diagnosis of diabetes, International Classification of Diseases (ICD) codes for diabetes, self-reported diabetes by patients answering a questionnaire, patients on anti-diabetes medicationsa and laboratory records showing elevated fasting blood glucose values and elevated glycosylated hemoglobin values. Few studies have prospectively screened patients for diabetes using glucose tolerance tests or measuring fasting glucose values.
The prevalence of diabetes in pancreatic cancer has varied considerably (from 4% to 65%) in reported studies, variability that has largely resulted in ascertainment of diabetes noted above. In retrospective studies of medical records and studies relying on self-reported diabetes, the prevalence of diabetes in pancreatic cancer has varied from 4% to 20%.5 A recently published study using medical records to identify diabetes reported the prevalence of diabetes in pancreatic cancer to be 10% which was no different from that seen in the control non-cancer subjects.6
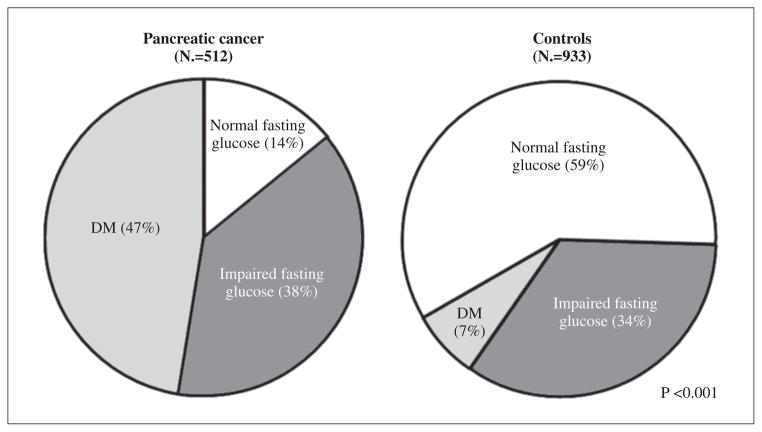
On the other hand, in studies where patients with pancreatic cancer were prospectively screened for diabetes or where fasting blood glucose data on patients was reviewed, the prevalence of diabetes has been shown to be very high (45% and 75%),7–9 a prevalence many times greater than that reported in the general population. A study by Permert et al.7 using glucose tolerance tests in patients with newly diagnosed pancreatic cancer showed that 75% of patients met criteria for diabetes. Pannala et al.10 used fasting blood glucose values or previous use of antidiabetic medications to define diabetes in patients with pancreatic cancer (N.=512) and age-matched control non-cancer subjects attending primary care clinics (N.=933) (Figure 1). They reported a nearly seven-fold higher prevalence of diabetes in pancreatic cancer patients compared to controls (47% vs. 7%). In a retrospective study using similar criteria, Chari et al. found the prevalence of diabetes in pancreatic cancer patients to be 40%.11
Figure 1.
Distribution of fasting blood glucose among pancreatic cancer cases and controls. From Pannala et al.10
In a recent study Agrawal et al.12 examined the reasons for the discrepancy in prevalence rates of diabetes in pancreatic cancer in retrospective epidemiologic studies and prospective clinical studies The study cohort consisted of 111 consecutive primary care clinic patients who were subsequently diagnosed with pancreatic cancer. Diabetes was defined if fasting glucose values met ADA criteria.4 In nearly one-third of pancreatic cancer patients who met the criteria for diabetes, the diabetes was never diagnosed by the clinician. It is well known that type 2 remains undiagnosed for a long duration before clinical diagnosis. In an NIDDK study, Harris et al.13 extrapolated the relation between retinopathy and diabetes duration to estimate the latency period and reported that diabetes may be present as long as 9 to 12 years prior to clinical diagnosis. Similarly, using a non-linear model, Thomson et al.14 estimated the latency period to be 7.6 years in an Egyptian population. In pancreatic cancer, the majority of diabetes is new onset (<3 years in duration) (see below). It is, therefore, not surprising that pancreatic cancer is diagnosed before the associated diabetes is diagnosed. Furthermore, in pancreatic patients in whom the diagnosis of diabetes and cancer are made concurrently, the diabetes diagnosis does not enter the medical records, as the grave prognosis of cancer makes the diagnosis of diabetes inconsequential. Thus, studies relying on medical records grossly underestimate the prevalence of diabetes in pancreatic cancer.
Since diabetes is a chronic disease, longstanding disease is eventually diagnosed and enters the medical records. The prevalence of long standing diabetes and its association with pancreatic cancer is therefore likely to be reasonably accurate (there are issues with accurate diagnosis of pancreatic cancer which are not addressed here). The difference in actual and reported prevalence of diabetes has greater implications for assessing the association between recent onset diabetes and pancreatic cancer. While most epidemiologic studies report an inverse duration-dependent risk of pancreatic cancer in diabetes, the relative risk of pancreatic cancer for diabetes of <3 years in these studies is grossly underestimated as, for calculation risck, they rely on diabetes diagnosed and reported by physicians. This fact is important as we review the reported risk of pancreatic cancer in subjects with diabetes.
Risk of pancreatic cancer in subjects with diabetes
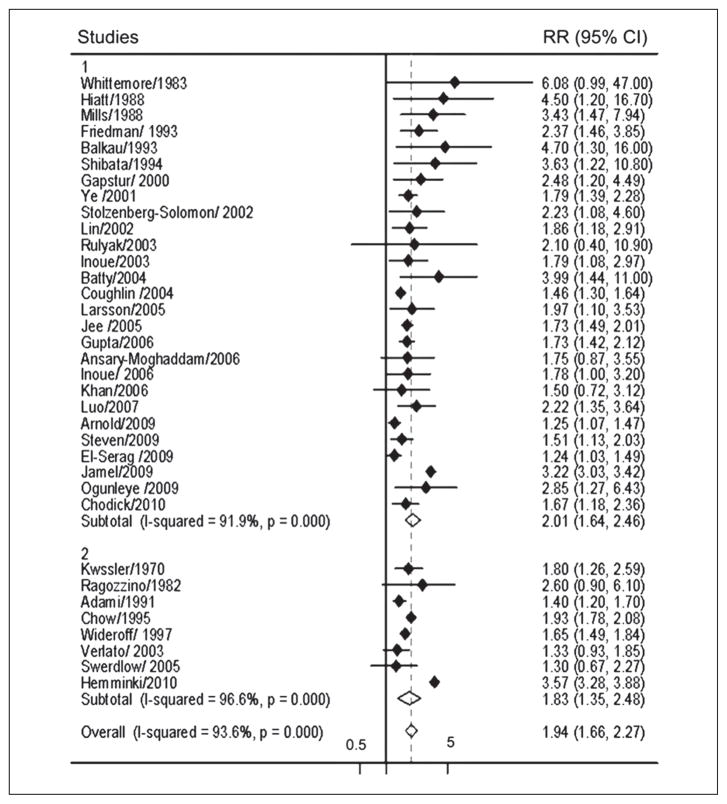
There have been numerous epidemiologic studies, both cohort and case-control, on the association between diabetes and pancreatic cancer. The studies have stratified the risk of cancer based on duration of diabetes. As is to be expected, a number of meta-analyses of these studies have been published (Everhart, Huxley).15, 16 The latest meta-analysis, in 2011 by Ben et al., includes 35 case-control and nested case-control studies from 1966 to 2010 17 (Figure 2) Studies with potential type 1 diabetes (defined as diabetes onset at early age, <30 years) patients and gestational diabetes were excluded.
Figure 2.
Relationship between type 2 diabetes and risk of pancreatic cancer in case-control and nested case control studies.
Diamond: point estimate representing study-specific relative risks or summary relative risks with 95% CIs. Horizontal lines: represent 95% confidence intervals (CIs). Test for heterogeneity among studies: P<0.001, I2=93.6%. 1, cohort studies (N.=27) use incidence or mortality rate as the measurements of relative risk; 2, cohort studies (N.=8) use standardized incidence/mortality rate as the measurement of relative risk. From Ben et al.17
The meta-analysis concluded that diabetes was associated with a twofold increased risk of pancreatic cancer (RR 1.94). Though there was significant heterogeneity among the studies, the RR was always >1. The increased risk of pancreatic cancer was independent of confounding factors such as sex, alcohol consumption, body mass index (BMI) and smoking status. Statistical analysis showed that individuals with the shorter duration of diabetes (1 to 4 years) had higher risk of developing pancreatic cancer than individuals who had duration of diabetes of 5 to 9 years (RR 1.95 versus 1.49) and more than 10 years (RR, 1.47) but significantly lower risk than in individuals who had diabetes less than 1 year (RR 5.38) (Table I). Thus, the RR of pancreatic cancer inversely correlated with duration of diabetes and the highest risk was found among patients who had been diagnosed with diabetes within less than 1 year.
Table I.
Relative risk of pancreatic cancer by duration of diabetes.
| Duration of diabetes | Number of studies | Relative risk (95% CI) |
|---|---|---|
| <1 year | 3 | 5.38 |
| 1–4 years | 5 | 1.95 |
| 5–9 years | 4 | 1.49 |
| ≥10 years | 4 | 1.47 |
| >1 | 14 | 1.96 |
| >5 | 11 | 1.83 |
Potential pathogenetic mechanisms for development of pancreatic cancer in long-standing type 2 diabetes
Diabetes is a disease associated with increased intracellular oxidative stress, as well as accelerated biochemical aging.18 Diabetes is also associated with shorter telomere length.19 These data would suggest that diabetes would predispose to cancer in general. In addition, diabetes and pancreatic cancer share common risk factors. Type 2 diabetes is strongly associated with obesity, insulin resistance and high levels of insulin. Obesity is an independent, albeit modest, risk factor for pancreatic cancer.20–23 Similarly, insulin resistance is seen in pancreatic cancer even in the absence of diabetes.24 High insulin levels have been associated with pancreatic cancer.25 A recent genetic case-control study showed an association between a single nucleotide polymorphism in the glucokinase receptor (GCKR) and pancreatic cancer.26 Though changes in diabetes could potentially predispose to cancer and pancreatic cancer and diabetes share a number of risk factors, a cause and effect relationship between diabetes and pancreatic cancer has not been firmly established.
Risk of pancreatic cancer is modified by anti-diabetes medications
A number of studies have been published looking at the risk of pancreatic cancer in diabetics, stratified by anti-diabetic medications. The studies suggest that while metformin is protective against development of pancreatic cancer, insulin and sulfonylureas increase the risk. However, the results should be interpreted with caution. The studies vary considerably in their methodology and the manner in which diabetes medications were ascertained. For example while some studies used patient recall to ascertain drug use,27 while others used pharmacy data.6 While some classified medication use as ever/never,27 others chose to classify by number of prescriptions issued.6 Also, some acknowledged that pancreatic cancer can cause or worsen diabetes (and, therefore, its treatment), most did not. The way studies dealt with the issue of pancreatic cancer-induced glucose intolerance also varies. While one study moved the index date by 2 years in cases and controls 6 to avoid cancer-induced medication changes, another study excluded those with diabetes <2 years in duration.27
Metformin use and risk of pancreatic cancer
A number of studies have reported pancreatic cancer incidence in metformin users. A recent meta-analysis was reported on pooled data from published studies.28 Although it concluded that metformin had a protective effect on overall cancer mortality (pooled risk ratio 0.66 [95% CI 0.49, 0.88]), the site specific analysis for pancreatic cancer using pooled data from 6 six studies showed a non-significant effect (pooled RR 0.48 [95% CI 0.2, 1.17]). A study by Bodmer et al.6 published after the meta-analysis by Ben et al. found no association between metformin use and pancreatic cancer. Overall, we believe that studies are too heterogeneous to provide reliable pooled data to clarify the association between metformin and pancreatic cancer risk.
Insulin and cancer risk
Insulin therapy is often required in longstanding type 2 diabetes. Insulin-glargine, the long-acting insulin analog, is the most widely used long acting insulin. Insulin-glargine has potential mitogenic and anti-apoptotic effects on cultured cancer cells through activation of the IGF-1 pathway.29–31 Several studies have evaluated the risk of cancer in patients on insulin-glargine.32–40 A heated debated has resulted from the conflicting results from these studies.
To determine if insulin glargine affected cardiovascular and other health outcomes, the ORIGIN (Outcome Reduction with Initial Glargine Intervention) study was initiated.41, 42 It is an international clinical trial assessing the effect of long-term (>6 years) therapy with basal insulin (supplied as insulin glargine) on cardiovascular and other health outcomes in pre-diabetes or type 2 diabetes). It is a large (12537 participants), multicenter (573 sites), multinational (40 countries) study where cancer outcomes were also assessed. The study has recently reported no difference in the incidence of cardiovascular events, cancers or other health outcomes in the insulin glargine and standard care groups (2.94 and 2.85 per 100 person-years; P=0.63) (http://origintrial.org/Default.aspx).
Chronic hyperglycemia and antidiabetic medications both have the potential to influence cell proliferation and resultant cancer risk. Therefore, the relationship among diabetes, anti-diabetes medications and cancer is indeed intriguing. The fact that this association has been the subject of a debate for decades and yet there are no clear answers suggests that the link is most certainly weak to modest and difficult to tease out due to the complexity of the factors involved. For most practitioners it remains an academic debate to date.
New-onset diabetes and pancreatic cancer
There is epidemiologic, clinical and experimental evidence to suggest that pancreatic cancer causes diabetes.
Onset of diabetes is temporally related to diagnosis of pancreatic cancer
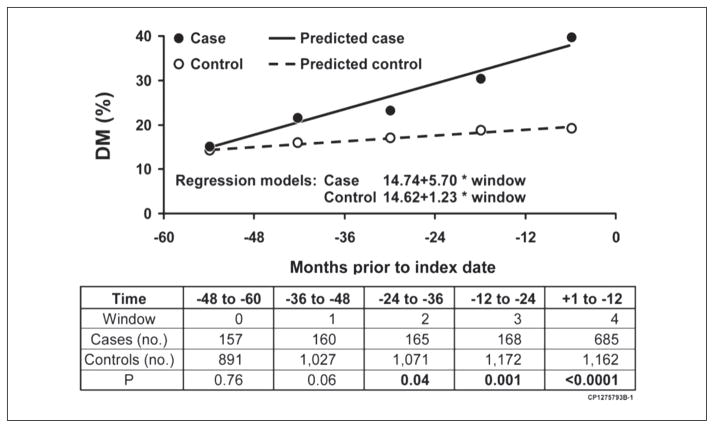
As already noted there is a very high prevalence of diabetes in pancreatic cancer. One epidemiologic study compared the prevalence of diabetes in pancreatic cancer with that in the general population yearly for up to 60 months before index date (date of cancer diagnosis in cases).11 The prevalence was similar in the two groups >36 months prior to index date and progressively increased starting 24 to 36 months prior to index date. Therefore, it appears that onset of cancer-induced diabetes is as long as 2 to 3 years before cancer diagnosis (Figure 3).
Figure 3.
Observed and expected prevalence of diabetes mellitus in 5 years before the pancreatic cancer diagnosis. From Chari et al.11
Majority of diabetes in pancreatic cancer is new-onset
When prospectively screened nearly half to two-thirds of pancreatic cancer patients have diabetes.7, 43–45 Nearly 75% of diabetes in pancreatic cancer is of recent onset. Pannala et al.10 compared the prevalence and clinical characteristics of diabetes in 512 newly diagnosed pancreatic cancer cases and 933 matched controls and found a high prevalence of diabetes (47% vs. 7%) and diabetes was predominantly of new onset (<2-year duration) (74% vs. 53%) among pancreatic cancer cases compared with controls. In another study, Agarwal et al.12 retrospectively reviewed the records of diabetic patients from 1995 to 2009 who were eventually diagnosed with pancreatic cancer. Of 51 diabetic pancreatic cancer patients, 30 (58%) had new-onset diabetes.
New-onset diabetes in pancreatic cancer resolves with resection of cancer
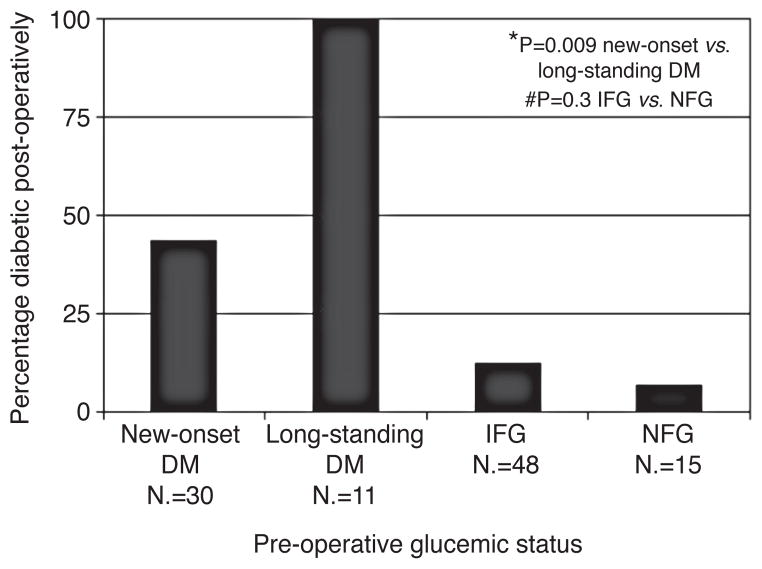
Three studies have reported that there is improvement in glucose tolerance following resection of pancreatic cancer. In a study using hyperglycemic glucose clamp and glucagon stimulation of seven pancreatic cancer patients Permert et al.46 found that six of them had diabetes before surgery and demonstrated improvement in the diabetic status after 85% subtotal pancreatectomy in all these patients. Similarly Fogar et al.43 reported improvement in oral glucose tolerance in 11 out of 15 patients (73%) after the tumor removal. Pannala et al. studied the effect of pancreatico-duodenectomy on diabetes status in 41 pancreatic cancer patients. Whereas diabetes resolved in 57% of patients with new-onset diabetes (N.=30), its prevalence remained unchanged in patients with longstanding diabetes (N.=11) (Figure 4).
Figure 4.
Diabetes mellitus (diabetes) prevalence after pancreaticoduodenectomy for pancreatic cancer (pancreatic cancer). From Pannala et al.10
Is new onset diabetes in pancreatic cancer a paraneoplastic phenomenon?
The data presented suggests that pancreatic cancer causes diabetes. The diabetes is not due to destruction of the gland as is seen in chronic pancreatitis. This is evident from the fact that at the onset of diabetes, the CT scan often shows a normal-appearing pancreas.47 Also, insulin levels in pancreatic cancer are high 24, 48 due to insulin resistance, rather than low, as would be seen if the diabetes had resulted from beta cell destruction. Therefore, diabetes must be a paraneoplastic phenomenon caused by tumor secreted products. This is also supported by the fact that supernatant from pancreatic cancer cell lines is metabolically active.49, 50 Pancreatic cancer cell lines appear to secrete diabetogenic substances that inhibit insulin release and cause insulin resistance. Pancreatic islets exposed to culture media conditioned by pancreatic cancer cell lines pANC-1 and HpAF II cells demonstrated decrease in insulin release.51 Also, in vitro studies of human skeletal muscle from patients with pancreatic cancer when compared with controls showed impaired insulin-mediated PI 3-kinase activity and glucose transport which in-turn contributed to the insulin resistance in these patients.52
Pancreatic cancer-induced diabetes is not type 3c diabetes mellitus
According to Center for Disease Control (CDC) (2011) diabetes affects 25.8 million people (8.3% of US population).53 Common forms of diabetes are type 1 (auto-immune) diabetes and type 2. The frequent association of exocrine and endocrine pancreas disease is consistent with an increased prevalence of pancreatic diabetes which is also called apancreatic diabetes or type 3 Diabetes Mellitus (type 3 diabetes).
It is known that the prevalence of diabetes is high in patients with exocrine pancreatic insufficiency. Ewald et al.54 investigated 1868 patients diagnosed with diabetes mellitus and showed that chronic pancreatitis is the most common cause of type 3c diabetes. In their study, 172 (9.2%) patients were classified as type 3c diabetes mellitus. Of these, 135 were diagnosed with chronic pancreatitis (78.5%), 12 with hereditary haemochromatosis, 14 with pancreatic cancer and 7 with cystic fibrosis. Thus, diabetes mellitus due to chronic pancreatitis occurred in this cohort in 7.2% of all diabetic subjects. Most type 3c diabetes patients were initially misclassified as type 2 diabetes (69/84).
Although the American Diabetic association classifies diabetes secondary to diseases of exocrine pancreas as type 3 c,4 the mechanisms and pathogenesis of diabetes in chronic pancreatitis and pancreatic cancer are totally different. Type 3c pancreatogenous diabetes or apancreatic diabetes is usually seen after resection of the pancreas 55 or secondary to severe chronic pancreatitis.54
With the exception of pancreatic cancer, other causes of pancreatogenous (type 3c) diabetes result from extensive damage or surgical removal of pancreas. This results in insulin and pancreatic polypeptide (PP) deficiency. The fact that even small pancreatic adenocarcinomas have been associated with diabetes implies a mechanism other than loss of beta-cell mass. The mediators of diabetes in pancreatic cancer are yet unknown.
New-onset diabetes in pancreatic cancer: implications for screening for cancer
Screening for sporadic pancreatic cancer has so far been considered unrealistic for two reasons: its incidence in the general population is low, and there is not yet a well-defined high-risk group to screen for this sporadic disease. Since type 2 diabetes is a very prevalent condition, screening for pancreatic cancer in this population will not be feasible. However, while type 2 diabetes is common, new-onset incident diabetes is far less common. The cohort of subjects with new-onset diabetes provides an intriguing opportunity to explore the possibility of screening for pancreatic cancer in these patients. However, a number of questions remain.
What is the prevalence of pancreatic cancer in new-onset diabetes?
Three studies shed some light on the question of prevalence of pancreatic cancer in new diabetes. Damiano et al.56 studied 115 patients aged over 50 who were hospitalized for new-onset (<30 days) diabetes as they required insulin. Routine imaging revealed pancreatic cancer in 5.2% of patients. However, the subset of patients who were investigated was symptomatic with acute abdominal symptoms. Not surprisingly, the majority of cancers identified were unresectable.56 Ogawa et al. (2002) 57 studied 87 patients with onset of diabetes at >55 years with elevation of serum CA 19-9 or CEA and underwent endoscopic retrograde cholangio pancreatography (ERCP). They found that neither CEA, CA 19-9 in pancreatic juice was useful in the diagnosis of pancreatic carcinoma and, obviously, did not help in early diagnosis. They also reported that the prevalence of pancreatic carcinoma in patients with diabetes mellitus of less than 3 years was 13.9%. In this study patients screened were not necessarily asymptomatic.
To estimate the probability of pancreatic cancer following diabetes Chari et al.58 assembled a population-based cohort of 2122 subjects with incident diabetes who were aged >50 years and identified those who developed pancreatic cancer within 3 years of meeting criteria for diabetes. Of the 2122 subjects with diabetes, 18 (0.85%) were diagnosed with pancreatic cancer. When compared with age matched controls in the general population, subjects with new onset diabetes were 8 times more likely to develop pancreatic cancer within three years of onset of diabetes.56 Interestingly ~50% were diagnosed >6 months after first meeting criteria for diabetes and this probability was more pronounced among those >70 years of age. The findings of this study remain to be confirmed.
Does diabetes occur early enough to be a marker of resectable pancreatic cancer?
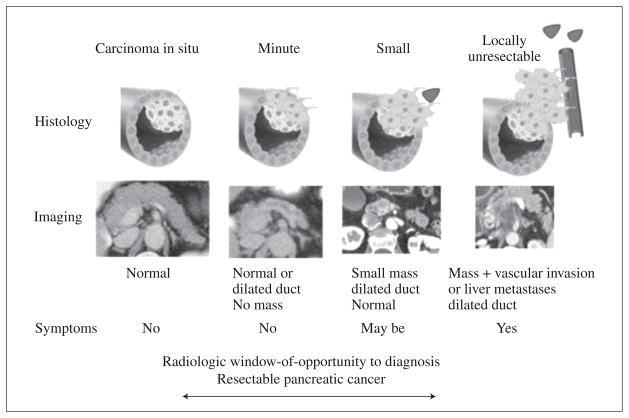
Retrospective review of CT scans done prior to diagnosis suggests that pancreatic cancer resectability may be significantly improved if detected as few as 6 months before clinical diagnosis of cancer.59 Another retrospective study 47 of CT scans suggested that pancreatic cancer is not visible at the time of onset of diabetes. In careful studies done on patients with serial blood sugars it has been observed that the median duration of diabetes prior to diagnosis of cancer is 6.5 months.12 In a recent study Agrawal et al.12 estimated that in ~25% of pancreatic cancer the onset of diabetes was >6 months prior to diagnosis of cancer, at a time when subjects had no cancer-related symptoms (Figure 5). Impaired fasting glucose is seen even earlier, but is far less predictive of cancer than of diabetes.
Figure 5.
Progression of pancreatic cancer. From Chari S.59
What is a potential strategy to screen for pancreatic cancer in new-onset diabetes?
The approach to screening new-onset diabetes for pancreatic cancer would include: 1) identifying patients with truly incident diabetes; 2) further risk stratifying using secondary screening; 3) performing imaging studies on the highest risk group to identify pancreatic cancer.
Step 1
Identifying subjects with new onset diabetes in the general population is the first step towards defining a high risk population for pancreatic cancer. This would require screening asymptomatic individuals of age >50 years for diabetes.9 For the strategy to succeed it would also require excluding people with prevalent, long-standing diabetes (even if it is not physician documented). This could be done by carefully reviewing previous fasting glucose values.
Step 2
Further enrichment of this cohort will be required before conducting invasive tests to identify the cancer. If the screening with a second filter is positive further confirmation would be done by more invasive radiological imaging and endoscopic ultrasound (Figure 6). The second filter could be a clinical phenotype, a unique serological marker or an abnormal non-invasive imaging for pancreatic cancer.
Figure 6.
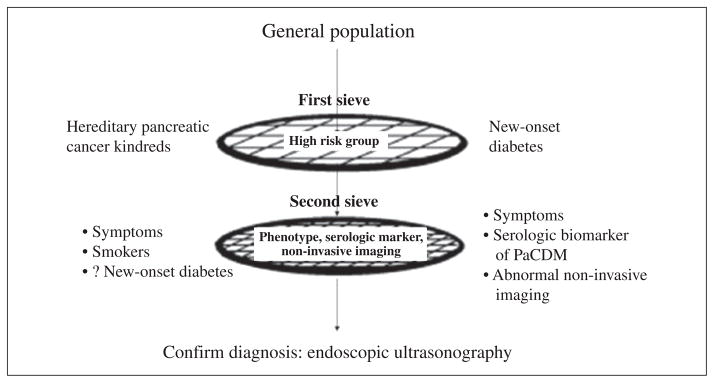
Model for screening for pancreatic cancer with new onset diabetes as the first filter. From Chari S.59
Step 3
The role of CT or MRI to detect asymptomatic small cancer is unknown. It may well require endoscopic ultrasound to identify small cancers associated with diabetes.
For the strategy to use new-onset diabetes to diagnose small pancreatic cancer, one will have to use additional clues to enrich the cohort of new-onset diabetes for pancreatic using clinical, biochemical markers or non-invasive imaging.
Clinical differences between pancreatic cancer-induced diabetes and type 2 diabetes
Some authors have suggested that pancreatic cancer-associated diabetes should be suspected if patient is lean and/or has no family history of diabetes.5 In other words atypical diabetes, occurring in the absence of canonical risk factors for diabetes, should raise suspicion for pancreatic cancer. However, when Pannala et al.10 carefully compared the clinical profiles of type 2 diabetes and pancreatic cancer associated diabetes they found no differences in normal adult body mass index or family history of diabetes. However, one clinical difference that has been observed is that pancreatic cancer patients with diabetes lose more weight than those without diabetes.12, 60 To examine this in greater detail, Hart et al.60 compared body weight (kg) and fasting blood glucose (mg/dL) at diabetes onset, 1 to 2 years before and at index date of cancer diagnosis in 29 pancreatic cancer patients and 43 type 2 diabetes subjects. Both groups had serial fasting blood glucose measurements, new-onset diabetes, and no cancer-specific symptoms at onset of diabetes. However at onset of diabetes, 59% of pancreatic cancer diabetes subjects lost weight compared to only 30% of type 2 diabetes subjects. On the other hand, 56% of type 2 diabetes subjects gained weight prior to onset of diabetes compared to 31% of diabetes subjects who had cancer. This study suggested that while weight gain is a typical feature which precedes type 2 diabetes, weight loss frequently precedes onset of diabetes in pancreatic cancer. They concluded that the paradoxical development of diabetes during ongoing weight loss may be a clue that the diabetes could be due to pancreatic cancer rather than type 2 diabetes.
Serological markers of pancreatic cancer
Attempts have been to identify serologic markers of pancreatic cancer, especially pancreatic cancer associated diabetes. In 1994, Permert et al.48 measured islet amyloid polypeptide (IAPP) (also called amylin) in plasma from 30 patients with pancreatic cancer, 46 patients with other cancers, 23 patients with diabetes, and 25 normal subjects. They reported that plasma IAPP concentrations were elevated in patients with pancreatic cancer, especially those who had diabetes. They hypothesized that since IAPP may cause insulin resistance, its overproduction may contribute to the diabetes that occurs in these patients. In other studies they showed that these patients had multiple hormone elevation in fasting state and that IAPP and glucagon, but not somatostatin, normalized following subtotal pancreatectomy.61 The animal studies carried out by Permert et al. in 2001 further confirmed that plasma IAPP (along with hyperglycemia and hyperinsulinemia) raises 27 weeks after the ductal-cell-specific carcinogen.62 However, in the same year Chari et al. published their results after measuring fasting serum glucose, IAPP, and CA 19-9 in 130 subjects with pancreatic cancer and 366 non-cancer subjects and concluded that IAPP is elevated in pancreatic cancer but is not sensitive enough to replace or complement existing tests.63
CA19-9
Among all the tumor associated antigens CA 19-9 is the most widely studied biomarker in pancreatic cancer. CA19-9 was initially found in colorectal cancer; however it is widely secreted in normal pancreas, stomach, and biliary epithelial cells. Adult pancreata normally express CA19-9 in about 80% of cases, usually in the ductal cells but not in the acinar and Langerhans islets.64 CA19-9 is elevated in cholestasis and when there are damaged bilio-pancreatic ducts, though the mechanisms are still obscure.65 This makes diagnostic value questionable when a patient has an obstructive jaundice.64 Also, CA19-9 levels are of limited value in differentiating whether cystic tumors of the pancreas are benign or malignant.66 Although CA19-9 is not accurate enough to be used in screening asymptomatic subjects for pancreatic cancer, it remains the best studied marker of pancreatic cancer with a sensitivity ranging from 70% to 90% and a specificity from 68% to 91% (Table II).66–92
Table II.
Sensitivity and specificity for biomarkers for pancreatic cancer.
| Biomarker | Study | Sensitivity | Specificity | N. |
|---|---|---|---|---|
| CA19-9 | Goonetilleke 68 | 79 | 82 | Meta-analysis |
| Steinberg 69 | 81 | 90 | Meta-analysis | |
| CA125 | Duraker 85 | 57 | 78 | 123 |
| Haguland 86 | 45 | 76 | 95 | |
| CEA | Ni 87 | 45 | 75 | 68 |
| Haglund 86 | 54 | 76 | 95 | |
| Zhao 88 | 25 | 86 | 143 | |
| Duraker 85 | 39 | 91 | 123 | |
| SPan-1 | Kiriyama 74 | 81 | 76 | 64 |
| Chung 89 | 92 | 83 | 67 | |
| Kobayashi 90 | 82 | 85 | 200 | |
| Du-PAN 2 | Satake 83 | 48 | 85 | 239 |
| Sawabu 91 | 72 | 94 | 32 | |
| Kawa 92 | 64 | - | 200 |
Other carbohydrate and glycoprotein antigens
Several other carbohydrate antigens have been studied in pancreatic cancer. The sensitivity and specificity of CA50, CA195, CA494 and CA125 are less than that of CA 19-9. CA125 has been found to be identical to the gene product of MUC16 of the MUC protein family.70 However, it cannot be used for screening pancreatic cancer with such low sensitivity and specificity and false elevations in other GI conditions like liver cirrhosis, pancreatitis and hepatitis.71 Carcinoembryonic antigen (CEA) 72 and pancreatic oncofetal antigen (POA) 73 are other glycoproteins studied in pancreatic cancer and they are not useful as biomarkers. Mucin glycoproteins such as Du-PAN 2, SPan-1 and CAM17.1 are also found in the sera of patients with pancreatic cancer.74 Du-PAN 2 is particularly interesting as it is seen in patients with Lewis a-b blood group type individuals where CA 19-9 may be absent.75 However, the sensitivity and specificity were less than that of CA 19-9. Span-1 has better sensitivity of 82% to 92%, but it does not improve the diagnostic accuracy obtained from CA19-9.76
Enzymes and hormones
Several enzymatic proteins and hormones like tumor-associated trypsin inhibitor (TATI),77 tumor M2-Pyruvate Kinase,78 galactosyltransferase Isoenzyme II,79 CEACAM1,80 macrophage inhibitory cytokine 1 (MIC-1),81 osteopontin (OPN) have been studied in pancreatic cancer. However, none of them performs better than CA 19-9. Despite so many markers being studied, none of these are very specific or yield greater diagnostic accuracy.82
Most tumor markers show a low sensitivity and specificity in the early stage of pancreatic carcinoma and are not appropriate for detecting early cancer. Even in early stage cancers CA 19-9 remains the best marker.83
Changes in non-invasive imaging
Retrospective review of computed tomography (CT) scans performed by prior to diagnosis suggests that pancreatic cancer resectability may be significantly improved if detected as few as 6 months before clinical diagnosis. However, the same studies suggest that computerized tomography (CT) may have limited value for detecting pancreatic cancers in asymptomatic subject. Pancreatic duct dilatation and cut-off are the only early findings associated with asymptomatic pancreatic cancer. However, such typical signs were seen only in 7% (1/15) of the scans obtained more than 18 months before diagnosis.84 Performing CT scans on all the patients with diabetes may therefore not be reasonable, and the search for an ideal biomarker with high sensitivity is still under way. Newer imaging techniques hold promise for detecting lesions not visible on standard cross sectional imaging.
Conclusions
Several studies have shown the high prevalence of diabetes in pancreatic cancer and demonstrated the temporal relationship of onset of recent diabetes and diagnosis of pancreatic cancer. The risk of developing pancreatic cancer is higher in new-onset diabetes with preceding weight loss. However, a biomarker or non-invasive imaging modality is needed to make further progress in screening for sporadic pancreatic cancer in new-onset diabetes.
Acknowledgments
Funding.—Dr. Chari was funded by grants from NIH (R01 CA 100685) and the Mayo Clinic Pancreas Cancer SPORE (P50 CA 102701).
References
- 1.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 2.Pancreatic cancer statistics. 1/13/2012. American Cancer Society; 2012. cancer.org. [Google Scholar]
- 3.Berrino F, De Angelis R, Sant M, Rosso S, Bielska-Lasota M, Coebergh JW, et al. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995–99: results of the EURO-CARE-4 study. Lancet Oncol. 2007;8:773–83. doi: 10.1016/S1470-2045(07)70245-0. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noy A, Bilezikian JP. Clinical review 63: Diabetes and pancreatic cancer: clues to the early diagnosis of pancreatic malignancy. J Clin Endocrinol Metab. 1994;79:1223–31. doi: 10.1210/jcem.79.5.7962312. [DOI] [PubMed] [Google Scholar]
- 6.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol. 2012;107:620–6. doi: 10.1038/ajg.2011.483. [DOI] [PubMed] [Google Scholar]
- 7.Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnqvist HJ, Larsson J. Pancreatic cancer is associated with impaired glucose metabolism. Eur J Surg. 1993;159:101–7. [PubMed] [Google Scholar]
- 8.Cersosimo E, Pisters PW, Pesola G, McDermott K, Bajorunas D, Brennan MF. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67:486–93. doi: 10.1002/1097-0142(19910115)67:2<486::aid-cncr2820670228>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10:88–95. doi: 10.1016/S1470-2045(08)70337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–7. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chari ST, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal G, Rabe KG, Petersen GM, Chari ST. New-onset diabetes in pancreatic cancer: A study in the primary care setting. Pancreatology. 2012;12:156–61. doi: 10.1016/j.pan.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4-7 yr before clinical diagnosis. Diabetes Care. 1992;15:815–9. doi: 10.2337/diacare.15.7.815. [DOI] [PubMed] [Google Scholar]
- 14.Thompson TJ, Engelgau MM, Hegazy M, Ali MA, Sous ES, Badran A, et al. The onset of NIDDM and its relationship to clinical diagnosis in Egyptian adults. Diabet Med. 1996;13:337–40. doi: 10.1002/(SICI)1096-9136(199604)13:4<337::AID-DIA71>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 15.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273:1605–9. [PubMed] [Google Scholar]
- 16.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W, et al. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer. 2011;47:1928–37. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Lyons TJ. Glycation, carbonyl stress, EAGLEs, and the vascular complications of diabetes. Semin Vasc Med. 2002;2:175–89. doi: 10.1055/s-2002-32041. [DOI] [PubMed] [Google Scholar]
- 19.Salpea KD, Humphries SE. Telomere length in atherosclerosis and diabetes. Atherosclerosis. 2010;209:35–8. doi: 10.1016/j.atherosclerosis.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arslan AA, Helzlsouer KJ, Kooperberg C, Shu XO, Steplowski E, Bueno-de-Mesquita HB, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan) Arch Intern Med. 2010;170:791–802. doi: 10.1001/archinternmed.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genkinger JM, Spiegelman D, Anderson KE, Bernstein L, van den Brandt PA, Calle EE, et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int J Cancer. 2011;129:1708–17. doi: 10.1002/ijc.25794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiao L, Berrington de Gonzalez A, Hartge P, Pfeiffer RM, Park Y, Freedman DM, et al. Body mass index, effect modifiers, and risk of pancreatic cancer: a pooled study of seven prospective cohorts. Cancer Causes Control. 2010;21:1305–14. doi: 10.1007/s10552-010-9558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 24.Chari ST, Zapiach M, Yadav D, Rizza RA. Beta-cell function and insulin resistance evaluated by HOMA in pancreatic cancer subjects with varying degrees of glucose intolerance. Pancreatology. 2005;5:229–33. doi: 10.1159/000085276. [DOI] [PubMed] [Google Scholar]
- 25.Stolzenberg-Solomon RZ, Graubard BI, Chari S, Limburg P, Taylor PR, Virtamo J, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294:2872–8. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 26.Prizment AE, Gross M, Rasmussen-Torvik L, Peacock JM, Anderson KE. Genes related to diabetes may be associated with pancreatic cancer in a population-based case-control study in Minnesota. Pancreas. 2012;41:50–3. doi: 10.1097/MPA.0b013e3182247625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–8. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7:e33411. doi: 10.1371/journal.pone.0033411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azar M, Lyons TJ. Diabetes, insulin treatment, and cancer risk: what is the evidence? F1000 Med Rep. 2010:2. doi: 10.3410/M2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer D, Shukla A, Enzmann H. Proliferative effects of insulin analogues on mammary epithelial cells. Arch Physiol Biochem. 2008;114:38–44. doi: 10.1080/13813450801900645. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev. 2009;25:41–9. doi: 10.1002/dmrr.912. [DOI] [PubMed] [Google Scholar]
- 32.Liefvendahl E, Arnqvist HJ. Mitogenic effect of the insulin analogue glargine in malignant cells in comparison with insulin and IGF-I. Horm Metab Res. 2008;40:369–74. doi: 10.1055/s-2008-1062739. [DOI] [PubMed] [Google Scholar]
- 33.Staiger K, Hennige AM, Staiger H, Haring HU, Kellerer M. Comparison of the mitogenic potency of regular human insulin and its analogue glargine in normal and transformed human breast epithelial cells. Horm Metab Res. 2007;39:65–7. doi: 10.1055/s-2007-957352. [DOI] [PubMed] [Google Scholar]
- 34.Hemkens LG, Grouven U, Bender R, Gunster C, Gutschmidt S, Selke GW, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–44. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonasson JM, Ljung R, Talback M, Haglund B, Gudbjornsdottir S, Steineck G. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia. 2009;52:1745–54. doi: 10.1007/s00125-009-1444-2. [DOI] [PubMed] [Google Scholar]
- 36.Colhoun HM. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia. 2009;52:1755–65. doi: 10.1007/s00125-009-1453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–77. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 38.Rosenstock J, Fonseca V, McGill JB, Riddle M, Halle JP, Hramiak I, et al. Similar risk of malignancy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: findings from a 5 year randomised, open-label study. Diabetologia. 2009;52:1971–3. doi: 10.1007/s00125-009-1452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Home PD, Lagarenne P. Combined randomised controlled trial experience of malignancies in studies using insulin glargine. Diabetologia. 2009;52:2499–506. doi: 10.1007/s00125-009-1530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dejgaard A, Lynggaard H, Rastam J, Krogsgaard Thomsen M. No evidence of increased risk of malignancies in patients with diabetes treated with insulin detemir: a meta-analysis. Diabetologia. 2009;52:2507–12. doi: 10.1007/s00125-009-1568-4. [DOI] [PubMed] [Google Scholar]
- 41.Gerstein HYS, Riddle MC, Ryden L, Bosch J. ORIGIN (Outcome Reduction with Initial Glargine Intervention) 2008. Hamilton, Canada: 2008. [Google Scholar]
- 42.Gerstein H, Yusuf S, Riddle MC, Ryden L, Bosch J. Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention) Am Heart J. 2008;155:26–32. e1–6. doi: 10.1016/j.ahj.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Fogar P, Pasquali C, Basso D, Sperti C, Panozzo MP, Tessari G, et al. Diabetes mellitus in pancreatic cancer follow-up. Anticancer Res. 1994;14:2827–30. [PubMed] [Google Scholar]
- 44.Permert J, Larsson J, Ihse I, Pour PM. Diagnosis of pancreatic cancer. Alteration of glucose metabolism. Int J Pancreatol. 1991;9:113–7. doi: 10.1007/BF02925586. [DOI] [PubMed] [Google Scholar]
- 45.Gullo L, Ancona D, Pezzilli R, Casadei R, Campione O. Glucose tolerance and insulin secretion in pancreatic cancer. Ital J Gastroenterol. 1993;25:487–9. [PubMed] [Google Scholar]
- 46.Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnquist HJ, Larsson J. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg. 1993;80:1047–50. doi: 10.1002/bjs.1800800841. [DOI] [PubMed] [Google Scholar]
- 47.Pelaez-Luna M, Takahashi N, Fletcher JG, Chari ST. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol. 2007;102:2157–63. doi: 10.1111/j.1572-0241.2007.01480.x. [DOI] [PubMed] [Google Scholar]
- 48.Permert J, Larsson J, Westermark GT, Herrington MK, Christmanson L, Pour PM, et al. Islet amyloid polypeptide in patients with pancreatic cancer and diabetes. N Engl J Med. 1994;330:313–8. doi: 10.1056/NEJM199402033300503. [DOI] [PubMed] [Google Scholar]
- 49.Basso D, Brigato L, Veronesi A, Panozzo MP, Amadori A, Plebani M. The pancreatic cancer cell line MIA PaCa2 produces one or more factors able to induce hyperglycemia in SCID mice. Anticancer Res. 1995;15:2585–8. [PubMed] [Google Scholar]
- 50.Basso D, Valerio A, Brigato L, Panozzo MP, Miola M, Lucca T, et al. An unidentified pancreatic cancer cell product alters some intracellular pathways of glucose metabolism in isolated rat hepatocytes. Pancreas. 1997;15:132–8. doi: 10.1097/00006676-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Wang F, Larsson J, Abdiu A, Gasslander T, Westermark P, Adrian TE, et al. Dissociated secretion of islet amyloid polypeptide and insulin in serum-free culture media conditioned by human pancreatic adenocarcinoma cell lines. Int J Pancreatol. 1997;21:157–64. doi: 10.1007/BF02822387. [DOI] [PubMed] [Google Scholar]
- 52.Isaksson B, Strommer L, Friess H, Buchler MW, Herrington MK, Wang F, et al. Impaired insulin action on phosphatidylinositol 3-kinase activity and glucose transport in skeletal muscle of pancreatic cancer patients. Pancreas. 2003;26:173–7. doi: 10.1097/00006676-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 53.CDC National Diabetes Fact Sheet. Center for Disease Control Center for Disease Control; 2011. January 10, 2012 ed. [Google Scholar]
- 54.Ewald N, Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c) Diabetes Metab Res Rev. 2012;28:338–42. doi: 10.1002/dmrr.2260. [DOI] [PubMed] [Google Scholar]
- 55.Slezak LA, Andersen DK. Pancreatic resection: effects on glucose metabolism. World J Surg. 2001;25:452–60. doi: 10.1007/s002680020337. [DOI] [PubMed] [Google Scholar]
- 56.Damiano J, Bordier L, Le Berre JP, Margery J, Dupuy O, Mayaudon H, et al. Should pancreas imaging be recommanded in patients over 50 years when diabetes is discovered because of acute symptoms? Diabetes Metab. 2004;30:203–7. doi: 10.1016/s1262-3636(07)70111-8. [DOI] [PubMed] [Google Scholar]
- 57.Ogawa Y, Tanaka M, Inoue K, Yamaguchi K, Chijiiwa K, Mizumoto K, et al. A prospective pancreatographic study of the prevalence of pancreatic carcinoma in patients with diabetes mellitus. Cancer. 2002;94:2344–9. doi: 10.1002/cncr.10493. [DOI] [PubMed] [Google Scholar]
- 58.Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504–11. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chari ST. Detecting early pancreatic cancer: problems and prospects. Semin Oncol. 2007;34:284–94. doi: 10.1053/j.seminoncol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hart PA, Kamada P, Rabe KG, Srinivasan S, Basu A, Aggarwal G, et al. Weight loss precedes cancer-specific symptoms in pancreatic cancer-associated diabetes mellitus. Pancreas. 2011;40:768–72. doi: 10.1097/MPA.0b013e318220816a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Permert J, Larsson J, Fruin AB, Tatemoto K, Herrington MK, von Schenck H, et al. Islet hormone secretion in pancreatic cancer patients with diabetes. Pancreas. 1997;15:60–8. doi: 10.1097/00006676-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 62.Permert J, Herrington M, Kazakoff K, Pour PM, Adrian TE. Early changes in islet hormone secretion in the hamster pancreatic cancer model. Teratog Carcinog Mutagen. 2001;21:59–67. [PubMed] [Google Scholar]
- 63.Chari ST, Klee GG, Miller LJ, Raimondo M, DiMagno EP. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology. 2001;121:640–5. doi: 10.1053/gast.2001.27210. [DOI] [PubMed] [Google Scholar]
- 64.Eskelinen M, Haglund U. Developments in serologic detection of human pancreatic adenocarcinoma. Scand J Gastroenterol. 1999;34:833–44. doi: 10.1080/003655299750025273. [DOI] [PubMed] [Google Scholar]
- 65.Rhodes JM, Ching CK. Serum diagnostic tests for pancreatic cancer. Baillieres Clin Gastroenterol. 1990;4:833–52. doi: 10.1016/0950-3528(90)90022-9. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 67.Audisio RA, Veronesi P, Maisonneuve P, Chiappa A, Andreoni B, Bombardieri E, et al. Clinical relevance of serological markers in the detection and follow-up of pancreatic adenocarcinoma. Surg Oncol. 1996;5:49–63. doi: 10.1016/s0960-7404(96)80001-6. [DOI] [PubMed] [Google Scholar]
- 68.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266–70. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 69.Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. Am J Gastroenterol. 1990;85:350–5. [PubMed] [Google Scholar]
- 70.Ringel J, Lohr M. The MUC gene family: their role in diagnosis and early detection of pancreatic cancer. Mol Cancer. 2003;2:9. doi: 10.1186/1476-4598-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herlyn M, Sears HF, Steplewski Z, Koprowski H. Monoclonal antibody detection of a circulating tumor-associated antigen. I. Presence of antigen in sera of patients with colorectal, gastric, and pancreatic carcinoma. J Clin Immunol. 1982;2:135–40. doi: 10.1007/BF00916897. [DOI] [PubMed] [Google Scholar]
- 72.Podolsky DK. Serologic markers in the diagnosis and management of pancreatic carcinoma. World J Surg. 1984;8:822–30. doi: 10.1007/BF01656021. [DOI] [PubMed] [Google Scholar]
- 73.Basso D, Fabris C, Panucci A, Del Favero G, Angonese C, Plebani M, et al. Tissue polypeptide antigen, galactosyltransferase isoenzyme II and pancreatic oncofetal antigen serum determination: role in pancreatic cancer diagnosis. Int J Pancreatol. 1988;3(Suppl 1):S95–100. [PubMed] [Google Scholar]
- 74.Kiriyama S, Hayakawa T, Kondo T, Shibata T, Kitagawa M, Ono H, et al. Usefulness of a new tumor marker, Span-1, for the diagnosis of pancreatic cancer. Cancer. 1990;65:1557–61. doi: 10.1002/1097-0142(19900401)65:7<1557::aid-cncr2820650718>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 75.Takasaki H, Uchida E, Tempero MA, Burnett DA, Metzgar RS, Pour PM. Correlative study on expression of CA 19-9 and DU-PAN-2 in tumor tissue and in serum of pancreatic cancer patients. Cancer Res. 1988;48:1435–8. [PubMed] [Google Scholar]
- 76.Frena A. SPan-1 and exocrine pancreatic carcinoma. The clinical role of a new tumor marker. Int J Biol Markers. 2001;16:189–97. doi: 10.1177/172460080101600306. [DOI] [PubMed] [Google Scholar]
- 77.Pasanen PA, Eskelinen M, Partanen K, Pikkarainen P, Penttila I, Alhava E. Tumour-associated trypsin inhibitor in the diagnosis of pancreatic carcinoma. J Cancer Res Clin Oncol. 1994;120:494–7. doi: 10.1007/BF01191804. [DOI] [PubMed] [Google Scholar]
- 78.Cerwenka H, Aigner R, Bacher H, Werkgartner G, el-Shabrawi A, Quehenberger F, et al. TUM2-PK (pyruvate kinase type tumor M2), CA19-9 and CEA in patients with benign, malignant and metastasizing pancreatic lesions. Anticancer Res. 1999;19:849–51. [PubMed] [Google Scholar]
- 79.Uemura M, Winant RC, Brandt AE. Immunoassay of serum galactosyltransferase isoenzyme II in cancer patients and control subjects. Cancer Res. 1988;48:5335–41. [PubMed] [Google Scholar]
- 80.Simeone DM, Ji B, Banerjee M, Arumugam T, Li D, Anderson MA, et al. CEACAM1, a novel serum biomarker for pancreatic cancer. Pancreas. 2007;34:436–43. doi: 10.1097/MPA.0b013e3180333ae3. [DOI] [PubMed] [Google Scholar]
- 81.Koopmann J, Rosenzweig CN, Zhang Z, Canto MI, Brown DA, Hunter M, et al. Serum markers in patients with resectable pancreatic adenocarcinoma: macrophage inhibitory cytokine 1 versus CA19-9. Clin Cancer Res. 2006;12:442–6. doi: 10.1158/1078-0432.CCR-05-0564. [DOI] [PubMed] [Google Scholar]
- 82.Harsha HC, Kandasamy K, Ranganathan P, Rani S, Ramabadran S, Gollapudi S, et al. A compendium of potential biomarkers of pancreatic cancer. PLoS Med. 2009;6:e1000046. doi: 10.1371/journal.pmed.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Satake K, Chung YS, Umeyama K, Takeuchi T, Kim YS. The possibility of diagnosing small pancreatic cancer (less than 4.0 cm) by measuring various serum tumor markers. A retrospective study. Cancer. 1991;68:149–52. doi: 10.1002/1097-0142(19910701)68:1<149::aid-cncr2820680127>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 84.Gangi S, Fletcher JG, Nathan MA, Christensen JA, Harmsen WS, Crownhart BS, et al. Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: retrospective review of CT scans obtained before diagnosis. AJR Am J Roentgenol. 2004;182:897–903. doi: 10.2214/ajr.182.4.1820897. [DOI] [PubMed] [Google Scholar]
- 85.Duraker N, Hot S, Polat Y, Hobek A, Gencler N, Urhan N. CEA, CA 19-9, and CA 125 in the differential diagnosis of benign and malignant pancreatic diseases with or without jaundice. J Surg Oncol. 2007;95:142–7. doi: 10.1002/jso.20604. [DOI] [PubMed] [Google Scholar]
- 86.Haglund C. Tumour marker antigen CA125 in pancreatic cancer: a comparison with CA19-9 and CEA. Br J Cancer. 1986;54:897–901. doi: 10.1038/bjc.1986.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ni XG, Bai XF, Mao YL, Shao YF, Wu JX, Shan Y, et al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 2005;31:164–9. doi: 10.1016/j.ejso.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 88.Zhao XY, Yu SY, Da SP, Bai L, Guo XZ, Dai XJ, et al. A clinical evaluation of serological diagnosis for pancreatic cancer. World J Gastroenterol. 1998;4:147–9. doi: 10.3748/wjg.v4.i2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chung YS, Ho JJ, Kim YS, Tanaka H, Nakata B, Hiura A, et al. The detection of human pancreatic cancer-associated antigen in the serum of cancer patients. Cancer. 1987;60:1636–43. doi: 10.1002/1097-0142(19871001)60:7<1636::aid-cncr2820600736>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 90.Kobayashi T, Kawa S, Tokoo M, Oguchi H, Kiyosawa K, Furuta S, et al. Comparative study of CA-50 (time-resolved fluoroimmunoassay), Span-1, and CA19-9 in the diagnosis of pancreatic cancer. Scand J Gastroenterol. 1991;26:787–97. doi: 10.3109/00365529108998600. [DOI] [PubMed] [Google Scholar]
- 91.Sawabu N, Toya D, Takemori Y, Hattori N, Fukui M. Measurement of a pancreatic cancer-associated antigen (DU-PAN-2) detected by a monoclonal antibody in sera of patients with digestive cancers. Int J Cancer. 1986;37:693–6. doi: 10.1002/ijc.2910370509. [DOI] [PubMed] [Google Scholar]
- 92.Kawa S, Oguchi H, Kobayashi T, Tokoo M, Furuta S, Kanai M, et al. Elevated serum levels of Dupan-2 in pancreatic cancer patients negative for Lewis blood group phenotype. Br J Cancer. 1991;64:899–902. doi: 10.1038/bjc.1991.422. [DOI] [PMC free article] [PubMed] [Google Scholar]