SUMMARY
The discovery that malignant gliomas produce an excessive amount of VEGF, a key mediator of angiogenesis, has heightened interest in developing drugs that block angiogenic pathways. These antiangiogenic drugs tend to decrease vascular permeability, thereby diminishing tumor contrast enhancement independent of anti-tumor effects. This has made the determination of tumor response difficult, since contrast enhancement on post-contrast T1-weighted images is standard for assessing therapy effectiveness. In light of these unique challenges in assessing antiangiogenic therapy, new biomarkers have been proposed, based on advanced magnetic resonance techniques and PET. This article outlines the challenges associated with the evaluation of antiangiogenic therapy in malignant gliomas and describes how new imaging biomarkers can be used to better predict response.
Practice Points.
In most glioblastoma patients, antiangiogenic therapy reduces the amount of T2 and fluid-attenuated inversion recovery (FLAIR) hyperintensity and contrast enhancement on post-contrast T1-weighted images; however, this may be independent of any actual ‘anti-tumor’ effects.
The Response Assessment in Neuro-Oncology Group and individual institutions have implemented changes in T2/FLAIR lesions as part of the definition of tumor progression in the context of antiangiogenic therapy, and suggested a 15–25% change in bidirectional measurements may be sufficient for defining disease progression.
Residual T2 or FLAIR hyperintensity and contrast enhancement after the initial round of antiangiogenic treatment is a simple yet powerful predictor of tumor burden and a surrogate of survival.
A decrease in cerebral blood volume, increase in cerebral blood flow, decrease in vascular mean transit time and decrease in vascular permeability (Ktrans) after initial therapy, as measured with perfusion MRI, suggest a favorable response to antiangiogenic therapy.
Recurrent malignant gliomas with a low apparent diffusion coefficient within areas of contrast enhancement prior to antiangiogenic therapy progress nearly twice as fast as tumors with a higher apparent diffusion coefficient.
PET assessment of tumors after initiation of antiangiogenic therapy is useful for predicting long-term response and survival.
Primary brain tumors occur in approximately 18.7 out of 100,000 people per year and approximately 63,000 new cases are diagnosed in the USA per year [101]. Approximately 38% of all primary brain tumors are malignant and 32% are gliomas, which account for 80% of malignant primary brain tumors [101]. Glioblastoma (GBM) is a very aggressive form of primary brain tumor with a dismal prognosis, having a mean survival ranging from 12 to 14 months under the current standard of care of chemoradiotherapy – radiotherapy (RT) combined with concurrent chemotherapy using the alkylating agent, temozolomide (TMZ), along with adjuvant TMZ [1,2]. GBMs are highly vascularized, in part due to excessive levels of VEGF. This has in turn led to the targeting of VEGF and other angiogenic growth factors by a new class of antiangiogenic drugs, many of which are currently being studied in randomized clinical trials [3–5].
The introduction of antiangiogenic therapies in the realm of GBM treatment has led to the reassessment [6] of conventionally defined tumor response criteria (i.e., the Macdonald criteria [7]). The Macdonald criteria use bidimensional measurements of enhancing tumor based on post-contrast T1-weighted MRI scans, taking advantage of the increased permeability observed in tumor vasculature. However, antiangiogenic therapies reduce the permeability of tumor capillaries, resulting in a reduction of contrast agent leakage into the extravascular, extracellular space [8]. At least half of GBM patients do not respond to antiangiogenic therapies, and the response time can vary widely [9], but almost all patients see a reduction in enhancement following anti-VEGF therapy. This decoupling of tumor response from changes in enhancing tumor volume has diminished the enthusiasm for standard imaging biomarkers for determining responses to antiangiogenic therapies in GBMs and other malignant gliomas. Research efforts in this area, with the purpose of improving medical decision-making and, ultimately, patient survival, are therefore needed.
Antiangiogenic therapies in malignant gliomas
Antiangiogenic therapies were originally hypothesized to eradicate tumors by destroying their underlying vasculature; however, studies have demonstrated that they are the most effective when combined with chemotherapy and RT [10]. In light of these results, a ‘vascular normalization’ theory has been proposed, where antiangiogenic therapies re-establish a more efficient vasculature by pruning away the most inefficient vessels [11,12]. Pericytes – cells that are crucial to the maintenance of the blood–brain barrier – are recruited when the VEGF receptor is blocked, thereby decreasing vascular permeability. The basement membrane seen in tumor vessels becomes thinner, increasing oxygenation of the tumor. These combined effects are thought to allow chemotherapeutics to perfuse the tumor more efficiently, resulting in a higher response rate.
Currently, only one antiangiogenic agent has been US FDA approved for use in the clinical setting: bevacizumab, a humanized monoclonal antibody for VEGF-A. In a 2009 Phase II trial of 167 recurrent GBM patients, bevacizumab was used alone or in combination with irinotecan, a topoisomerase inhibitor. Progression-free survival (PFS) rates at 6 months were 42.6 and 50%, respectively; this was a significant improvement over the historical baseline of 15% 6-month PFS with salvage chemotherapy [13]. Moreover, corticosteroid use was reduced over time in bevacizumab-treated patients [13]. As a consequence, bevacizumab was conditionally approved by the FDA in 2009 for use in patients with recurrent GBM [14,15].
Several additional antiangiogenic drugs have been explored in ongoing clinical trials. Cediranib, a small-molecule inhibitor of all VEGF subtypes, PDGF receptor and c-Kit [9], has a potential advantage over bevacizumab because of its oral bioavailability. In a Phase II trial of 31 patients, PFS at 6 months was 25.8% and a radiographic response was observed in 56.7% of patients [16]. Similar to bevacizumab, cediranib was associated with a reduction or discontinuation of corticosteroids. Another antiangiogenic agent currently under investigation is sorafenib, a small molecule that targets VEGF receptor, PDGF receptor and Raf kinase. A Phase II study in 2010 demonstrated no survival advantage when sorafenib was used in combination with RT and TMZ compared with RT and TMZ alone [17]. Pazopanib, a small-molecule inhibitor, was not shown to improve PFS in recurrent GBM patients [18]. Other antiangiogenic drugs currently under investigation include vatalanib [19] and vandetanib [20], which have undergone Phase I trials, both in combination with RT and TMZ in newly diagnosed GBM patients, and were generally well tolerated.
In summary, several antiangiogenic therapies appear to improve PFS compared with standard salvage chemotherapies. However, tumor progression is intimately tied to radiographic response via contrast enhancement, which is directly altered by the mechanism of antiangiogenic therapies, which is potentially independent of their anti-tumor effects. This underscores the need for new imaging biomarkers aimed at quantifying the response to and predicting early failure of antiangiogenic therapies in malignant gliomas.
Imaging biomarkers for malignant glioma response to antiangiogenic therapy
▪ Response Assessment in Neuro-Oncology
In 2010, Response Assessment in Neuro-Oncology (RANO) released the first white paper outlining updated response criteria for neuro-oncology [6] that aimed to address some of the limitations of the Macdonald criteria when evaluating antiangiogenic therapy. Specifically, the RANO criteria aimed to overcome the challenges associated with the significant reduction in contrast enhancement after bevacizumab treatment [13,21–23] by incorporating changes in T2-weighted or fluid-attenuated inversion recovery (FLAIR) images, as well as clinical variables, into the definition of tumor response; however, no specific thresholds for what constitutes ‘significant changes’ in T2/FLAIR images have been provided. In response to this lack of guidance in interpreting significant changes in T2/FLAIR hyperintense lesions, a few studies have attempted to verify and quantify the added benefit of incorporating specific thresholds for T2/FLAIR changes. For example, Radbruch et al. implemented two cut-offs, a 15 and 25% increase in T2 compared with baseline, or best response as a marker of tumor progression in 144 patients with recurrent malignant gliomas [24]. Results from this study suggested a threshold of a 15% change in bidirectional measurements of T2/FLAIR hyperintense lesions should be used for the evaluation of nonenhancing tumor growth. Additionally, Gállego Pérez-Larraya et al. performed a comprehensive comparative analysis of the Macdonald, Response Evaluation Criteria in Solid Tumors (RECIST), RANO and RECIST with FLAIR (RECIST + F) criteria [25]. In this study, investigators used a 20% increase in the largest diameter of the enhancing lesion for RECIST + F analysis and a 25% or more increase in the maximal cross-sectional area for RANO analysis. Results demonstrated that the inclusion of FLAIR reduced response rates by approximately 5% compared with their direct counterparts, and RANO and RECIST + F detected recurrence approximately 1 month before the Macdonald and RECIST criteria. These studies both indicate that incorporation of FLAIR into the RANO guidelines for tumor response appears to improve the accuracy of determining tumor progression; however, quantification of changes still remains an issue since precise quantification of infiltrating tumor from treatment effects or changes in edema can be challenging. Furthermore, the precise cut-off point for defining relevant T2 change remains controversial despite being critical for implementation. Choosing a cut-off point is a double-edged sword: a low cut-off for T2 changes may decrease the specificity by increasing the risk of misdiagnosis of progression (e.g., T2 changes due to radiation therapy, corticosteroids or postoperative changes) and a higher cut-off for T2 changes decreases the sensitivity by increasing the risk of not identifying nonenhancing tumor progression. A more sophisticated approach to determine the optimal cut-off (e.g., receiver operating characteristic curves) may be useful for resolving this debate.
Although most transient measures of treatment response for antiangiogenic therapies using standard magnetic resonance (MR) images are generally ascribed to changes in vascular permeability, there is also evidence for other radiographic changes that may reflect a favorable response to therapy. For example, subsets of bevacizumab patients (∼5%) develop and maintain necrosis within the main lesion site as well as vascular control. These patients may develop persistent restricted diffusion lesions on diffusion MRI with relatively low cerebral blood volume PET tracer uptake, and appear to have a survival advantage compared with matched patients without these lesions [26]. Additionally, an increase in T2 hyperintensity or contrast enhancement could possibly reflect postradiation changes (i.e., pseudoprogression), which typically have a favorable prognosis.
Changes in radiographic features are likely to reflect the underlying molecular and microenvironmental changes within the tumor. For example, patients and animals that have nonenhancing tumor progression while being treated with antiangiogenic agents typically maintain low levels of VEGF, but may show evidence of increased hypoxic lesions as well as an increase in IGFB2 and MMP2 expression, indicative of tumor cell invasion [27]. This observation of increased infiltrating, nonenhancing tumor as evidenced by T2 or FLAIR hyperintensity has been noted in many studies [27–29]; however, this topic remains controversial owing to other studies that support a lack of abnormal tumor invasion patterns [30,31]. Increased contrast enhancement, blood volume or vasculature during treatment with antiangiogenic agents may reflect activation of alternative angiogenic signaling pathways, such as bFGF, Tie-2 and DSF-1α, or recruitment of endothelial progenitor cells [32]. Because of these many confounds to traditional radiographic interpretation, integration of more sophisticated physiological imaging biomarkers or tumor segmentation techniques into the RANO criteria may be advantageous.
▪ Quantitative volumetric analysis
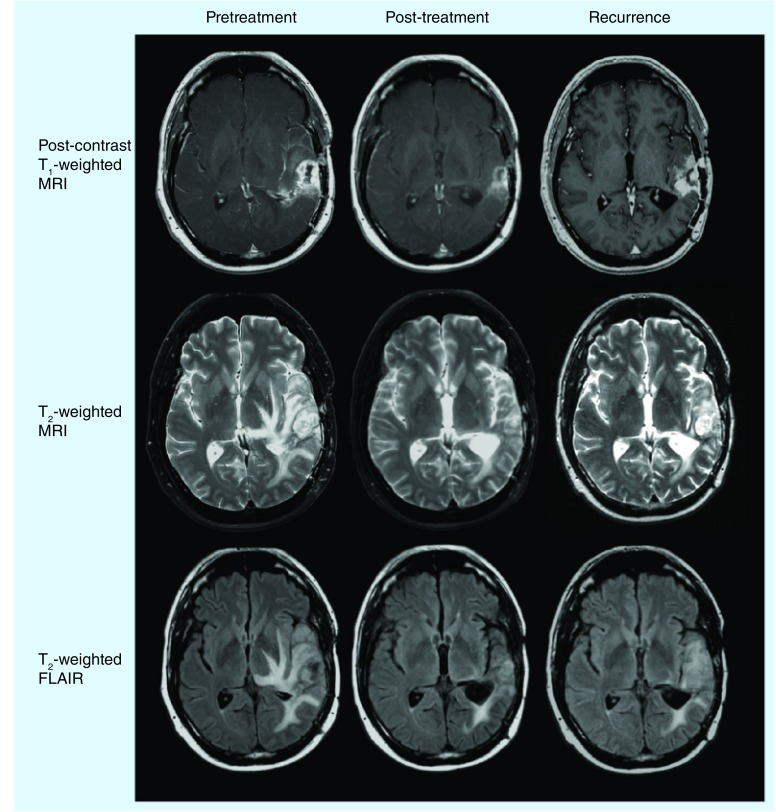
Multiple studies have demonstrated the dramatic effects of bevacizumab on standard MRI, such as the reduction in vasogenic edema on T2-weighted images and reduction in contrast enhancement (Figure 1) [29,33–35]. In a recent investigation into the volumetric changes in contrast enhancement and T2 hyperintensity in 84 patients with recurrent GBM, it was demonstrated that, in patients treated with bevacizumab, patients with a post-treatment, contrast-enhancing volume of more than 15 ml were statistically more likely to progress sooner than patients with a lower volume of contrast enhancement [36]. This same study also showed that the relative nonenhancing tumor ratio (rNTR) – the ratio of FLAIR volume to contrast-enhancing volume – was a significant predictor of response. Specifically, the median PFS was 88 days for patients with a high rNTR (≥7.5) and 162.5 days for the patients with a low rNTR, whereas the same threshold demonstrated a median overall survival (OS) of 260 and 352 days for the high and low rNTR groups, respectively.
Figure 1. Anatomical MRI response to antiangiogenic therapy.
FLAIR: Fluid-attenuated inversion recovery.
▪ T2 relaxometry
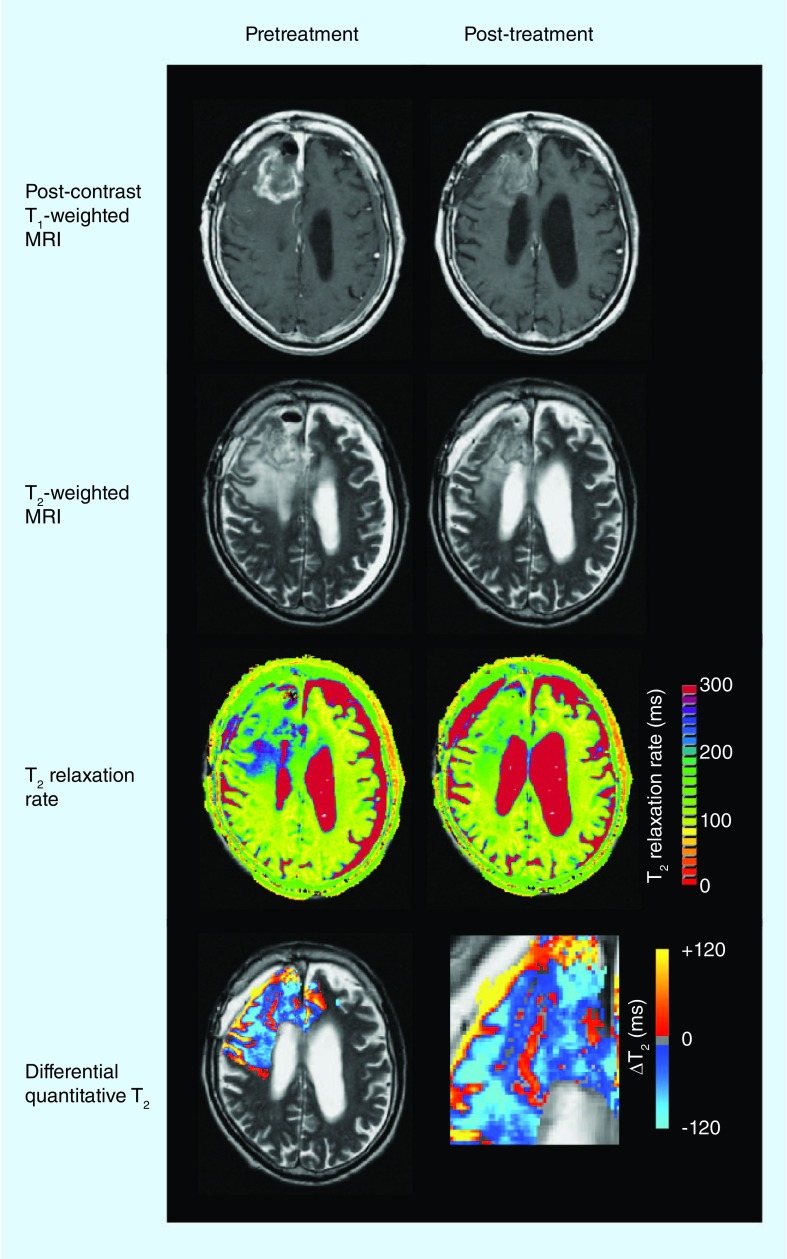
Antiangiogenic therapy results in a reduction of T2 hyperintense volume on standard T2-weighted or FLAIR images; however, this reduction in observed hyperintense volume is directly related to changes in T2 relaxation rates. In a recent study, a voxelwise subtraction technique was used to create differential quantitative T2 maps (Figure 2) [37]. This study demonstrated that patients with a larger decrease in T2 following first treatment with bevacizumab were more likely to have a longer PFS and OS. Furthermore, median post-treatment T2 linearly correlated with PFS and OS. In general, however, the inability to predict clinical end points using the change in T2 may suggest that the change in water concentration from vasogenic edema does not reflect changes in the tumor itself, but rather the change in vascular permeability. Regardless, differential quantitative T2 maps may be advantageous for evaluating changes in tissue water content and provide another perspective from which to approach the assessment of the efficacies of antiangiogenic therapies.
Figure 2. Differential quantitative T2 response to antiangiogenic therapy.
T2 relaxation rate color maps are calculated using multiecho fast spin-echo acquisition. Differential quantitative T2 images illustrate the voxelwise difference in T2 measurements. Note that T2 has increased along the right boundary of the contrast-enhancing lesion, as shown in red.
▪ Perfusion-weighted MRI biomarkers
Given that malignant gliomas thrive by co-opting the pre-existing vasculature and by inducing new vessel formation, perfusion-weighted MR techniques are an intriguing tool for uncovering the change in vascularity that may result from antiangiogenic therapy; however, very few studies have actually employed perfusion-weighted MRI to study antiangiogenic therapies. Dynamic susceptibility contrast-enhanced MRI utilizes the first pass of MRI (paramagnetic) contrast to estimate the relative cerebral blood volume (rCBV) and relative cerebral blood flow (rCBF). Pechman et al. have used rCBV as a measure of therapeutic responses to bevacizumab and combination therapy in a U87 brain tumor murine model [38]. In one study, they used different concentrations of bevacizumab, 0.0, 2.5, 5.0 and 10.0 mg/kg, to determine the potential effects on tumor volume and rCBV [38]. Results suggested that rCBV decreased with treatment as early as 2 days after therapy, but changes in enhancing tumor volumes from post-contrast T1-weighted images were delayed in comparison. In a subsequent study they compared bevacizumab (5 mg/kg) to combination therapy with irinotecan (20.83 mg/kg) and observed a rCBV decrease between 4 and 6 days after therapy, followed by a rebound effect [38]. The authors suggest this may be indicative of the ‘vascular normalization window’, which was further supported by histological visualization of the vessel densities, which were found to be similar between the bevacizumab and control groups. Overall, results from these preclinical studies show the potential utility of rCBV as a biomarker for changes in vascularity known to accompany antiangiogenic therapy.
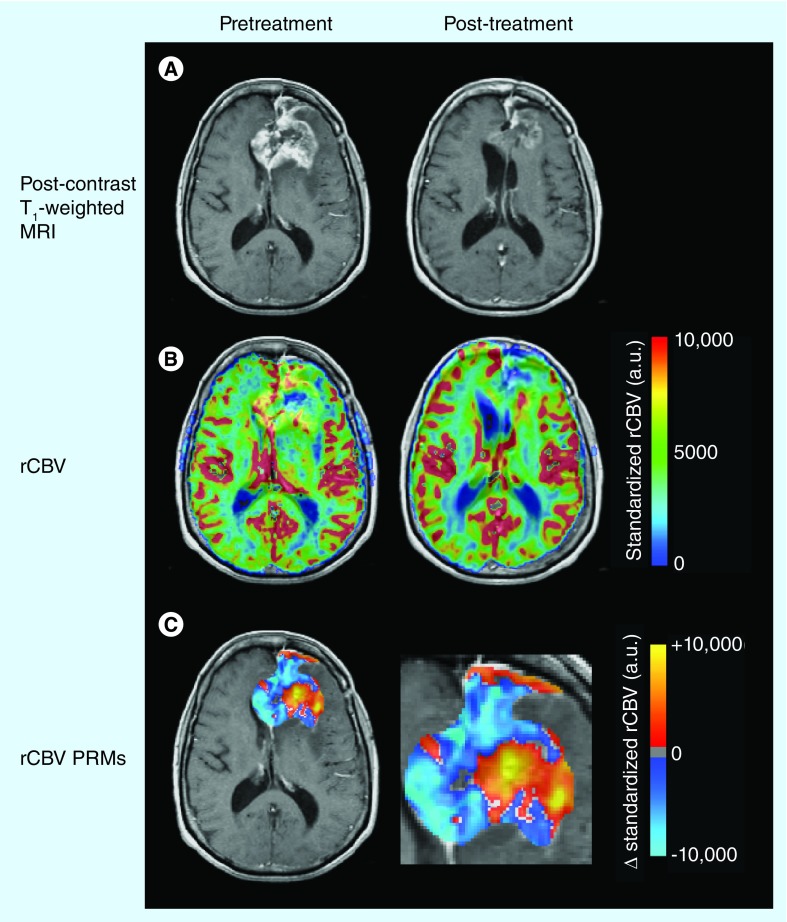
Although only a single human study has directly examined the prognostic capabilities of rCBV and rCBF to predict response to antiangiogenic therapies [39], unpublished data from our institution suggest that a decrease in rCBV in the weeks following bevacizumab treatment reflects a favorable patient response (rCBV maps and cerebral blood volume parametric response maps after bevacizumab treatment are illustrated in Figure 3) [Leu K et al., Unpublished Data]. Sorensen et al. noted that patients with an increase in rCBF have a favorable prognosis due to the normalization of abnormal blood vasculature leading to more efficient perfusion [39], which has been evidenced in other studies [40]. This may seem to contradict our observations of rCBV, but both rCBF and rCBV are related to each other via the mean transit time (MTT) of blood through the image voxel (rCBV = rCBF × MTT). Thus, the observation that a decrease in rCBV and an increase in rCBF are implicated in better prognosis intrinsically implies a reduction in MTT, probably as a result of decreased tortuosity of tumor neovasculature after antiangiogenic therapy. Additionally, data from our institution show that recurrence after bevacizumab treatment results in increased rCBV relative to the first post-treatment assessment (but lower than recurrence when antiangiogenic agents were not used). This increase in rCBV at recurrence probably reflects the aforementioned alternative angiogenic escape pathways and may provide an early indication of treatment failure.
Figure 3. Effects of antiangiogenic therapy on relative cerebral blood volume.
(A) Post-contrast T1-weighted magnetic resonance images. (B) Leakage-corrected and standardized rCBV color maps. (C) rCBV PRMs showing voxelwise changes in rCBV after antiangiogenic therapy. Red/yellow indicates an increase in rCBV, while blue/cyan indicates a decrease.
PRM: Parametric response map; rCBV: Relative cerebral blood volume.
As an alternative to traditional dynamic susceptibility contrast-enhanced MRI analysis, Essock-Burns et al. examined the ability of two MRI-derived parameters to predict the response to antiangiogenic therapies and identify early predictors of progression: relative peak height, a measure of vascularization; and percentage of signal intensity recovery, a gauge of capillary permeability [41]. The percentage recovery was defined as the relative return of the bolus enhancement curve to baseline. Essock-Burns et al. found that a unit increase in peak height above the 90th percentile during the first month was associated with a fivefold greater risk of progression. On the other hand, a greater than 25th percentile recovery at 2 months from baseline was correlated with a longer PFS. The authors reported that 4 months prior to progression, the heterogeneity of percentage recovery values within the tumor region increased, as assessed by the standard deviation of percentage recovery. The prediction of tumor progression may be particularly useful for clinicians, although the method of assessing heterogeneity of percentage recovery values should be tested in larger trials.
Dynamic contrast-enhanced MRI, another perfusion MRI technique, uses a simple pharmacokinetic model to estimate gadolinium contrast agent transfer rates and compartment volumes from dynamic T1-weighted images. Kreisl et al. used dynamic contrast-enhanced MRI as a secondary biomarker for evaluating the activity of single-agent bevacizumab in patients with recurrent anaplastic gliomas [42]. As soon as 4 days after administration of bevacizumab, there was a 30.8% decrease in the transfer rate from the intravascular space to the extravascular space (Ktrans), which is a surrogate for vascular permeability [43], and a 21.4% decrease in fractional plasma volume. By 4 weeks, Ktrans had decreased by an average of 51.9% and the mean fractional plasma volume decreased by 45.9% compared with pretreatment levels. Despite the significant reduction in quantitative vascular parameters, neither Ktrans nor fractional plasma volume were predictive of patient outcome or survival. Sorensen et al. showed a similar decrease in Ktrans and fractional plasma volume following administration of cediranib, along with a weak association between change in Ktrans and patient survival [44]. They also found that by combining Ktrans with biological assays, they could estimate the vascular normalization window and better predict patient survival than with quantitative imaging parameters alone. By combining Ktrans, biological assays and collagen IV levels, Sorensen and colleagues demonstrated a multiparametric tool for predicting early response to antiangiogenic therapy and estimating patient survival.
Farrar et al., using an orthotopic mouse glioma model, strove to demonstrate the sensitivity of the MRI perfusion biomarkers that putatively predict outcomes [45]. They found that the most sensitive measures were T2, rCBV, relative microvascular blood volume (rMBV) and Ktrans. The T2 changes that are assumed to reflect the differences in tumor water content were indeed correlated with ex vivo measurements of tumor water. Intravital optical microscopy measures were used to confirm the sensitivity of rCBV and Ktrans values. In cediranib-treated mice, the authors observed that the rMBV decreased more than rCBV did. On the other hand, rCBV increased and rMBV decreased for the untreated mice. This suggests that cediranib may preferentially prune the smaller, less well-developed tumor blood vessels. This proof-of-principle study confirms that perfusion-based MR biomarkers are indeed sensitive to changes in tumor vascularity and may be useful for measuring response to new antiangiogenic agents.
Although an extensive review is outside the scope of this article, the development of new customized contrast agents, nanospheres, functional agents and novel blood pool agents offers the possibility of further characterizing the vascular pores in normal and tumor vasculature [46]. For example, Henderson et al. developed a technique for using two different gadolinium contrast agents with very different molecular weights (gadolinium-diethylene triamine pentaacetic acid, 0.6 kDa, and 24-gadolinium-macrocyclic dendrimer, 17 kDa) to estimate blood flow, volume and vascular permeability in breast lesions [47]. Alternatively, the use of iron oxide particles with different sizes, charges and surface structures could provide insight into changes in vascular permeability beyond that of traditional contrast agents.
▪ Diffusion-weighted MRI biomarkers
Diffusion-sensitive MRI techniques are another imaging method that has shown promise in predicting response to standard cytotoxic as well as modern antiangiogenic therapies. Diffusion-weighted imaging is sensitive to microscopic, subvoxel water motion for which an apparent diffusion coefficient (ADC) can be estimated, reflecting the magnitude of water motion. ADC has been shown to be inversely correlated with tumor cell density [48–55], largely as a result of restriction of extracellular water motion caused by tightly packed tumor cells. Given that brain neoplasms have a higher cell density than normal tissues, they have lower ADC values. On the other hand, edema and necrosis, which are associated with lower cell densities, have relatively higher ADC values.
Consistent with this hypothesis, Pope et al. utilized the distribution of ADC values within pretreatment contrast-enhancing regions to predict the response to bevacizumab [56]. Specifically, this study fit a double Gaussian mixture model to the ADC histogram extracted from pretreatment contrast-enhancing regions and noted that the mean of the lower ADC histogram (ADCL) was a significant predictor of PFS. Specifically, this study noted that patients with a lower mean ADCL were more likely to develop resistance to bevacizumab treatment earlier than patients with a higher mean ADCL. In a follow-up multicenter study, Pope et al. applied the double Gaussian mixture model in 97 recurrent GBM patients [57]. Consistent with the smaller study, lower mean ADCL values were correlated with shortened survival. Meanwhile, a combined mean ADCL score <1.209 µm2/ms and a lower curve proportion >0.71 was associated with a 2.28-fold reduction in median time to progression and a 1.42-fold decrease in median OS. This study is the first to confirm the potential clinical usefulness of ADC histogram analysis; although standardizing the imaging methodology and submitting it to further prospective evaluations can help optimize this biomarker. Interestingly, Pope et al. also applied this same technique to newly diagnosed GBM patients treated with bevacizumab and found somewhat opposite trends [58]. Specifically, GBM patients with a higher ADCL actually had a lower PFS compared with patients with a lower ADCL. These results were attributed to patients with a lower ADCL being more likely to have the MGMT promoter methylated, which is favorable for chemoradiotherapy; however, the basis for this discrepancy still warrants investigation.
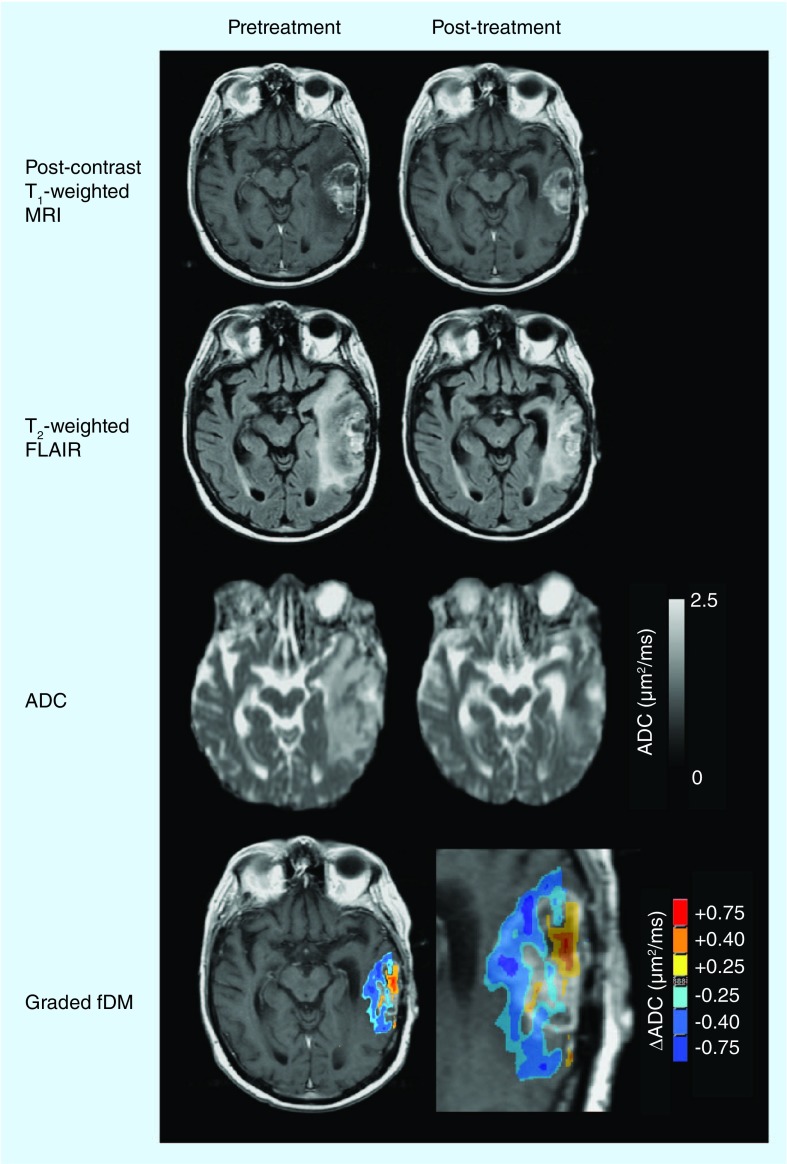
Voxelwise changes in ADC have also been utilized as a potential biomarker for response to antiangiogenic therapy using a technique termed functional diffusion mapping [59,60]. Specifically, functional diffusion maps (fDMs) are created by quantifying the voxelwise changes in ADC after co-registration of ADC maps from different time points. Although fDMs have been applied to cytotoxic therapy, Ellingson et al. were the first to use fDMs to assess antiangiogenic therapy [61,62]. In one study, the investigators used ‘graded’ fDMs, as shown in Figure 4, in which multiple ΔADC thresholds were used to generate fDMs to demonstrate that a decrease in ADC between 0.25 and 0.40 µm2/ms recorded in a larger volume than that seen in the group median within the FLAIR regions of interest had a poor prognosis. Graded fDMs also produced a higher sensitivity (58%) and specificity (67%) than the traditional fDMs (56 and 63%, respectively) with respect to the 12-month OS in recurrent GBM patients treated with bevacizumab.
Figure 4. Functional diffusion map response to antiangiogenic therapy.
ADC maps were calculated from diffusion magnetic resonance images. Graded fDMs show a decrease in ADC along the edge of the enhancing tumor. Blue/cyan indicates a decrease in ADC, while yellow/red indicates an increase.
ADC: Apparent diffusion coefficient; fDM: Functional diffusion map; FLAIR: Fluid-attenuated inversion recovery.
In another fDM study, Ellingson et al. explored the possible utility of a nonlinear registration scheme, registering the post-treatment ADC map to the pretreatment one, and vice versa [63]. This study found that the ‘pre-to-post’ nonlinear registration scheme applied to FLAIR regions provided the best stratification between short- and long-term PFS and OS, and had the highest hazard ratio. Furthermore, the ‘pre-to-post’ scheme had a higher sensitivity (64%) and specificity (73%) for 6-month PFS and 12-month OS in recurrent GBM patients treated with bevacizumab than linear fDMs (59 and 67%, respectively). Interestingly, the volume fraction of tissue within the FLAIR regions of interest having an increased ADC was significantly different between linear and nonlinear registration techniques, further improving the ability of the fDMs to predict response to bevacizumab in recurrent GBMs.
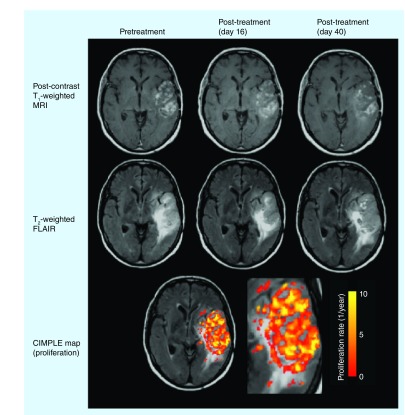
Since ADC is believed to be a surrogate for tumor cell density, serial ADC maps can be used to generate estimates of tumor cell proliferation and invasion rate using cell invasion, motility and proliferation level estimate (CIMPLE) maps [62,64]. Figure 5 shows proliferation maps for a patient treated with bevacizumab. Investigators retrospectively studied 26 recurrent GBM patients treated with bevacizumab and noted a linear correlation between the mean proliferation rate, PFS and OS. A mean proliferation rate of 3.73 per year was used to stratify patients, resulting in a median PFS of 100.5 days for highly proliferative tumors and 401 days for tumors with a lower proliferation rate. Similarly, patients with a high proliferation rate had a median OS of 286 days, while patients with a low proliferation rate had a median OS of approximately 711.5 days. Most notably, CIMPLE maps were also able to spatially predict regions of future tumor recurrence in nearly a third of patients, which demonstrates the potential of CIMPLE maps as a predictive biomarker in antiangiogenic therapy.
Figure 5. Cell invasion, motility and proliferation level estimate map response to antiangiogenic therapy.
CIMPLE map estimates of proliferation rate show elevated proliferation within the region of contrast enhancement.
CIMPLE: Cell invasion, motility and proliferation level estimate; FLAIR: Fluid-attenuated inversion recovery.
▪ MR spectroscopic biomarkers
MR spectroscopy can be used to quantify the levels of important biochemical metabolites within tumors, including choline (Cho), a molecule associated with cell turnover and proliferation; N-acetyl aspartate (NAA), a molecule associated with healthy neurons; and lipids and lactate, molecules associated with degradation of myelin and cell membrane structures. Kim et al. investigated the MR spectral profiles of 31 patients with recurrent GBM during and after treatment with cediranib, noting a ratio of NAA:Cho in contrast-enhancing tumor regions of 2.4 and 5.0, respectively, in normal-appearing brain tissue [65]. During the first 28 days of cediranib treatment, the time period believed to be associated with the vascular normalization window, investigators observed consistency of the NAA:Cho ratio, suggesting tumor cells are not destroyed during this time frame, but rather cediranib acts solely by decreasing vascular permeability. A significant increase in NAA:Cho occurred after 28 days and patients with a positive change in NAA:Cho had a better OS than those with negative values at days 28 and 56, supporting the notion of using MR spectroscopy to monitor antiangiogenic treatment response.
▪ PET imaging biomarkers
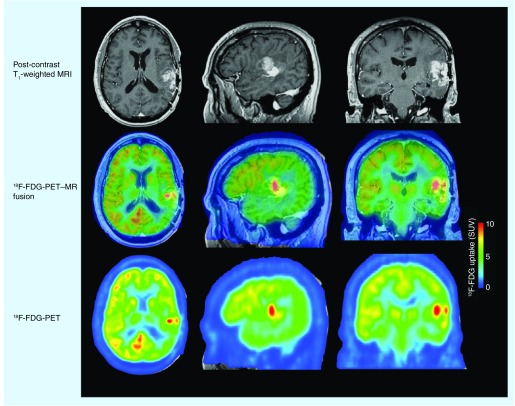
PET scans have also been explored in the context of identifying biomarkers for response to antiangiogenic therapies. For many malignant tumors, 18F-fluorodeoxyglucose (18F-FDG) is used as the radiotracer of choice for PET scans as it can be used to quantify glucose uptake and metabolism. Colavolpe et al. recently used pretreatment 18F-FDG to predict survival in 25 recurrent high-grade glioma patients treated with bevacizumab and irinotecan [66]. They investigated two different PET parameters: the tumor maximal standardized uptake value within a region of interest (SUVmax) and the ratio between tumor and symmetric contralateral SUVmax (T:CL). Univariate analysis showed that SUVmax >7 and T:CL ratio >1.348 were statistically significant for PFS and OS. Multivariate analysis confirmed SUVmax and T:CL ratio as predictors of PFS and OS, regardless of histological grade. 18F-FDG was also recently used as a secondary imaging biomarker in a Phase II trial of bevacizumab in 31 recurrent anaplastic gliomas [42]. Contradictory to the findings by Colavolpe et al. [66], this study did not find pretreatment 18F-FDG uptake to be a significant predictor of PFS or OS when performing multivariate analysis; however, the 18F-FDG uptake 4 weeks after starting therapy was found to be a significant predictor of PFS, but not OS. Interestingly, average 18F-FDG uptake was only approximately 4% lower 4 weeks following bevacizumab and a decrease in uptake was only observed in approximately 50% of patients. The change in uptake was also not found to be a significant predictor of OS. Unpublished data from our institution suggest that 18F-FDG uptake is elevated at the time of radiographic recurrence in almost all patients on bevacizumab, regardless of their initial response [Leu K et al., Unpublished Data]. Figure 6 shows an example of 18F-FDG PET uptake in a patient who progressed on bevacizumab, showing elevated uptake in regions of recurrent tumor.
Figure 6. 18F-fluorodeoxyglucose PET uptake at recurrence after antiangiogenic therapy.
18F-FDG PET–MR fusion images show co-localization of elevated 18F-FDG in the medial contrast-enhancing nodule. 18F-FDG PET uptake images show elevated 18F-FDG uptake within the tumor areas.
18F-FDG: 18F-fluorodeoxyglucose; MR: Magnetic resonance; SUV: Standardized uptake value.
Another radiotracer that has been explored in the context of antiangiogenic therapy in malignant gliomas is the thymidine analog, 3´-deoxy-3´-18F-fluorothymidine (18F-FLT), which allows for more direct quantification of proliferation rates through expression of the enzyme thymidine kinase-1 during DNA synthesis [67]. In a pilot study, 18F-FLT was explored as an imaging biomarker for separating bevacizumab responders from nonresponders [68]. In a follow-up study, Schwarzenberg et al. compared 18F-FLT uptake with results from MRI scans in 30 patients with recurrent high-grade gliomas treated with bevacizumab and irinotecan [69]. A decrease in 18F-FLT uptake of ≥25% was deemed to be a successful treatment response. The authors found that the uptake changes at 2 and 6 weeks post-treatment were predictive of both PFS and OS. The patients identified as responders, according to 18F-FLT uptake, lived 3.3-times longer than their nonresponder counterparts, compared with a 1.4-fold increase in survival for MRI responders compared with nonresponders. Interestingly, there were discrepancies between the MRI and PET results. For example, of the seven patients that were classified as nonresponders to MRI but were responders to PET, survival was 12.3 months, in line with the responders’ survival times. On the other hand, the one responder to MRI, but not PET, survived only 2.8 months. This demonstrates that 18F-FLT PET may be capable of identifying treatment responders earlier than MRI. Figure 7 shows an example of 18F-FLT uptake in a patient treated with bevacizumab, illustrating an initial decrease in uptake following therapy.
Figure 7. 3’-deoxy-3’-18F-fluorothymidine PET response to antiangiogenic therapy.
18F-FLT PET images show reduced uptake after antiangiogenic therapy and a sustained decrease in uptake relative to baseline (pretreatment) levels.
18F-FLT: 3’-deoxy-3’-18F-fluorothymidine; FLAIR: Fluid-attenuated inversion recovery; SUV: Standardized uptake value.
In a recent study, Harris et al. demonstrated the power of examining voxelwise changes in PET uptake, termed PET parametric response maps, by examining changes in both 18F-FLT and 3,4-dihydroxy-6-18F-fluoro-L-phenylalanine (18F-FDOPA), an amino acid tracer [70]. Receiver operating characteristic analysis revealed that patients with a large volume of increased 18F-FDOPA uptake after treatment with bevacizumab had a shorter PFS and OS. Additionally, a high volume of decreased uptake in both tracers had a high sensitivity (91% for 18F-FDOPA and 90% for 18F-FLT) for predicting 3-month PFS and 6-month OS. These studies clearly demonstrate the potential for more sophisticated analyses of PET data to predict response to antiangiogenic therapy.
Conclusion & future perspective
Within the last few years, treatment strategies for malignant gliomas have combined RT and chemotherapy with new antiangiogenic drugs. This paradigm shift highlights the need for sensitive imaging biomarkers to identify changes in the tumor independent of contrast enhancement. To overcome this challenge, researchers have begun developing and testing a myriad of imaging biomarkers for patient treatment response by examining multiple biological perspectives and pathways. The intent of these studies is largely to provide new tools for evaluating new antiangiogenic drugs, provide clinicians with information to make earlier treatment decisions and, ultimately, to improve malignant glioma patient survival. Since a subset of patients in most clinical trials evaluating the use of antiangiogenic drugs have a complete radiographic response to therapy and may have significantly longer survival, this population may offer the chance to optimize imaging biomarkers to detect a ‘true’ complete response earlier than traditional techniques.
Given that most of these studies into new biomarkers were performed retrospectively in relatively small trials, prospective studies in larger clinical trials are necessary to validate and optimize the use of these imaging biomarkers so that they may enter clinical practice. Yet, given the modest success that antiangiogenic therapies have had thus far, glioma treatment methods will probably continue to evolve. As the landscape of tumor treatment changes over time, certain imaging modalities and measurements may need to be modified to reflect true tumor response. New imaging biomarkers must continue to take up the mantle to better reflect patient prognoses as our understanding of malignant glioma biology and treatment responses advances.
Footnotes
Financial & competing interests disclosure
The authors acknowledge the following funding: University of California, Los Angeles (UCLA) Institute for Molecular Medicine Seed Grant, UCLA Radiology Exploratory Research Grant, UCLA Cancer Research Coordinating Committee Grant and American College of Radiology Imaging Network (ACRIN) Young Investigator Initiative Grant to BM Ellingson; Art of the Brain, Ziering Family Foundation in memory of Sigi Ziering, Singleton Family Foundation and Clarence Klein Fund for Neuro-Oncology to TF Cloughesy; and NIH National Institute of General Medical Sciences (NIGMS) training grant GM08042 and UCLA Medical Scientist Training Program to K Leu. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪ of interest ▪▪ of considerable interest
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto Phase II clinical trials. J. Clin. Oncol. 1999;17(8):2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 3.Plate KH, Breier G, Risau W. Molecular mechanisms of developmental and tumor angiogenesis. Brain Pathol. 1994;4(3):207–218. doi: 10.1111/j.1750-3639.1994.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 4.Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367(6463):576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- 5.Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284(5422):1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 6.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology Working Group. J. Clin. Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]; ▪▪ Describes the new Response Assessment in Neuro-Oncology response criteria for use in assessing malignant glioma response to antiangiogenic therapy.
- 7.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for Phase II studies of supratentorial malignant glioma. J. Clin. Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 8.Pope WB, Young JR, Ellingson BM. Advances in MRI assessment of gliomas and response to anti-VEGF therapy. Curr. Neurol. Neurosci. Rep. 2011;11(3):336–344. doi: 10.1007/s11910-011-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norden AD, Drappatz J, Wen PY. Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol. 2008;7(12):1152–1160. doi: 10.1016/S1474-4422(08)70260-6. [DOI] [PubMed] [Google Scholar]
- 10.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 12.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64(11):3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 13.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 14.Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14(11):1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 15.Cloughesy TF, Prados MD, Wen PY, et al. A Phase II, randomized, non-comparative clinical trial of the effect of bevacizumab (BV) alone or in combination with irinotecan (CPT) on 6-month progression free survival (PFS6) in recurrent, treatment-refractory glioblastoma (GBM) J. Clin. Oncol. 2008;26(Suppl. 15) Abstract 2010b. [Google Scholar]
- 16.Batchelor TT, Duda DG, Di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J. Clin. Oncol. 2010;28(17):2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hainsworth JD, Ervin T, Friedman E, et al. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer. 2010;116(15):3663–3669. doi: 10.1002/cncr.25275. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto FM, Lamborn KR, Robins HI, et al. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02) Neuro Oncol. 2010;12(8):855–861. doi: 10.1093/neuonc/noq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerstner ER, Eichler AF, Plotkin SR, et al. Phase I trial with biomarker studies of vatalanib (PTK787) in patients with newly diagnosed glioblastoma treated with enzyme inducing anti-epileptic drugs and standard radiation and temozolomide. J. Neurooncol. 2011;103(2):325–332. doi: 10.1007/s11060-010-0390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drappatz J, Norden AD, Wong ET, et al. Phase I study of vandetanib with radiotherapy and temozolomide for newly diagnosed glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2010;78(1):85–90. doi: 10.1016/j.ijrobp.2009.07.1741. [DOI] [PubMed] [Google Scholar]
- 21.Batchelor TT, Sorensen AG, Di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 23.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radbruch A, Lutz K, Wiestler B, et al. Relevance of T2 signal changes in the assessment of progression of glioblastoma according to the Response Assessment in Neurooncology criteria. Neuro Oncol. 2012;14(2):222–229. doi: 10.1093/neuonc/nor200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gállego Pérez-Larraya J, Lahutte M, Petrirena G, et al. Response assessment in recurrent glioblastoma treated with irinotecan–bevacizumab: comparative analysis of the Macdonald, RECIST, RANO, and RECIST + F criteria. Neuro Oncol. 2012;14(5):667–673. doi: 10.1093/neuonc/nos070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mong S, Ellingson BM, Nghiemphu PL, et al. Persistent diffusion-restricted lesions in bevacizumab-treated malignant gliomas are associated with improved survival compared with matched controls. Am. J. Neuroradiol. 2012;33(9):1763–1770. doi: 10.3174/ajnr.A3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12(3):233–242. doi: 10.1093/neuonc/nop027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 30.Di Tomaso E, Snuderl M, Kamoun WS, et al. Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Res. 2011;71(1):19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pope WB, Xia Q, Paton VE, et al. Patterns of progression in patients with recurrent glioblastoma treated with bevacizumab. Neurology. 2011;76(5):432–437. doi: 10.1212/WNL.0b013e31820a0a8a. [DOI] [PubMed] [Google Scholar]
- 32.Arbab AS. Activation of alternative pathways of angiogenesis and involvement of stem cells following anti-angiogenesis treatment in glioma. Histol. Histopathol. 2012;27(5):549–557. doi: 10.14670/hh-27.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nghiemphu PL, Liu W, Lee Y, et al. Bevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experience. Neurology. 2009;72(14):1217–1222. doi: 10.1212/01.wnl.0000345668.03039.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73(15):1200–1206. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuniga RM, Torcuator R, Jain R, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J. Neurooncol. 2009;91(3):329–336. doi: 10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 36.Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Mischel PS, Pope WB. Quantitative volumetric analysis of conventional MRI response in recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(4):401–409. doi: 10.1093/neuonc/noq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellingson BM, Cloughesy TF, Lai A, et al. Quantification of edema reduction using differential quantitative T2 (DQT2) relaxometry mapping in recurrent glioblastoma treated with bevacizumab. J. Neurooncol. 2012;106(1):111–119. doi: 10.1007/s11060-011-0638-x. [DOI] [PubMed] [Google Scholar]; ▪ Describes the use of novel quantitative T2 relaxometry techniques to assess malignant glioma response to antiangiogenic therapy.
- 38.Pechman KR, Donohoe DL, Bedekar DP, Kurpad SN, Hoffmann RG, Schmainda KM. Characterization of bevacizumab dose response relationship in U87 brain tumors using magnetic resonance imaging measures of enhancing tumor volume and relative cerebral blood volume. J. Neurooncol. 2011;105(2):233–239. doi: 10.1007/s11060-011-0591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorensen AG, Emblem KE, Polaskova P, et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72(2):402–407. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubenstein JL, Kim J, Ozawa T, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2(4):306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Essock-Burns E, Lupo JM, Cha S, et al. Assessment of perfusion MRI-derived parameters in evaluating and predicting response to antiangiogenic therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13(1):119–131. doi: 10.1093/neuonc/noq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreisl TN, Zhang W, Odia Y, et al. A Phase II trial of single-agent bevacizumab in patients with recurrent anaplastic glioma. Neuro Oncol. 2011;13(10):1143–1150. doi: 10.1093/neuonc/nor091. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ First study to use both dynamic contrast-enhanced MRI and 18F-fluorodeoxyglucose PET as biomarkers for assessing response to bevacizumab.
- 43.Ferrier MC, Sarin H, Fung SH, et al. Validation of dynamic contrast-enhanced magnetic resonance imaging-derived vascular permeability measurements using quantitative autoradiography in the RG2 rat brain tumor model. Neoplasia. 2007;9(7):546–555. doi: 10.1593/neo.07289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorensen AG, Batchelor TT, Zhang WT, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69(13):5296–5300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Used a combined dynamic contrast-enhanced MRI imaging biomarker and biological assay to predict response to antiangiogenic therapy.
- 45.Farrar CT, Kamoun WS, Ley CD, et al. Sensitivity of MRI tumor biomarkers to VEGFR inhibitor therapy in an orthotopic mouse glioma model. PLoS ONE. 2011;6(3):e17228. doi: 10.1371/journal.pone.0017228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukumura D, Jain RK. Imaging angiogenesis and the microenvironment. APMIS. 2008;116(7–8):695–715. doi: 10.1111/j.1600-0463.2008.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henderson E, Sykes J, Drost D, Weinmann HJ, Rutt BK, Lee TY. Simultaneous MRI measurement of blood flow, blood volume, and capillary permeability in mammary tumors using two different contrast agents. J. Magn. Reson. Imaging. 2000;12(6):991–1003. doi: 10.1002/1522-2586(200012)12:6<991::aid-jmri26>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 48.Bode MK, Ruohonen J, Nieminen MT, Pyhtinen J. Potential of diffusion imaging in brain tumors: a review. Acta Radiol. 2006;47(6):585–594. doi: 10.1080/02841850600580598. [DOI] [PubMed] [Google Scholar]
- 49.Ellingson BM, Malkin MG, Rand SD, et al. Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J. Magn. Reson. Imaging. 2010;31(3):538–548. doi: 10.1002/jmri.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J. Magn. Reson. Imaging. 1999;9(1):53–60. doi: 10.1002/(sici)1522-2586(199901)9:1<53::aid-jmri7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 51.Lyng H, Haraldseth O, Rofstad EK. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn. Reson. Med. 2000;43(6):828–836. doi: 10.1002/1522-2594(200006)43:6<828::aid-mrm8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 52.Chenevert TL, Stegman LD, Taylor JM, et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J. Natl Cancer Inst. 2000;92(24):2029–2036. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 53.Guo AC, Cummings TJ, Dash RC, Provenzale JM. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002;224(1):177–183. doi: 10.1148/radiol.2241010637. [DOI] [PubMed] [Google Scholar]
- 54.Hayashida Y, Hirai T, Morishita S, et al. Diffusion-weighted imaging of metastatic brain tumors: comparison with histologic type and tumor cellularity. Am. J. Neuroradiol. 2006;27(7):1419–1425. [PMC free article] [PubMed] [Google Scholar]
- 55.Kinoshita M, Hashimoto N, Goto T, et al. Fractional anisotropy and tumor cell density of the tumor core show positive correlation in diffusion tensor magnetic resonance imaging of malignant brain tumors. Neuroimage. 2008;43(1):29–35. doi: 10.1016/j.neuroimage.2008.06.041. [DOI] [PubMed] [Google Scholar]
- 56.Pope WB, Kim HJ, Huo J, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009;252(1):182–189. doi: 10.1148/radiol.2521081534. [DOI] [PubMed] [Google Scholar]
- 57.Pope WB, Qiao XJ, Kim HJ, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J. Neurooncol. 2012;108(3):491–498. doi: 10.1007/s11060-012-0847-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ First study to demonstrate the utility of diffusion MRI biomarkers in a multicenter clinical trial setting.
- 58.Pope WB, Lai A, Mehta R, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free survival in newly diagnosed bevacizumab-treated glioblastoma. Am. J. Neuroradiol. 2011;32(5):882–889. doi: 10.3174/ajnr.A2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamstra DA, Chenevert TL, Moffat BA, et al. Evaluation of the functional diffusion map as an early biomarker of time-to-progression and overall survival in high-grade glioma. Proc. Natl Acad. Sci. USA. 2005;102(46):16759–16764. doi: 10.1073/pnas.0508347102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moffat BA, Chenevert TL, Lawrence TS, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc. Natl Acad. Sci. USA. 2005;102(15):5524–5529. doi: 10.1073/pnas.0501532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ellingson BM, Cloughesy TF, Lai A, et al. Graded functional diffusion map-defined characteristics of apparent diffusion coefficients predict overall survival in recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(10):1151–1161. doi: 10.1093/neuonc/nor079. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Used a novel diffusion MRI biomarker to evaluate the glioblastoma response to bevacizumab.
- 62.Ellingson BM, Laviolette PS, Rand SD, et al. Spatially quantifying microscopic tumor invasion and proliferation using a voxelwise solution to a glioma growth model and serial diffusion MRI. Magn. Reson. Med. 2011;65(4):1131–1143. doi: 10.1002/mrm.22688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Pope WB. Nonlinear registration of diffusion-weighted images improves clinical sensitivity of functional diffusion maps in recurrent glioblastoma treated with bevacizumab. Magn. Reson. Med. 2012;67(1):237–245. doi: 10.1002/mrm.23003. [DOI] [PubMed] [Google Scholar]
- 64.Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Pope WB. Cell invasion, motility, and proliferation level estimate (CIMPLE) maps derived from serial diffusion MR images in recurrent glioblastoma treated with bevacizumab. J. Neurooncol. 2011;105(1):91–101. doi: 10.1007/s11060-011-0567-8. [DOI] [PubMed] [Google Scholar]
- 65.Kim H, Catana C, Ratai EM, et al. Serial magnetic resonance spectroscopy reveals a direct metabolic effect of cediranib in glioblastoma. Cancer Res. 2011;71(11):3745–3752. doi: 10.1158/0008-5472.CAN-10-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colavolpe C, Chinot O, Metellus P, et al. FDG-PET predicts survival in recurrent high-grade gliomas treated with bevacizumab and irinotecan. Neuro Oncol. 2012;14(5):649–657. doi: 10.1093/neuonc/nos012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J. Nucl. Med. 2002;43(9):1210–1217. [PubMed] [Google Scholar]
- 68.Chen W, Delaloye S, Silverman DH, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J. Clin. Oncol. 2007;25(30):4714–4721. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 69.Schwarzenberg J, Czernin J, Cloughesy TF, et al. 3´-deoxy-3´-18F-fluorothymidine PET and MRI for early survival predictions in patients with recurrent malignant glioma treated with bevacizumab. J. Nucl. Med. 2012;53(1):29–36. doi: 10.2967/jnumed.111.092387. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Used 3´-deoxy-3´-18F-fluorothymidine, a nonfluorodeoxyglucose tracer that is sensitive to tumor cell proliferation, to evaluate malignant glioma response to bevacizumab.
- 70.Harris RJ, Cloughesy TF, Pope WB, et al. 18F-FDOPA and 18F-FLT positron emission tomography parametric response maps predict response in recurrent malignant gliomas treated with bevacizumab. Neuro Oncol. 2012;14(8):1079–1089. doi: 10.1093/neuonc/nos141. [DOI] [PMC free article] [PubMed] [Google Scholar]
▪ Website
- 101.www.cbtrus.org Central Brain Tumor Registry of the United States. Primary brain tumors in the United States 2004–2006 (2010)