Abstract
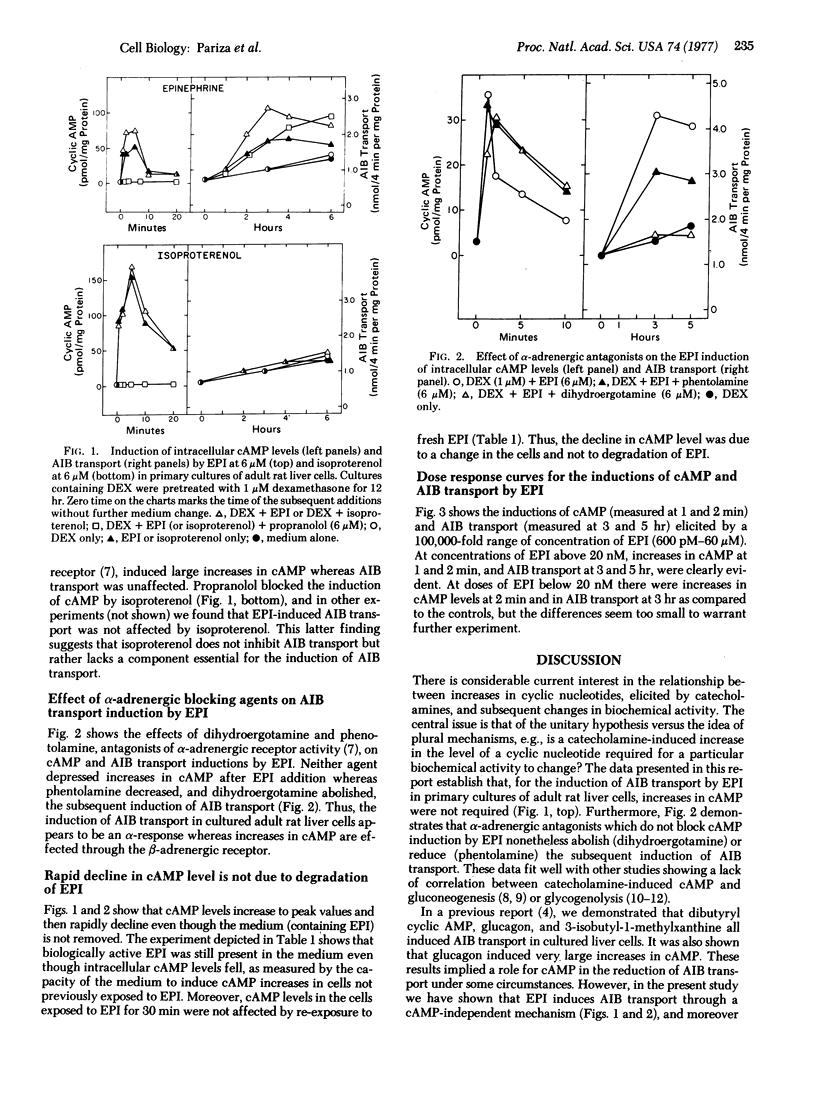
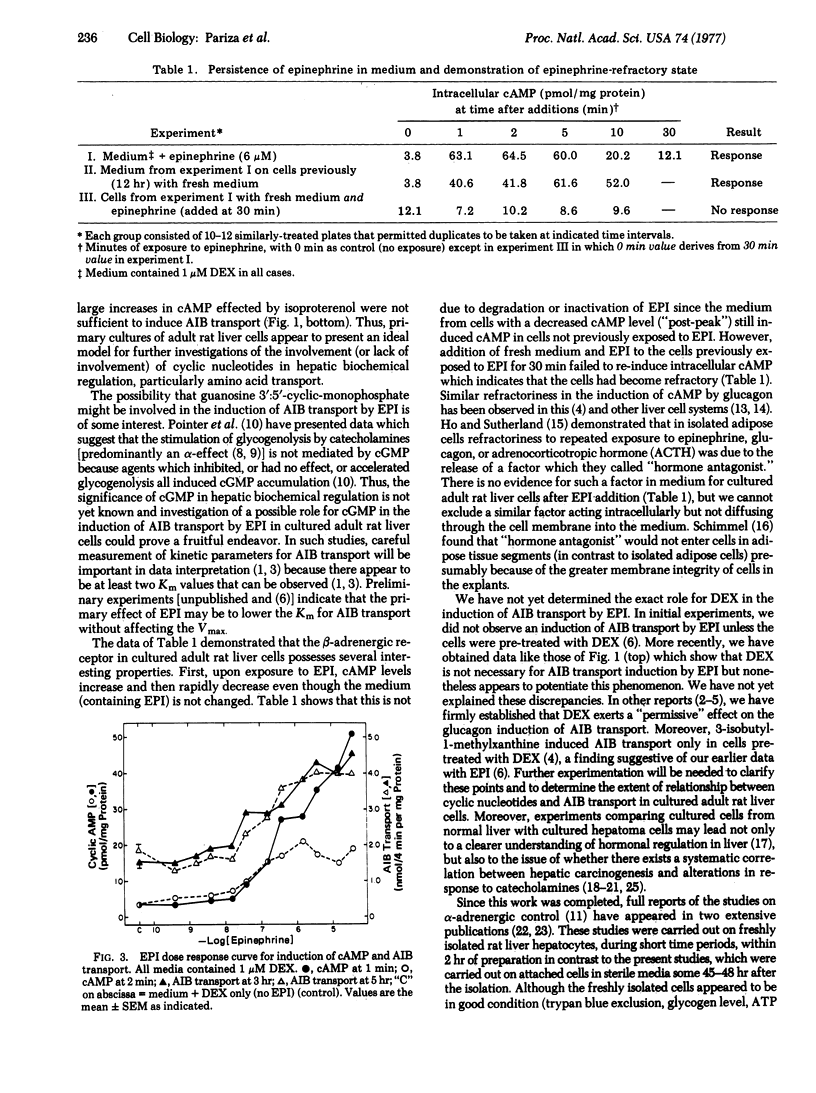
Liver cells were obtained from adult rats by a collagenase perfusion technique and cultured as monolayers in serum-free media. Epinephrine and isoproterenol both induced large increases in intracellular adenosine 3':5'-monophosphate (cAMP) within 1-2 min whereas epinephrine (but not isoproterenol) induced 2- to 3-fold increases in the rate of alpha-aminoisobutyric acid transport within 2-4 hr after a 1 hr lag. Propranolol abolished the increase in cAMP elicited by epinephrine and isoproterenol, but did not block the induction of alpha-aminoisobutyric acid transport by epinephrine. In contrast, dihydroergotamine abolished and phentolamine diminished the induction of alpha-aminoisobutyric acid transport by epinephrine but did not decrease the stimulation of cAMP levels by epinephrine. Epinephrine dose response curves for cAMP and alpha-aminoisobutyric acid transport were similar. Once exposed to epinephrine, cells became refractory to further stimulation of cAMP levels by epinephrine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd H., Martin T. J. Changes in catecholamine- and glucagon-responsive adenylate cyclase activity in preneoplastic rat liver. Mol Pharmacol. 1976 Mar;12(2):195–202. [PubMed] [Google Scholar]
- Brown H. D., Chattopadhyay S. K., Morris H. P., Pennington S. N. Adenyl cyclase activity in Morris hepatomas 7777, 7794A, and 9618A. Cancer Res. 1970 Jan;30(1):123–126. [PubMed] [Google Scholar]
- Cherrington A. D., Assimacopoulos F. D., Harper S. C., Corbin J. D., Park C. R., Exton J. H. Studies on the alpha-andrenergic activation of hepatic glucose output. II. Investigation of the roles of adenosine 3':5'-monophosphate and adenosine 3':5'-monophosphate-dependent protein kinase in the actions of phenylephrine in isolated hepatocytes. J Biol Chem. 1976 Sep 10;251(17):5209–5218. [PubMed] [Google Scholar]
- Christoffersen T., Morland J., Osnes J. B., Elgjo K. Hepatic adenyl cyclase: alterations in hormone response during treatment with a chemical carcinogen. Biochim Biophys Acta. 1972 Sep 15;279(2):363–366. doi: 10.1016/0304-4165(72)90153-5. [DOI] [PubMed] [Google Scholar]
- DeRubertis F. R., Craven P. Reduced sensitivity of the hepatic adenylate cyclase-cyclic AMP system to glucagon during sustained hormonal stimulation. J Clin Invest. 1976 Feb;57(2):435–443. doi: 10.1172/JCI108294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmelot P., Bos C. J. Studies on plasma membranes. XIV. Adenyl cyclase in plasma membranes isolated from rat and mouse livers and hepatomas, and its hormone sensitivity. Biochim Biophys Acta. 1971 Oct 12;249(1):285–292. doi: 10.1016/0005-2736(71)90106-4. [DOI] [PubMed] [Google Scholar]
- Fain J. N. Biochemical aspects of drug and hormone action on adipose tissue. Pharmacol Rev. 1973 Mar;25(1):67–118. [PubMed] [Google Scholar]
- Ho R. J., Sutherland E. W. Formation and release of a hormone antagonist by rat adipocytes. J Biol Chem. 1971 Nov 25;246(22):6822–6827. [PubMed] [Google Scholar]
- Hutson N. J., Brumley F. T., Assimacopoulos F. D., Harper S. C., Exton J. H. Studies on the alpha-adrenergic activation of hepatic glucose output. I. Studies on the alpha-adrenergic activation of phosphorylase and gluconeogenesis and inactivation of glycogen synthase in isolated rat liver parenchymal cells. J Biol Chem. 1976 Sep 10;251(17):5200–5208. [PubMed] [Google Scholar]
- Klein I., Levey G. S., Bricker L. A., Morris H. P. Glucagon and epinephrine activation of adenylate cyclase and glucagon binding in Morris hepatomas. Endocrinology. 1974 Jan;94(1):279–283. doi: 10.1210/endo-94-1-279. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Pariza M. W., Becker J. E., Potter V. R. A "permissive" effect of dexamethasone on the glucagon induction of amino acid transport in cultured hepatocytes. Nature. 1975 Jul 3;256(5512):46–47. doi: 10.1038/256046a0. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Pariza M. W., Becker J. E., Potter V. R., Butcher F. R. Induction of amino acid transport in primary cultures of adult rat liver parenchymal cells by insulin. J Biol Chem. 1976 May 25;251(10):3014–3020. [PubMed] [Google Scholar]
- Kneer N. M., Bosch A. L., Clark M. G., Lardy H. A. Glucose inhibition of epinephrine stimulation of hepatic gluconeogenesis by blockade of the alpha-receptor function. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4523–4527. doi: 10.1073/pnas.71.11.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariza M. W., Kletzien R. F., Butcher F. R., Potter V. R. Inductions by hormones added singly, simultaneously or sequentially: what cultured hepatocytes can tell us about metabolic regulation in the whole animal. Adv Enzyme Regul. 1976;14:103–115. doi: 10.1016/0065-2571(76)90009-1. [DOI] [PubMed] [Google Scholar]
- Pariza M. W., Yanagi S., Gurr J. A., Bushnell D. E., Morris H. P., Potter V. R. Ornithine decarboxylase activity and DNA synthesis in Morris hepatomas 5123-C and 7800. Life Sci. 1976 Jan 1;18(1):39–47. doi: 10.1016/0024-3205(76)90271-x. [DOI] [PubMed] [Google Scholar]
- Plas C., Nunez J. Glycogenolytic response to glucagon of cultured fetal hepatocytes. Refractoriness following prior exposure to glucagon. J Biol Chem. 1975 Jul 25;250(14):5304–5311. [PubMed] [Google Scholar]
- Pointer R. H., Butcher F. R., Fain J. N. Studies on the role of cyclic guanosine 3':5'-monophosphate and extracellular Ca2+ in the regulation of glycogenolysis in rat liver cells. J Biol Chem. 1976 May 25;251(10):2987–2992. [PubMed] [Google Scholar]
- Saitoh Y., Ui M. Stimulation of glycogenolysis and gluconeogenesis by epinephrine independent of its beta-adrenergic function in perfused rat liver. Biochem Pharmacol. 1976 Apr 1;25(7):841–845. doi: 10.1016/0006-2952(76)90156-8. [DOI] [PubMed] [Google Scholar]
- Schimmel R. J. Responses of adipose tissue to sequential lipolytic stimuli. Endocrinology. 1974 May;94(5):1372–1380. doi: 10.1210/endo-94-5-1372. [DOI] [PubMed] [Google Scholar]
- Schmidtke J., Wienker T., Flügel M., Engel W. In vitro inhibition of cyclic AMP phosphodiesterase by cortisol. Nature. 1976 Aug 12;262(5569):593–594. doi: 10.1038/262593a0. [DOI] [PubMed] [Google Scholar]
- Tolbert M. E., Butcher F. R., Fain J. N. Lack of correlation between catecholamine effects on cyclic adenosine 3':5'-monophosphate and gluconeogenesis in isolated rat liver cells. J Biol Chem. 1973 Aug 25;248(16):5686–5692. [PubMed] [Google Scholar]


