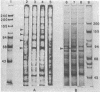
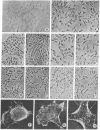
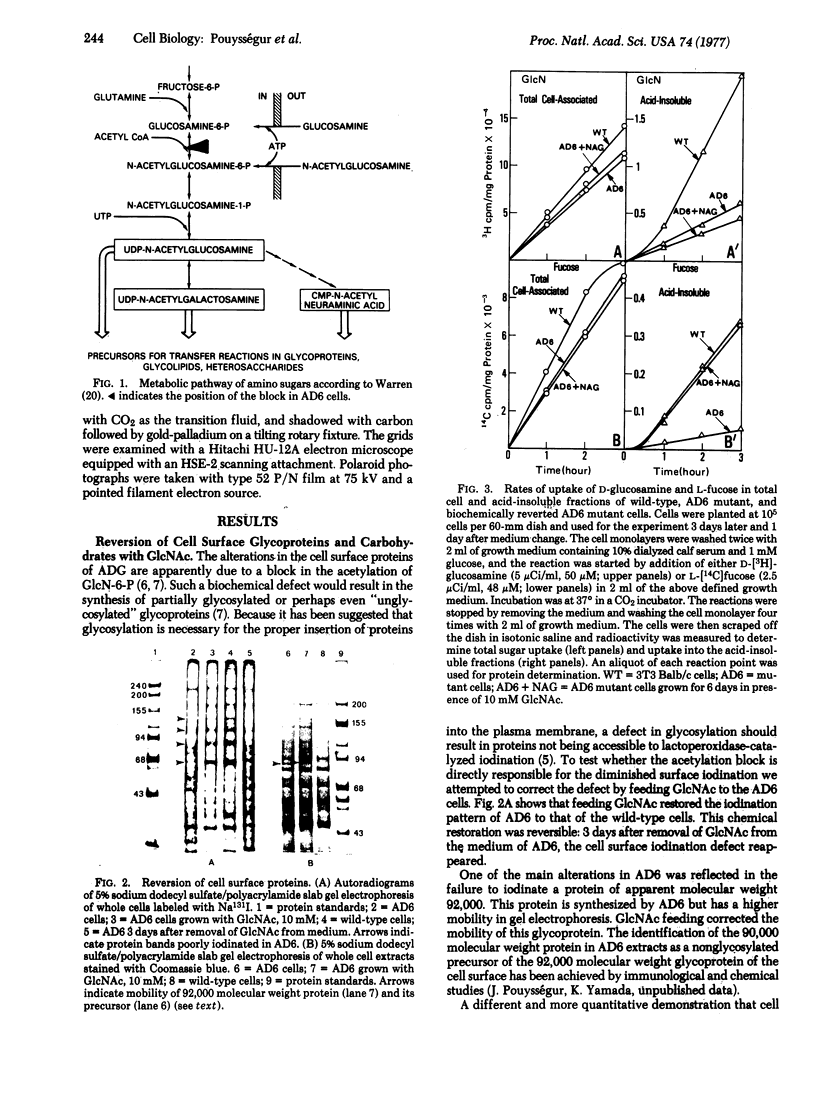
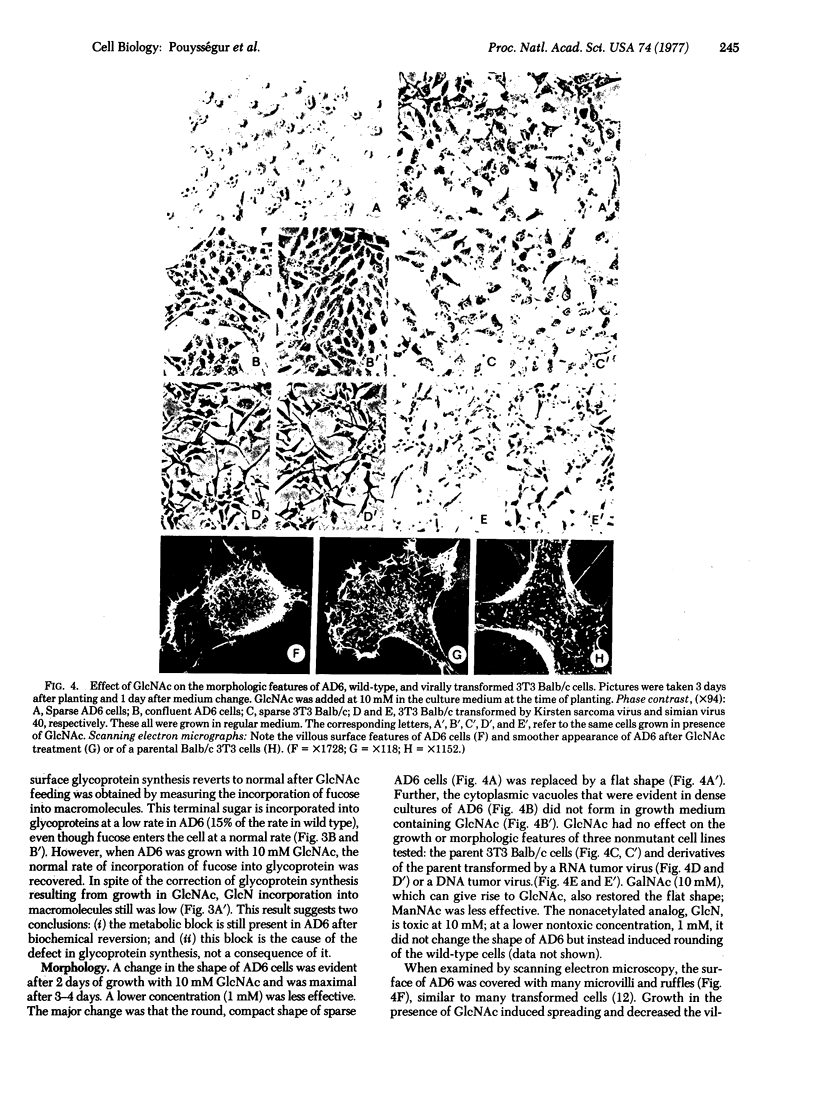
Abstract
AD6, a mutant derived from 3T3 Balb/c cells, is characterized by low adhesion to substratum, round shape, increase in surface microvilli, increase in agglutinability by concanavalin A, and loss of directional motility. These properties are often observed in transformed cells. However, the mutant has normal growth properties and anchorage-dependence of growth, and it does not form tumors. In AD6, the biosynthesis of complex carbohydrates and glycoproteins is impaired because of a block in the acetylation of GlcN-6-P. This defect is responsible for all the surface alterations because feeding of GlcNAc to AD6 cells corrects the defects in the synthesis of complex carbohydrates and the exposure of glycoproteins at the outer surface of the plasma membrane. Parallel to this biochemical reversion, there is full restoration of the altered biological properties. In contrast, GlcNAc has no effect on the morphologic features of two lines of transformed cells. Our results suggest that the carbohydrate portion of cell surface proteins has an important role in adhesion and related aspects of cell behavior. The fact that a defined alteration of the cell surface induces many properties often encountered in transformed cells, without affecting control of cell division, strongly suggests that these alterations in properties are not sufficient to account for the loss of growth regulation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie M., Heaysman J. E., Pegrum S. M. The locomotion of fibroblasts in culture. I. Movements of the leading edge. Exp Cell Res. 1970 Mar;59(3):393–398. doi: 10.1016/0014-4827(70)90646-4. [DOI] [PubMed] [Google Scholar]
- Bernard J., Caen J., Nurden A., Tobelem G., Jeanneau C. Défaut d'une glycoprotéine de membrane plaquettaire, base moléculaire de l'anomalie d'adhésion des plaquettes au sous-endothélium dans la dystrophie thrombocytaire hémorragique. C R Acad Sci Hebd Seances Acad Sci D. 1975 Jun 2;280(21):2517–2520. [PubMed] [Google Scholar]
- Emmelot P. Biochemical properties of normal and neoplastic cell surfaces; a review. Eur J Cancer. 1973 May;9(5):319–333. doi: 10.1016/0014-2964(73)90047-9. [DOI] [PubMed] [Google Scholar]
- Erickson C. A., Trinkaus J. P. Microvilli and blebs as sources of reserve surface membrane during cell spreading. Exp Cell Res. 1976 May;99(2):375–384. doi: 10.1016/0014-4827(76)90595-4. [DOI] [PubMed] [Google Scholar]
- Harris A. K. Cell surface movements related to cell locomotion. Ciba Found Symp. 1973;14:3–26. doi: 10.1002/9780470719978.ch2. [DOI] [PubMed] [Google Scholar]
- Hoover R. L. Surface characterization of two amoebae relative to cell adhesion. Exp Cell Res. 1974 Aug;87(2):265–276. doi: 10.1016/0014-4827(74)90480-7. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Trans-membrane control of the receptors on normal and tumor cells. II. Surface changes associated with transformation and malignancy. Biochim Biophys Acta. 1976 Apr 30;458(1):1–72. doi: 10.1016/0304-419x(76)90014-7. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. Specific roles for platelet surface glycoproteins in platelet function. Nature. 1975 Jun 26;255(5511):720–722. doi: 10.1038/255720a0. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Exposed protein on the intact human erythrocyte. Biochemistry. 1971 May 11;10(10):1766–1771. doi: 10.1021/bi00786a006. [DOI] [PubMed] [Google Scholar]
- Porter K. R., Todaro G. J., Fonte V. A scanning electron microscope study of surface features of viral and spontaneous transformants of mouse Balb-3T3 cells. J Cell Biol. 1973 Dec;59(3):633–642. doi: 10.1083/jcb.59.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J. M., Pastan I. Mutants of Balb/c 3T3 fibroblasts defective in adhesiveness to substratum: evidence for alteration in cell surface proteins. Proc Natl Acad Sci U S A. 1976 Feb;73(2):544–548. doi: 10.1073/pnas.73.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M., O'Neill C., Berryman S., Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer. 1968 Sep 15;3(5):683–693. doi: 10.1002/ijc.2910030517. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. Cyclic AMP mediates the concanavalin A agglutinability of mouse fibroblasts. J Cell Biol. 1974 Oct;63(1):288–294. doi: 10.1083/jcb.63.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. Cyclic AMP modulates microvillus formation and agglutinability in transformed and normal mouse fibroblasts. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1263–1267. doi: 10.1073/pnas.72.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Ohanian S. H., Pastan I. Cell surface protein decreases microvilli and ruffles on transformed mouse and chick cells. Cell. 1976 Oct;9(2):241–245. doi: 10.1016/0092-8674(76)90115-x. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Weston J. A. Isolation of a major cell surface glycoprotein from fibroblasts. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3492–3496. doi: 10.1073/pnas.71.9.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]