Focal adhesion kinase (FAK) is a non-receptor protein tyrosine kinase critical in mediating adhesion and migration in many cell types via direct association with integrins. Considering its role in controlling events downstream of integrin activation, the function of FAK in platelet signaling has been well studied. Platelet adhesion to fibrinogen via integrin αIIbβ3 leads to rapid FAK phosphorylation in an agonist-dependent manner and on activation of protein kinase C [1,2]. FAK phosphorylation in response to co-stimulation with either fibrinogen or collagen and adenosine 5′-diphosphate (ADP) accompanies changes in platelet spreading [3]. However, as Fak deletion in mice is embryonic lethal at day 8.5, before the onset of significant hematopoiesis, the exact roles that FAK plays in platelet function in vivo remain elusive.
To fully evaluate the role of FAK in platelet function, we successfully ablated Fak expression specifically in megakaryocytes and platelets by crossing conditional Fak-floxed mice [4] with megakaryocyte lineage–specific platelet factor 4 (Pf4)-Cre mice [5]. We found that Pf4-Cre/Fak-floxed (Fak−/−) mice exhibit increased bleeding times by tail bleed assays and attenuated platelet spreading. Recently, it was demonstrated an FAK inhibitor, PF-573,228, significantly attenuated platelet aggregation, spreading, and calcium release [6], suggesting that targeting FAK with specific pharmacological inhibitors may prevent thrombosis in high-risk patients.
In this study, we further investigate the role of FAK in platelet function using platelet-specific Fak knockout mice and determine the effectiveness of FAK inhibitors, PF-573,228 (PF-228) and PF-573,271 (PF-271), in mediating platelet activity, in the presence and absence of FAK.
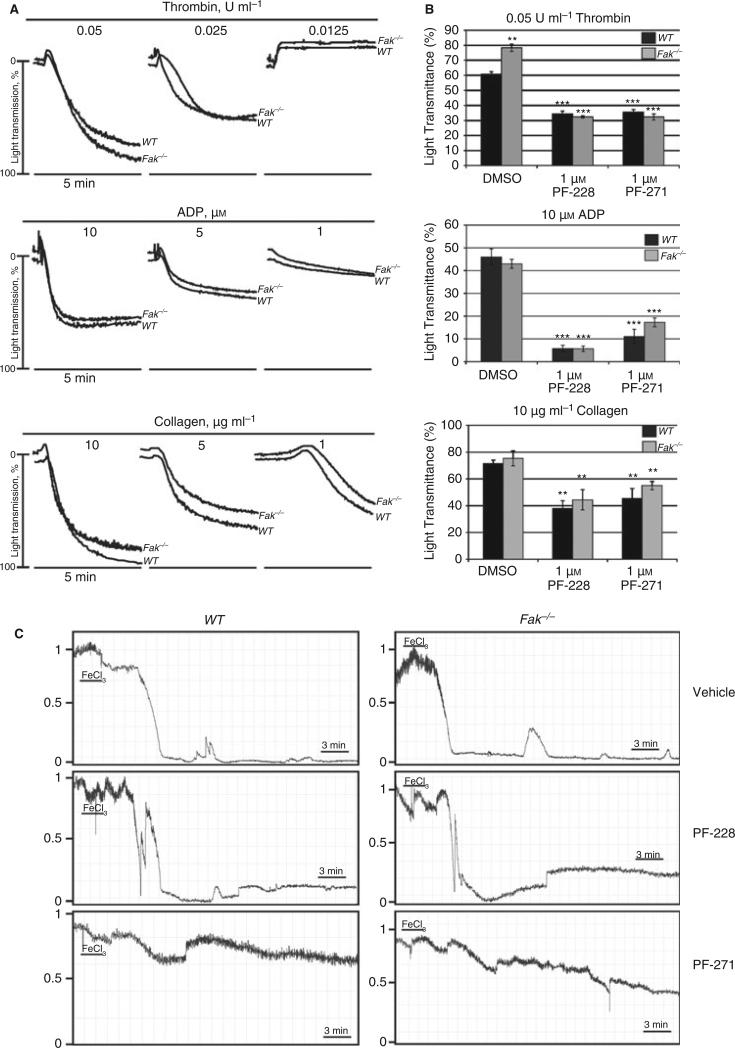
We found that platelet aggregation was not significantly different in WT and Fak−/− mice in response to thrombin, ADP, or collagen (Fig. 1A). Platelet integrin expression was also not significantly different between the two groups (data not shown). Next, we determined the effects of FAK inhibitors PF-228 and PF-271 on platelet function in vitro. PF-228 and PF-271 both completely inhibited thrombin-mediated FAK phosphorylation in isolated WT platelets, while FAK was absent in Fak−/− platelets (data not shown). Despite the absence of FAK, however, PF-228 and PF-271 significantly inhibited platelet aggregation in response to thrombin, ADP, and collagen in Fak−/− platelets as well as WT (Fig. 1B).
Fig. 1.
Effects of Fak ablation and FAK inhibitors on platelet function and thrombosis. Animal procedures were performed in accordance to protocols approved by the Institutional Animal Care and Use Committee, Stony Brook University. (A) Platelet aggregation was determined using washed platelets stimulated with decreasing concentrations of thrombin, adenosine 5′diphosphate (ADP), and collagen. Data are representative of at least three separate experiments. (B) Platelet aggregation was determined in the absence and presence of FAK inhibitors in WT and Fak−/− platelets following stimulation with maximal concentrations of thrombin, ADP, and collagen. Data is representative of the SEM of three independent experiments (**P < 0.01; ***P < 0.005). (C) Carotid artery occlusion assays were used to determine the effects of FAK inhibitors on in vivo thrombosis. Mice were treated with vehicle or PF-228 or PF-271 (50 mg/kg−1) for 30 min before occlusion assay. Data are representative of four mice per group.
Arterial occlusion times were measured using age-matched WT and Fak−/− mice (8–12 weeks) after injury with 7.5% FeCl3 to the carotid artery as described previously [7]. At 30 min before artery occlusion assays, mice received 50 mg/kg−1 PF-228 or PF-271 solubilized in cremephore DL/DMSO/ethanol (3:2:3) via intraperitoneal injection. Control mice received vehicle alone. Arterial occlusion time was not different between vehicle-treated WT and Fak−/− mice (WT, 545 ± 87 s; Fak−/−, 552 ± 71 s; Fig. 1C). Significantly, PF-271–treated WT and Fak−/− mice failed to occlude following injury throughout the 30-min test period. However, PF-228 had no effect on arterial thrombosis in vivo (Fig.1C).
We have shown that the absence of FAK has no significant effects on arterial thrombosis following injury or platelet aggregation in response to ADP, collagen, or thrombin. One potential explanation for the apparent lack of platelet phenotype in Fak−/− mice is the compensatory role of the FAK homologue protein Pyk2. A number of reports describe increased expression and phosphorylation of Pyk2 when Fak is ablated and the increase in Pyk2 function is able to compensate for the absence of FAK [8,9]. Similarly, we observed that Pyk2 phosphorylation and expression are significantly increased in Fak−/− platelets (data not shown). Importantly, a recent publication determined the importance of Pyk2 in regulating integrin αIIbβ3 outside-in signaling in platelets, showing that Pyk2 ablation inhibited platelet adhesion and spreading on fibrinogen [10], further supporting the significance of Pyk2 in platelet function.
Given the roles of FAK in cellular motility, adhesion, invasion, metastasis, and angiogenesis, the potential of FAK inhibitors as antioncogenic drugs has received considerable attention [11]. Both of the FAK inhibitors we have used in our studies, which directly affect the ATP binding site and thereby lower FAK kinase activity, have been shown to inhibit tumor growth in murine models [12,13]. However, the development of these drugs for clinical trials has been complicated by the structural similarities of the ATP-binding domain of many kinases, resulting in off-target effects of the inhibitors. We have shown that the FAK inhibitors have a significant effect on platelet aggregation in response to thrombin, collagen, and ADP, similar to the conclusions made previously [6]. However, we have shown that these effects are observed in both the presence and the absence of FAK.
These data confirm that attenuation of platelet activity by treating with FAK inhibitors PF-228 and PF-271 is due to off-target effects rather than FAK inhibition. Considering PF-271 is now in phase I clinical trials, the significant inhibitory effects on platelet function should be considered as a potential side effect, although currently there are no reports of bleeding diatheses in treated patients.
Acknowledgements
We thank Radek Skoda (Basel University Hospital, Switzerland) and Hillary Beggs (University of California San Francisco) for kindly providing the Pf4-Cre and Fak-floxed mice, respectively. Research was supported by the American Heart Association (10BGIA4030034).
Footnotes
To cite this article: Roh ME, Cosgrove M, Gorski K, Hitchcock IS. Off-targets effects underlie the inhibitory effect of FAK inhibitors on platelet activation: studies using Fak-deficient mice. J Thromb Haemost 2013; 11: 1776–8.
Addendum
M. E. Roh designed and performed research, analyzed data, and wrote the manuscript. M. Cosgrove performed research and analyzed data. K. Gorski performed research and analyzed data. I. S. Hitchcock designed and performed research, analyzed data, and wrote the manuscript.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interests.
References
- 1.Haimovich B, Kaneshiki N, Ji P. Protein kinase C regulates tyrosine phosphorylation of pp 125FAK in platelets adherent to fibrinogen. Blood. 1996;87:152–61. [PubMed] [Google Scholar]
- 2.Shattil SJ, Haimovich B, Cunningham M, Lipfert L, Parsons JT, Ginsberg MH, Brugge JS. Tyrosine phosphorylation of pp 125FAK in platelets requires coordinated signaling through integrin and agonist receptors. J Biol Chem. 1994;269:14738–45. [PubMed] [Google Scholar]
- 3.Haimovich B, Lipfert L, Brugge JS, Shattil SJ. Tyrosine phosphorylation and cytoskeletal reorganization in platelets are triggered by interaction of integrin receptors with their immobilized ligands. J Biol Chem. 1993;268:15868–77. [PubMed] [Google Scholar]
- 4.Hitchcock IS, Fox NE, Prevost N, Sear K, Shattil SJ, Kaushansky K. Roles of focal adhesion kinase (FAK) in megakaryopoiesis and platelet function: studies using a megakaryocyte lineage specific FAK knockout. Blood. 2008;111:596–604. doi: 10.1182/blood-2007-05-089680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109:1503–6. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- 6.Jones ML, Shawe-Taylor AJ, Williams CM, Poole AW. Characterization of a novel focal adhesion kinase inhibitor in human platelets. Biochem Biophys Res Commun. 2009;389:198–203. doi: 10.1016/j.bbrc.2009.08.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitsios JV, Prevost N, Kasirer-Friede A, Gutierrez E, Groisman A, Abrams CS, Wang Y, Litvinov RI, Zemljic-Harpf A, Ross RS, Shattil SJ. What is vinculin needed for in platelets? J Thromb Haemost. 2010;8:2294–304. doi: 10.1111/j.1538-7836.2010.03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration. EMBO J. 1998;17:5933–47. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weis SM, Lim ST, Lutu-Fuga KM, Barnes LA, Chen XL, Gothert JR, Shen TL, Guan JL, Schlaepfer DD, Cheresh DA. Compensatory role for Pyk2 during angiogenesis in adult mice lacking endothelial cell FAK. J Cell Biol. 2008;181:43–50. doi: 10.1083/jcb.200710038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cipolla L, Consonni A, Guidetti G, Canobbio I, Okigaki M, Falasca M, Ciraolo E, Hirsch E, Balduini C, Torti M. The proline-rich tyrosine kinase Pyk2 regulates platelet integrin alphaIIbbeta3 outside-in signaling. J Thromb Haemost. 2013;11:345–56. doi: 10.1111/jth.12099. [DOI] [PubMed] [Google Scholar]
- 11.McLean GW, Avizienyte E, Frame MC. Focal adhesion kinase as a potential target in oncology. Expert Opin Pharmacother. 2003;4:227–34. doi: 10.1517/14656566.4.2.227. [DOI] [PubMed] [Google Scholar]
- 12.Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, Richter D, Emerson E, Lin J, Kath J, Coleman K, Yao L, Martinez-Alsina L, Lorenzen M, Berliner M, Luzzio M, Patel N, Schmitt E, LaGreca S, Jani J, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68:1935–44. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 13.Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, Luzzio MJ, Cooper B, Kath JC, Roberts WG, Parsons JT. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282:14845–52. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]