Abstract
The lateral habenula (LHb) has recently emerged as a key brain region in the pathophysiology of depression. However the molecular mechanism by which LHb becomes hyperactive in depression remains unknown. Through a quantitative proteomic screen, we found that βCaMII expression was significantly upregulated in the LHb of animal models of depression, and downregulated by antidepressants. Increasing the levels of β- but not α-CamKII in the LHb strongly enhanced the synaptic efficacy and spike output of LHb neurons, and was sufficient to produce profound depressive symptoms including anhedonia and behavioral despair. Downregulation of βCaMKII levels, blocking its activity or its target molecule GluR1, reversed the depressive symptoms. These results identify βCaMKII as a powerful regulator of LHb neuron function and a key molecular determinant of depression.
Major depressive disorder (MDD), one of the most prevalent and disabling mental disorders, is characterized by low mood, loss of motivation, feelings of despair, and an inability to feel pleasure, also known as anhedonia (1). Modern views on the cause of MDD suggest that the neural activity of specific brain circuits are altered in response to external stimuli such as stress, as a result of maladaptive molecular and cellular changes (2, 3). Recently, the lateral habenula (LHb), a nucleus that relays information from the limbic forebrain to multiple monoamine centers, has emerged as a key brain region in aversive behaviors and the pathophysiology of depression (4–10). LHb neurons are activated by aversive emotional cues, including stress, disappointment, fear or anticipation of a negative reward (4–6). Consistently, neuroimaging studies have identified heightened habenula activity in the depressed state (11–13). Furthermore, synaptic activity and spike output of LHb neurons were enhanced in animal models of depression (14). However, what molecular mechanisms underlie these aberrant cellular processes in LHb and how depression-inducing stimuli lead to these changes are yet to be determined.
We conducted an unbiased, mass spectrometry-based, quantitative proteomic screening, to compare habenular protein expression of wild-type control and congenitally learned helpless (cLH) rats, a well-accepted model of depression (15)). cLH rats were selectively bred for the phenotype of learned helplessness (16), displaying significantly reduced escape from escapable foot shocks, which was reversible by chronic antidepressant treatment (imipramine, i.p.,10mg/kg, 14 days, Fig. 1A). cLH rats also showed increased immobility in the forced swim test (Fig. 1A), another animal model of depression that reflects behavioral despair (17), though basic motor and cognitive functions are normal (15).
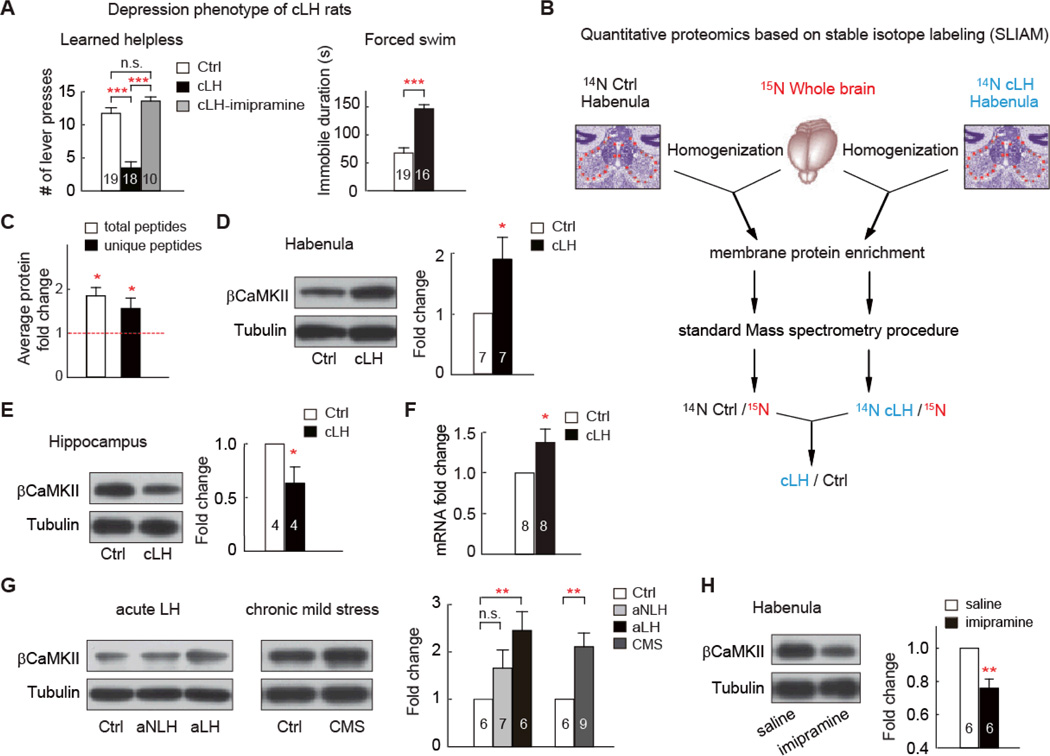
Fig. 1. βCaMKII is upregulated in the LHb of animal models of depression.
(A) Depression phenotypes of cLH rats. Numbers in the bars indicate number of animals used. Note in LH test, maximal number of bar presses is 15. (B) Experimental outline of the high-throughput quantitative proteomics based on stable isotope labeling. Briefly, habenula of unlabeled (14N) WT or cLH rats were dissected, homogenized, and mixed in a 1:1 ratio with total brain homogenate from a 15N-labeled rat. Membrane fraction was enriched, and 100 µg protein sample was used for standard mass spectra analysis. 14N /15N ratio for each identified peptide was calculated. Peptide ratios for each protein were then compared between cLH and control sample. Details see methods. (C) Proteomic analysis of βCaMKII, based either on total peptides, or unique peptides (peptides not shared by other CaMKII family members) identified in 3 independent proteomic runs. (D, E) Western blot analysis showing change of βCaMKII in membrane fraction of habenula (D) or hippocampus (E) of cLH rats. Tissue amounts of tubulin were used as loading control. Protein expression was normalized by control amount. (F) qPCR analysis of βCaMKII mRNA in habenula. (G) βCaMKII level increase in acute learned helpless and chronic mild stress (CMS) depression models. aLH and aNLH were rat groups subjecting to LH stress but did (aLH), or did not (aNLH) display LH symptom. (H) Western blot analysis showing level of βCaMKII in membrane fraction of habenula of cLH rats treated with saline or antidepressant imipramine. Data are mean ± SEM. * p < 0.05 ** p < 0.01, *** p < 0.001 compared to control group, n.s., not significant, two-tailed Student’s t-tests for two-group comparison, one-way ANOVA with Bonferroni post hoc analysis for multiple-group comparison.
We micro-dissected the habenuli of cLH and wild-type control rats and extracted protein for quantitative proteomic analysis based on 15N stable isotope labeling (Fig. 1B, 18). To reduce sample complexity, the membrane fraction was extracted and three independent sets of samples were analyzed (figs. S1-3, table S1). We identified βCaMKII as significantly upregulated in the habenula of cLH rats (1.9-fold of wild-type control, p = 0.01, Fig.1C). Other CaMKII family isoforms were also examined: αCamKII levels varied widely across samples although an increasing trend was observed; δCaMKII remained unchanged; γCaMKII showed a 1.3-fold increase (p = 0.0013, fig. S4). As βCaMKII is more enriched in the brain than γCaMKII (19, 20), we focused on this CaMKII isoform. Secondary validation by western blot analysis confirmed that βCaMKII in the membrane fraction of cLH habenular protein samples increased to 1.86-fold of the control level (p = 0.03, Fig. 1D). In contrast, the βCaMKII protein level in cLH hippocampal samples decreased (63% of control, p = 0.048, Fig. 1E), probably due to neural atrophy and spine loss in the hippocampus associated with depression (21, 22). Levels of βCaMKII mRNA in cLH habenula increased to 1.37-fold of control (p = 0.04) as measured by quantitative real-time PCR (Fig. 1F), suggesting that transcriptional regulation contributed to at least part of the protein level change. Immunohistochemical staining of habenular brain slices revealed that the CaMKII protein level increase occurred in the lateral part of the habenula (fig. S6).
We further examined βCaMKII level in two additional depression models, acute learned helpless, induced by repeated inescapable and uncontrollable foot shocks (16), and chronic mild stress, induced by prolonged exposure to unpredictable mild stressors (23). The βCaMKII levels were also significantly increased in these two stress paradigms (Fig. 1G). Furthermore, chronic antidepressant treatment with imipramine, which reversed the depressive phenotypes of cLH rats (Fig. 1A), caused significant downregulation of βCaMKII protein in the habenula of cLH rats (Fig. 1H).
We next investigated to what extent the change of βCaMKII levels in LHb is necessary or sufficient to cause depressive behaviors, or whether it is merely a biological marker of the depressive state. We first constructed viral vectors (adeno-associated virus 2 (AAV2)) to overexpress β- or α- CamKII in the LHb of wild-type rats and mice, and tested the effects on various depression models (Figs. 2A-C). We used the ubiquitin promoter to drive ubiquitous, high-level gene expression, as LHb neurons are almost uniformly glutamatergic (24). To estimate the level of overexpression, we injected CaMKII-expressing viruses into one side of the habenula. At 14 days post injection, CaMKII levels in the injected side were 4.4 ± 0.6-fold for AAV-βCaMKII and 4.6 ± 0.9-fold for AAV- αCaMKII of the non-injected side respectively (fig. S6). We then injected virus into both sides of LHb and allowed expression for 10 days before proceeding to behavioral tests of depression (Figs. 2B, 2C, fig. S7).
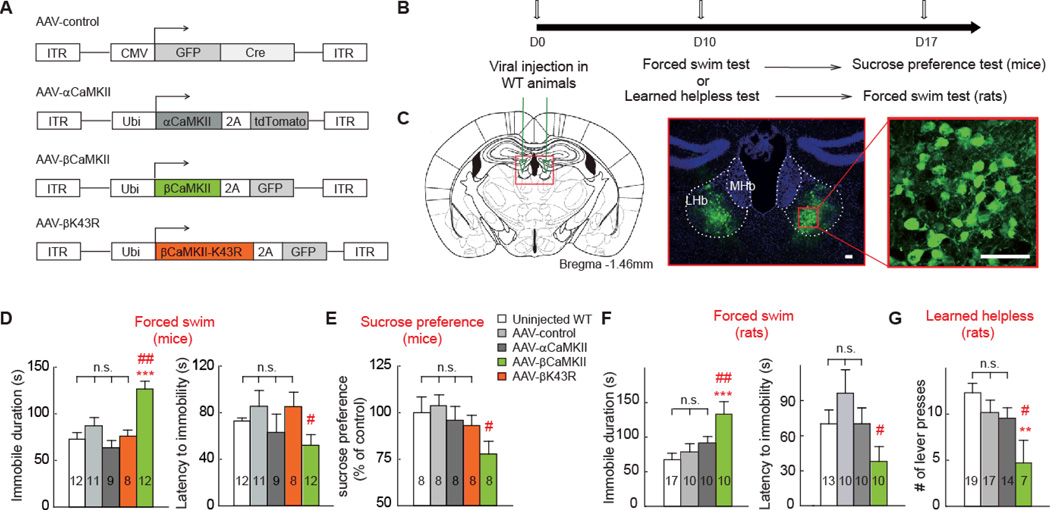
Fig. 2. Overexpression of βCaMKII but not αCaMKII in LHb caused depressive-like behaviors in both mice and rats.
A) Schematics of AAV vectors engineered to overexpress a control construct, βCaMKII, αCaMKII, or a kinase-dead mutant of βCaMKII. ITR, inverted terminal repeats; CMV, cytomegalovirus promoter; Ubi: ubiquitin promoter; 2A: viral 2A linker peptide allowing translation of multiple unfused proteins. (B) Experimental paradigm for behavioral testing of WT mice or rats. (C) Illustration of bilateral viral injection of AAV-βCaMKII in mouse LHb (counter-stained with anti-GFP and nuclear marker Hoechst). Scale bars, 50 µm. (D-G) Behavioral effects of expressing various viral constructs in LHb in animal models of depression in mice (D-E) or rats (H, I). * p < 0.05, ** p < 0.01, *** p < 0.001 compared with non-injected WT, # p < 0.05, ## p < 0.01 compared with AAV-control, one-way ANOVA with Bonferroni post hoc analysis.
Overexpression of βCaMKII in the LHb of unstressed mice produced significantly increased immobility time and decreased latency to immobility onset in the forced swim test, compared with the injection control (Fig. 2D). Locomotor activities of these mice were not significantly different (fig. S8), indicating that the immobility was unlikely due to motor defects. In addition, βCaMKII overexpression also caused anhedonia, evident from a significant reduction in the preference for the sucrose solution (Fig. 2E). To estimate the minimal infection rate required to produce the depression phenotype, we bilaterally injected an additional group of mice with 1:10 diluted AAV-βCaMKII virus. Unlike the normal injection group (infection rate = 38 ± 3%), this sparse injection group (infection rate = 5 ± 0.6%) did not exhibit depressive phenotypes (fig. S9). Overexpression of αCaMKII, or a control GFP-Cre construct, at a similar infection rate (36 ± 2.5% for αCaMKII, 36 ± 3% for GFP-Cre) did not cause similar depressive effects (Fig. 2 D-E). βCaMKII can act both as a kinase and a structural scaffolding protein at the synapses (25). A kinase-dead version of βCaMKII, βK43R (26), even when overexpressed at a similar level as wild-type βCaMKII (4.6 ± 0.5-fold, infection rate = 40 ± 3%, fig. S6), did not cause depressive-like phenotypes (Figs. 2D, 2E), suggesting that the kinase function of βCaMKII was required to produce depression. Furthermore, in rats, overexpression of βCaMKII in the LHb significantly increased immobility in the forced swim test and reduced the escape behavior, as indicated by number of bar pressing to terminate foot shocks in the learned helplessness test (Figs. 2F, 2G).
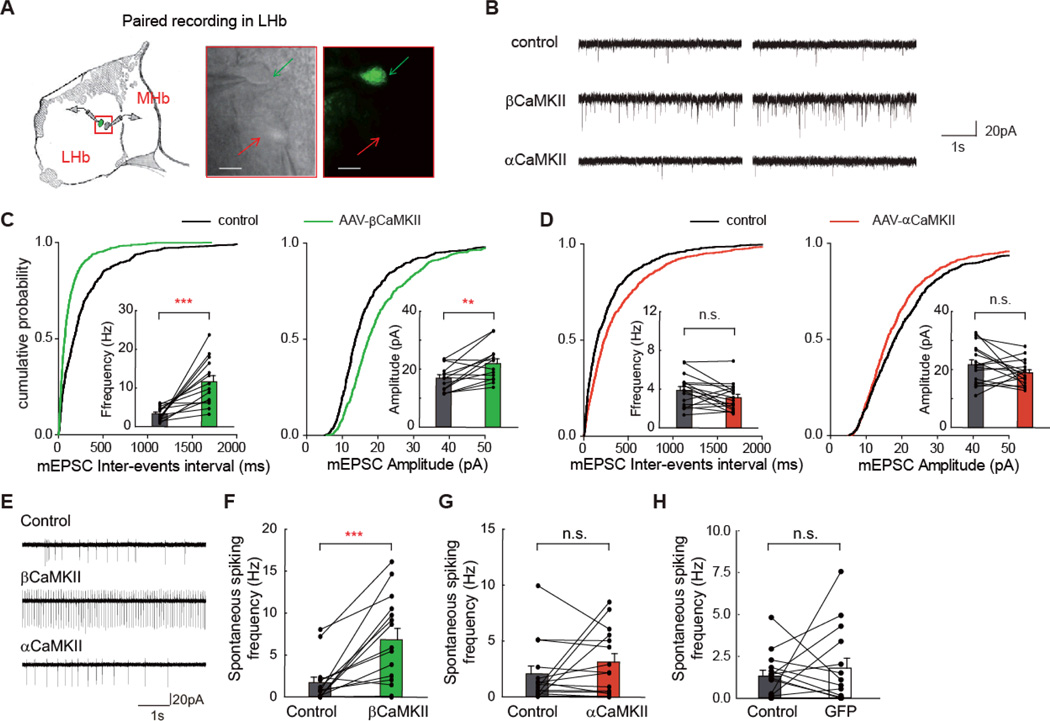
To investigate the cellular mechanism by which βCaMKII overexpression alters LHb neuron activity and function, we performed paired whole-cell patch-clamp recordings on viral-infected and neighboring uninfected LHb neurons in acute brain slices of wild-type rats (Fig. 3A). First, to examine the synaptic property of LHb neurons, we measured the miniature excitatory postsynaptic currents (mEPSCs), which are mediated by the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) subtype of glutamate receptors and reflect individual synaptic responses onto recorded neurons. In non-infected brain slices, neighboring LHb neuron pairs showed highly similar mEPSC frequency, despite heterogeneity across different sub-regions of the LHb (fig. S10). In neurons infected by AAV-βCaMKII, mEPSC frequency was greatly increased (351 ± 51% of neighboring controls, p = 0.0001), as was mEPSC amplitude (130 ± 10% of controls, p = 0.007, Figs. 3B, 3C). In contrast, AAV-αCaMKII infection caused a slight decrease in mEPSC frequency (81 ± 9% of controls, p = 0.08), and no change in mEPSC amplitude (87 ± 5% of controls, p = 0.16, Figs. 3B, 3D). The effects on mEPSC frequency largely resembled what has been shown in hippocampal neurons for these two CaMKII isoforms (27). Further, to examine the output of these LHb neurons, we measured the spontaneous spiking rate in a cell-attached configuration. The AAV-βCaMKII-infected neurons exhibited a 3.0-fold increase in spiking rate compared with neighboring uninfected neurons (p = 0.0004, Figs. 3E, 3F), whereas no such change was detected for AAV-αCaMKII (p = 0.7, Fig. 3G), or AAV-GFP-infected (p = 0.95, Fig. 3H) neurons.
Fig. 3. Overexpression of βCaMKII increased synaptic activity and spike output of LHb neurons.
(A) Schematics of paired recording configuration in LHb (left). Right: paired patching of a βCaMKII-infected LHb neuron (pointed by green arrow) and a neighboring uninfected neuron (pointed by red arrow) under transmitted and fluorescent light microscopy. Scale bars, 10 µm. (B) Example mEPSC traces, measured in a whole-cell configuration, from LHb neurons of control neurons, or neurons infected by AAV-βCaMKII or AAV-αCaMKII. (C, D) Cumulative distribution of mEPSC inter-events interval and average frequency (left), or mEPSC amplitude (right) of neurons infected by AAV-βCaMKII (C) or AAV-αCaMKII (D). Each line represents values from a pair of control and neighboring viral-infected neurons. (E) Example traces of spontaneous spiking, measured in a cell-attached configuration, from LHb neurons of control neurons, or neurons infected by AAV-βCaMKII or AAV-αCaMKII. (F - H) Average spontaneous spiking frequency of neurons infected by AAV-βCaMKII (F), AAV-αCaMKII (G), or AAV-GFP (H). ** p < 0.01, *** p < 0.001, Wilcoxon signed-rank test.
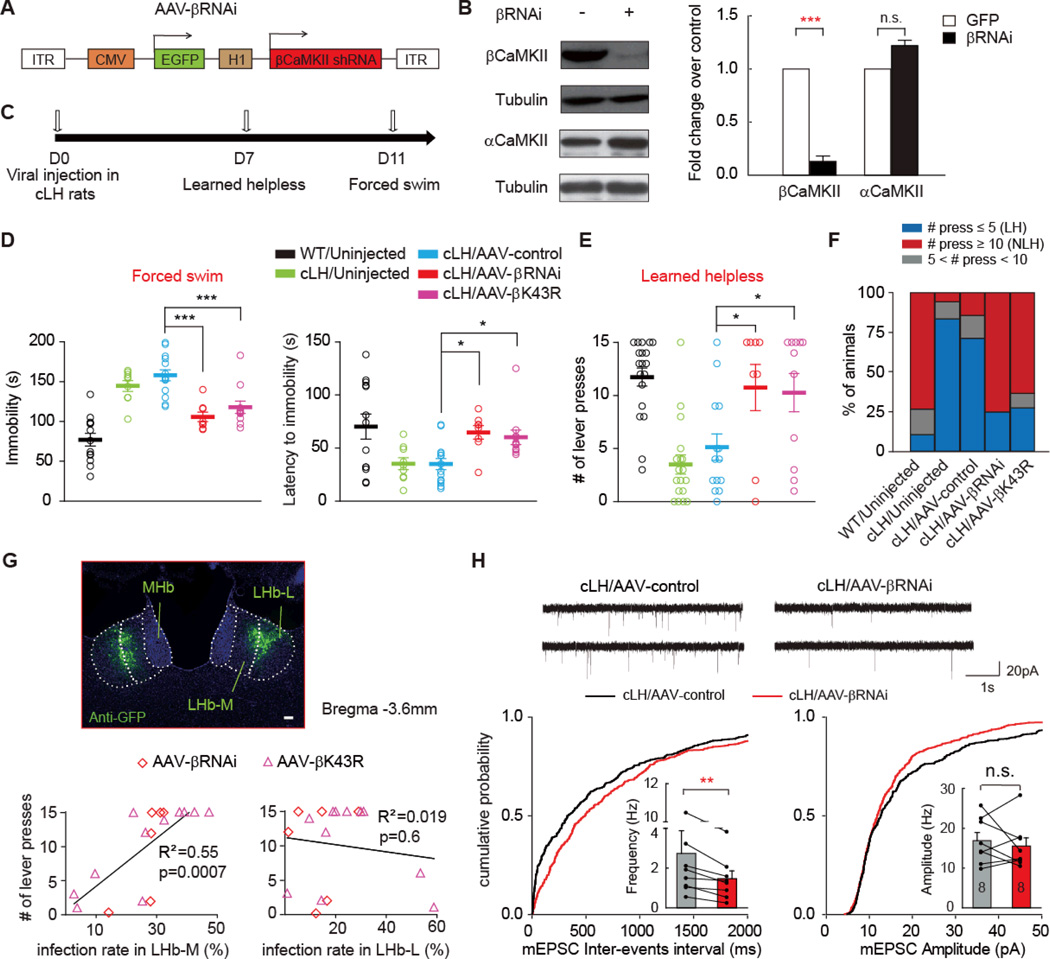
To determine whether down-regulation of βCaMKII levels or blockade of βCaMKII functions in LHb reversed depressive phenotypes, we first employed RNA interference (RNAi) to knock down βCaMKII protein level (Fig. 4A). A previously reported short hairpin RNA specifically targeting the βCaMKII transcript can effectively reduce βCaMKII protein levels without affecting αCaMKII (28, Fig. 4B). We then expressed this RNAi form of βCaMKII in AAV virus (AAV-βRNAi) and targeted its expression to the LHb of cLH rats by bilateral stereotactic injection (Fig. 4C). In cLH rats infected by AAV-βRNAi, the immobility time in the forced swim test was markedly reduced (p < 0.001) and the escape behavior in the learned helpless test significantly increased (p < 0.05, Figs. 4D, 4E). The percentage of learned helpless animals (defined as those with less than 5 bar pressing) dropped from 83.3% to 25% (Fig. 4F).
Fig. 4. Knocking-down of βCaMKII in LHb rescued depression-like phenotypes of cLH rats and reduced synaptic activity of LHb neurons.
(A) Schematics of the AAV vector engineered to overexpress an RNAi form of βCaMKII. H1: human H1 promoter. (B) Specific knocking down of βCaMKII but not αCaMKII by the βCaMKII RNAi construct. pSuper-βCaMKII-RNAi construct was co-transfected with AAV-βCaMKII or AAV-αCaMKII plasmid in 293TN cells, and expressed for 48 hrs before Western analysis. Left: representative western blot. Right: quantification of knock down efficiency. (C) Experimental paradigm for behavioral testing of cLH rats infected by virus. (D-E) Behavioral effects of expressing AAV-βRNAi and AAV-βK43R in the LHb of cLH rats in forced swim (D) or learned helpless test (EF). * p < 0.05, *** p < 0.001, one-way ANOVA with Bonferroni post hoc test. (F) Percentage of animals in each category. LH: learned helpless rats with ≤ 5 lever presses. NLH: non-learned helpless rats with ≥10 lever presses. (G) Sub-regional characterization of viral infection. Top: illustration of the division of lateral LHb (LHb-L) and medial LHb (LHb-M) in an AAV-βRNAi virus infected cLH rat brain slice. Scale bar, 100 µm. Bottom: Behavioral response in the learned helpless test plotted against infection rates in the LHb-L and LHb-M of cLH rats. (H) mEPSCs of AAV-βRNAi infected LHb neurons from cLH rats were altered. Top: example mEPSC traces. Cumulative distribution of mEPSC inter-events interval and average frequency (bottom left) or mEPSC amplitude (bottom right) of cLH LHb neurons infected by AAV-βRNAi. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with cLH/AAV-control, Wilcoxon signed-rank test.
To rule out potential off-target effects by RNAi, we further tested a kinase-dead version of βCaMKII, βK43R, which acts as a dominant-negative to block endogenous βCaMKII function when overexpressed (26, Fig. 2A). When AAV-βK43R virus was injected into the LHb of cLH rats, similar anti-depression effects as AAV-βRNAi were observed in both the forced swim and learned helplessness tests (Figs. 4D-4F). Intriguingly, an infection rate as low as 17 ± 3% for AAV-βRNAi or 24 ± 3% for AAV-βK43R was sufficient to achieve these strong anti-depression effects, suggesting that LHb neural network has little redundancy, and that altering the activity of a relatively small percentage of LHb neurons is sufficient to ameliorate depression. We thus further analyzed infection within sub-regions of LHb, and found that infection rates in the medial, but not lateral part of LHb were strongly correlated with the rescuing effects on learned helplessness (Fig. 4G). In the LHb brain slices of cLH rats, neurons infected by the AAV-βRNAi showed significantly reduced frequency of mEPSC (53.5 ± 15.3% of neighboring uninfected controls, p = 0.008, Fig. 4H).
What could be the downstream molecular targets of βCaMKII in mediating the LHb hyperactivity in depression? Upregulation of βCaMKII increases the synaptic expression and delivery of GluR1-type AMPAR in cultured hippocampal neurons (29). Thus, we examined the level of GluR1 in the LHb of cLH rats, and found that the membrane fraction of GluR1 was upregulated (222 ± 41.7% of controls, p < 0.01, fig. S11A). Conversely, in cLH rats treated with antidepressant imipramine, the membrane GluR1 level was decreased (72 ± 8% of controls, p = 0.003, fig. S11B). To test the role of GluR1 in βCaMKII–mediated depression, we co-expressed βCaMKII with a dominant negative form of GluR1, GluR1Ct, which blocks the synaptic trafficking of GluR1 (30, 31), using an AAV-βCaMKII-2A-GluR1Ct viral construct. Expression level tests based on unilateral injection revealed that βCaMKII exhibited a 3.8-fold and GluR1Ct a 3.7-fold overexpression of endogenous levels (fig. S11C). When this virus was bilaterally injected into the LHb of wild-type mice (infection rate 37 ± 2%), mice performed normally in both the forced swim and sucrose preference tests (figs. S11, D, E). Therefore coexpression of GulR1Ct prevented the depressive effects of βCaMKII overexpression.
Here we identified βCaMII as a key molecular determinant of habenular hyperactivity and behavioral depression, using a combination of molecular, behavioral and electrophysiological approaches. Our results point to a model in which stress-induced upregulation of βCaMII in the LHb causes more GluR1 insertion into synapses, resulting in increased synaptic efficacy. In addition, βCaMII may regulate other channels and membrane properties of LHb neurons to enhance spike output, which work in concert to cause LHb hyperactivity, leading to enhanced suppression of downstream monoamine centers (fig. S12A). Previous studies have implicated changes in CaMKIIs related to stress and antidepressant response (32–35). However it was unclear whether these changes are necessary or sufficient for depression etiology, and which brain region or isoform is crucial (36). βCaMKII is about eightfold more sensitive to Ca/calmodulin than αCaMKII (37), and possesses an actin-binding motif that is absent in αCamKII (38). It will be interesting to pinpoint in the future which feature of βCaMII renders this isoform-specific function in depression.
An important future question is how depressive stimuli and antidepressants lead to bidirectional changes in βCaMII levels in the LHb. Aversive emotional stimuli activate LHb neurons (4–6), whereas serotonin boosted by antidepressants suppresses excitatory inputs onto the LHb (7). Thus it is tempting to speculate that βCaMII levels are regulated by LHb neural activity. During the onset of depression, prolonged activation of LHb neurons may cause the upregulation of βCaMII to reach a threshold level, which can lead to further LHb neural activation. Conversely, antidepressant-caused suppression of the LHb may downregulate βCaMII levels, which can further lower LHb activity. These self-reinforcing feedback processes may eventually drive long-term adaptive changes in emotional states. Whether and how neuronal activity regulates βCaMII expression awaits further investigation.
In the context of our finding that the medial part of the LHb (LHb-M) is critical for the rescue of learned helplessness behavior (Fig. 4G), it is relevant to note that stress-induced c-fos activation is relatively confined to the LHb-M (4), and that there were more neurons with higher mEPSC frequency in LHb-M than those in LHb-L (fig. S10A). Further, LHb-M and LHb-L have different circuit wiring, which could contribute to their differential involvement in aversive behaviors and depressive symptoms (fig. S12B). Molecular manipulations in the nucleus accumbens (NAc) specifically mediate anhedonia but not behavioral despair (39). We found manipulation of βCaMII levels in the LHb affected both of these symptoms, suggesting that LHb may function upstream of NAc in the depression-related circuitry to control multiple aspects of depressive symptoms. Hence, the molecular targets identified in the LHb in this study should provide new insights for therapies that treat both of these core symptoms of depression.
Supplementary Material
Acknowledgements
We wish to thank Daniela Schulz for communication on cLH rats, Dafeng Xu and Jianbo Xiu for advice on AAV virus packaging, Lisa Monteggia for gift of the AAV-GFP-Cre plasmid and Bo Li for communicating unpublished results. We thank Xingyu Pan, Changpeng Wang, Pengcheng Shen, Dali Tong, Yong Zhang and Xiaodi Zhang for technical help. The work was supported by Chinese 973 Program (2011CBA00400), the “Strategic Priority Research Program (B) of the Chinese Academy of Sciences (XDB02030004), the Hundreds of Talents Program, the Outstanding Youth Grant and the Shanghai Pujiang Talent Program (to H. H.), the NIH P41 GM103533 and R01 MH067880 (to J. R. Y.).
References
- 1.Charney DS, Nestler EJ. Neurobiology of Mental Illness. Oxford University Press, USA: 2005. [Google Scholar]
- 2.Vaidya VA, Duman RS. Depresssion--emerging insights from neurobiology. Br Med Bull. 2001;57:61–79. doi: 10.1093/bmb/57.1.61. [DOI] [PubMed] [Google Scholar]
- 3.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 4.Wirtshafter D, Asin KE, Pitzer MR. Dopamine agonists and stress produce different patterns of Fos-like immunoreactivity in the lateral habenula. Brain research. 1994;633:21–26. doi: 10.1016/0006-8993(94)91517-2. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 6.Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008;28:11825–11829. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012;74:475–481. doi: 10.1016/j.neuron.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, Henn FA, Meyer-Lindenberg A. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biological psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Caldecott-Hazard S, Mazziotta J, Phelps M. paper presented at the J Neurosci. 1988 Jun; doi: 10.1523/JNEUROSCI.08-06-01951.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999;10:163–172. doi: 10.1006/nimg.1999.0455. [DOI] [PubMed] [Google Scholar]
- 13.Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963:274–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- 14.Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henn FA, Vollmayr B. Stress models of depression: forming genetically vulnerable strains. Neurosci Biobehav Rev. 2005;29:799–804. doi: 10.1016/j.neubiorev.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Maier SF. Learned helplessness and animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:435–446. [PubMed] [Google Scholar]
- 17.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 18.Liao L, Park SK, Xu T, Vanderklish P, Yates JR., 3rd Quantitative proteomic analysis of primary neurons reveals diverse changes in synaptic protein content in fmr1 knockout mice. Proc Natl Acad Sci U S A. 2008;105:15281–15286. doi: 10.1073/pnas.0804678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 21.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biological psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- 22.Sheline YI. Hippocampal atrophy in major depression: a result of depression-induced neurotoxicity? Molecular psychiatry. 1996;1:298–299. [PubMed] [Google Scholar]
- 23.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 24.Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H. Molecular characterization of the subnuclei in rat habenula. J Comp Neurol. 2012;520:4051–4066. doi: 10.1002/cne.23167. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci U S A. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wayman GA, Tokumitsu H, Davare MA, Soderling TR. Analysis of CaM-kinase signaling in cells. Cell Calcium. 2011;50:1–8. doi: 10.1016/j.ceca.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiagarajan TC, Piedras-Renteria ES, Tsien RW. alpha- and betaCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 28.Wheeler DG, Barrett CF, Groth RD, Safa P, Tsien RW. CaMKII locally encodes L-type channel activity to signal to nuclear CREB in excitation-transcription coupling. J Cell Biol. 2008;183:849–863. doi: 10.1083/jcb.200805048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groth RD, Lindskog M, Thiagarajan TC, Li L, Tsien RW. Beta Ca2+/CaM-dependent kinase type II triggers upregulation of GluA1 to coordinate adaptation to synaptic inactivity in hippocampal neurons. Proc Natl Acad Sci U S A. 2011;108:828–833. doi: 10.1073/pnas.1018022108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 31.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 32.Popoli M, Gennarelli M, Racagni G. Modulation of synaptic plasticity by stress and antidepressants. Bipolar Disord. 2002;4:166–182. doi: 10.1034/j.1399-5618.2002.01159.x. [DOI] [PubMed] [Google Scholar]
- 33.Xing G, Russell S, Hough C, O'Grady J, Zhang L, Yang S, Zhang LX, Post R. Decreased prefrontal CaMKII alpha mRNA in bipolar illness. Neuroreport. 2002;13:501–505. doi: 10.1097/00001756-200203250-00029. [DOI] [PubMed] [Google Scholar]
- 34.Suenaga T, Morinobu S, Kawano K, Sawada T, Yamawaki S. Influence of immobilization stress on the levels of CaMKII and phospho-CaMKII in the rat hippocampus. Int J Neuropsychopharmacol. 2004;7:299–309. doi: 10.1017/S1461145704004304. [DOI] [PubMed] [Google Scholar]
- 35.Novak G, Seeman P, Tallerico T. Increased expression of calcium/calmodulin-dependent protein kinase IIbeta in frontal cortex in schizophrenia and depression. Synapse. 2006;59:61–68. doi: 10.1002/syn.20211. [DOI] [PubMed] [Google Scholar]
- 36.Du J, Szabo ST, Gray NA, Manji HK. Focus on CaMKII: a molecular switch in the pathophysiology and treatment of mood and anxiety disorders. Int J Neuropsychopharmacol. 2004;7:243–248. doi: 10.1017/S1461145704004432. [DOI] [PubMed] [Google Scholar]
- 37.Brocke L, Chiang LW, Wagner PD, Schulman H. Functional implications of the subunit composition of neuronal CaM kinase II. The Journal of biological chemistry. 1999;274:22713–22722. doi: 10.1074/jbc.274.32.22713. [DOI] [PubMed] [Google Scholar]
- 38.Shen K, Teruel MN, Subramanian K, Meyer T. CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron. 1998;21:593–606. doi: 10.1016/s0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- 39.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.