Abstract
Williams syndrome, is caused by the deletion of 26-28 genes, including elastin, on human chromosome 7. Elastin insufficiency leads to the cardiovascular hallmarks of this condition, namely focal stenosis and hypertension. Extrapolation from the Eln+/− mouse suggests that affected persons may also have stiff vasculature, a risk factor for stroke, myocardial infarction and cardiac death. NCF1, one of the variably deleted Williams genes, is a component of the NAD(P)H oxidase complex and is involved in the generation of oxidative stress, making it an interesting candidate modifier for vascular stiffness. Using a case-control design, vascular stiffness was evaluated by pulse wave velocity in 77 Williams cases and matched controls. Cases had stiffer conducting vessels than controls (p<0.001), with increased stiffness observed in even the youngest Williams children. Pulse wave velocity increased with age at comparable rates in cases and controls and, although the degree of vascular stiffness varied, it was seen in both hypertensive and normotensive Williams participants. Use of anti-hypertension medication and extension of the Williams deletion to include NCF1 were associated with protection from vascular stiffness. These findings demonstrate that vascular stiffness is a primary vascular phenotype in Williams syndrome and that treatment with anti-hypertensives and/or agents inhibiting oxidative stress may be important in managing patients with this condition, potentially even those who are not overtly hypertensive.
Keywords: Elastin, NADPH Oxidase, Williams Syndrome, Vascular stiffness, Pulse wave velocity
Introduction
Vascular stiffness is an independent risk factor for multiple negative cardiovascular outcomes in normally aging adults, including stroke, myocardial infarction and sudden death1. Whether vascular stiffness occurs in developmental cardiovascular disorders, and portends similar adverse cardiovascular outcomes, is not well documented. Previous investigations in mouse models have consistently linked haploinsufficiency for elastin, an extracellular matrix protein that provides recoil to elastic tissues, to increased vascular stiffness and hypertension (reviewed in 2). Comparable data on the effect of elastin deficiency on vascular stiffness in humans are lacking and, thus, warrant study in naturally occurring elastin deficiency disorders.
Loss of function defects in the human elastin gene (ELN) cause focal arterial stenosis, generalized vascular narrowing, and hypertension in a rare condition called nonsyndromic supravalvular aortic stenosis 3-5 (SVAS, MIM# 185500, prevalence 1:20,000). Deletion of an entire ELN allele, as part of the contiguous gene deletion disorder, Williams syndrome (WS, MIM#194050, prevalence 1:8000-10,000), leads to the same vascular phenotype6.
To test the hypothesis that elastin insufficiency is associated with vascular stiffness in humans, we initiated the multi-institutional Williams Syndrome-Skin And Vessel Elasticity (WS-SAVE) study. Using applanation tonometry, we collected pulse wave velocity (PWV) measurements in the single largest WS cohort studied to date, consisting of 77 affected individuals, aged 7-62 years. Vascular stiffness normally increases with age and with comorbid conditions such as hypertension and diabetes7. Consequently, this robust sample size and broad age range allowed us to evaluate the presence of vascular stiffness in WS throughout the lifespan and also to identify covariates that modify arterial stiffness in this population. In particular, we investigate both pharmacologic interventions and genetic alterations that influence arterial stiffness and identify treatments associated with lower PWV. Differences in the WS deletion, affecting copy number for NCF1, an NAD(P)H oxidase subunit, have been shown to affect hypertension risk in WS8 but investigation of the gene's impact on arterial stiffness has not been investigated. As individuals with WS are at markedly increased risk of sudden death9, 10, possibly due, in part, to increased vascular stiffness, the modifiers examined here may be critical to improving their health and longevity. In addition, these findings could inform the management of other conditions that lead to pediatric vascular stiffness such as diabetes and chronic renal failure11, 12
Subjects and Methods
Please see supplemental methods for additional details.
Study Populations
Recruitment of cases and controls was performed as part of Institutional Review Board (IRB)-approved studies (WS cases through Washington University School of Medicine (WUSM) and the Massachusetts General Hospital, adult controls through WUSM and pediatric controls through Semmelweis University.) Each participant (adult controls) or their parent/caregiver (pediatric controls and all WS cases) consented to participation in the study. Procedures followed were in accordance with institutional guidelines. Historical medical and surgical data were obtained through use of a questionnaire. Medical records including echocardiogram were reviewed to validate questionnaire data when possible. WS participants underwent cardiovascular physical examination and WS phenotype was verified on physical exam by experienced clinical geneticists. Molecular confirmation of WS was achieved either by review of clinical testing results (FISH or microarray) or by research quantification of ELN copy number (Figure S1).
Pulse Wave Velocity
Height and weight were formally measured and individuals were then placed in a supine position and allowed to rest quietly. Blood pressure was manually assessed in both arms. PWV was determined from contour analysis of arterial waveforms recorded by applanation tonometry using a highly reproducible technique 7, 13, 14.
NCF1 gene and pseudogene copy number determination
The Williams syndrome critical region on chromosome 7 is flanked by three regions of low copy number repeats8, 15, each containing the NCF1 gene or one of its two NCF1 pseudogenes (NCF1B and NCF1C. See Figure S2 for diagram). The absence of two base pairs (ΔGT) at the beginning of exon 2 in the pseudogenes distinguishes them from the NCF1 gene16. To calculate relative copy number for the NCF1 gene to its pseudogenes, both the genes and pseudogenes were amplified together using PCR primers surrounding the ΔGT region. Following PCR amplification, the product was gel purified and Sanger sequenced. At the ΔGT, the gene and pseudogene sequences diverge and the relative peak heights of the next 27 bases are determined from the tracings as previously described17. NCF1 gene and pseudogene copy number were assigned using the ratio table in Table S1 and NCF1 gene number was used in subsequent statistical analysis.
Statistical Analyses
PWV in WS vs matched controls: As PWV normally increases with age 7, 14, 18, our initial analysis evaluated the pediatric (n=36) and adult (n=41) cohorts separately. Cases and controls were matched and PWV readings were compared using results from paired t-tests. To determine if PWV increases with age at the same rate in WS participants vs. controls, we also performed regression in the full matched data set (n=77) with age as the covariate.
Covariate analysis (PWV): To identify additional features associated with higher PWV in the WS population, we performed regression on PWV data from WS participants using diabetes, hypertension, anti-hypertension medication, or NCF1 copy number status as the covariate. For the NCF1 copy number analysis, because of the observed protective effect of anti-hypertensives on PWV in WS (see Figure 2B) and others19, individuals known to use these medications were excluded from this analysis.
Covariate analysis (hypertension): The Fisher's exact test was used to compare the prevalence of hypertension in WS individuals with NCF1 gene copy number (CN) of 1 vs ≥ 2. Hypertension was defined as any participant who had received a diagnosis of hypertension (treated or untreated).
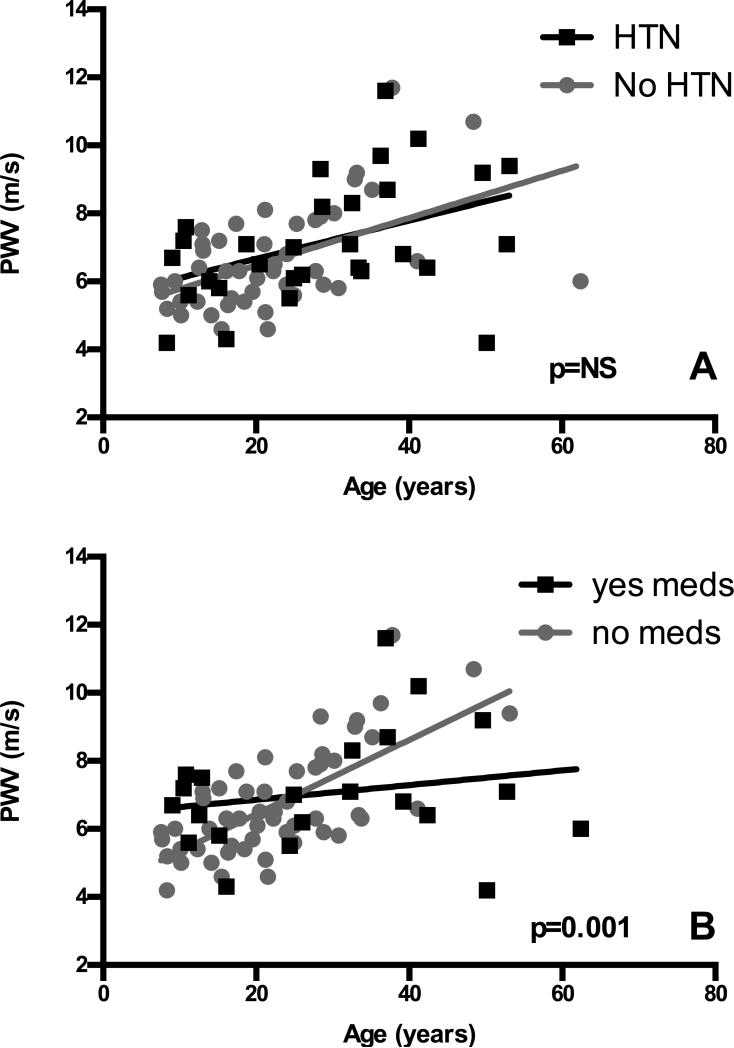
Figure 2. Use of anti-hypertensives but not hypertension influences the severity of vascular stiffness in WS.
Regression analyses were performed by plotting WS participant data points by age and PWV and dividing the cohort by history of hypertension (Panel A, N=76, 30 with hypertension (HTN) and 46 without) or use of anti-hypertensive medications (Panel B, N=76, 22 using medication (meds) and 54 not using medication). Data from participants without the described feature are gray and those with the phenotype are black. Regression lines are shown. The slope of the regression line was significantly different only in Panel B, where anti-hypertensive use appears to be protective from increases in PWV (p=0.001).
For each analysis, only participants with the necessary data components were analyzed and N for each study is reported. All statistical analyses were performed using Prism statistical software.
Results
Study Populations
WS Cases
103 individuals with WS (aged 7-62 years) were consented for the WS-SAVE study. Quality PWV measurements were obtained in 77 of the 103 (74.8%)WS participants. Rates of vascular disease in those with successful vs. and unsuccessful PWV measurements were similar for most phenotypes assessed (See Table S2 for p values.), with the unsuccessful cohort having a higher percentage of females, individuals with elevated BMI (>35) and SVAS repair. No participants were fully excluded from the study but individuals were excluded from portions of the analysis in which relevant data were not available. For example, history of hypertension was assessed in 101/103 participants. Likewise, DNA was of sufficient quality to calculate NCF1 copy number in 101/103 individuals.
Controls
Adult controls (N= 41, aged 21.5- 62.4 years). Adult controls were ambulatory subjects selected from among participants in IRB-approved cardiovascular studies at WUSM. Subjects were excluded for any of the following: left ventricular systolic dysfunction (ejection fraction < 55%), reported or suspected coronary artery disease, pulmonary hypertension, stage C or worse chronic kidney disease, reported infection by the human immunodeficiency virus, and reported sickle cell disease. Adults were matched to WS participants, in aggregate, for body mass index (BMI), hypertension diagnosis, use of anti-hypertensives, and diabetes, in addition to age and gender (paired t-tests revealed non-significant results for these variables, Table S3).
Pediatric controls (N=36, aged 7.6-21.2 years). Pediatric controls were enrolled as part of an international study establishing reference values of PWV in children and adolescents18. Children with known history of hypertension or diabetes were excluded. Children were matched by age (mean difference ± SD (WS-C): 0.04 ± 0.19 years), gender and height (mean difference (WS-C) -6 ± 7 cm) to WS youth.
Clinical characteristics of the WS-SAVE cohort
To determine whether the increased arterial stiffness noted in elastin insufficient mice20-22 extrapolates to humans with elastin insufficiency, we attempted PWV in a cohort of 103 individuals with WS. Clinical features for the 77 subjects successfully completing the PWV portion of the study are included in Table S4. 69% of those with successful PWV measurements have a history of vascular stenosis (any location). 54% had SVAS but only 10% had SVAS requiring surgical intervention. 40% of the cohort reported a history of hypertension but only 25% had hypertension that was treated with anti-hypertension medication. 10% reported a diagnosis of diabetes. Stroke (any type) was reported in 6% (3/48) of all individuals asked about this phenotype; among the subset with a successful PWV measurement,2 of 36 (6%) reported a history of stroke.
Children and adults with WS show stiffer vessels than matched controls
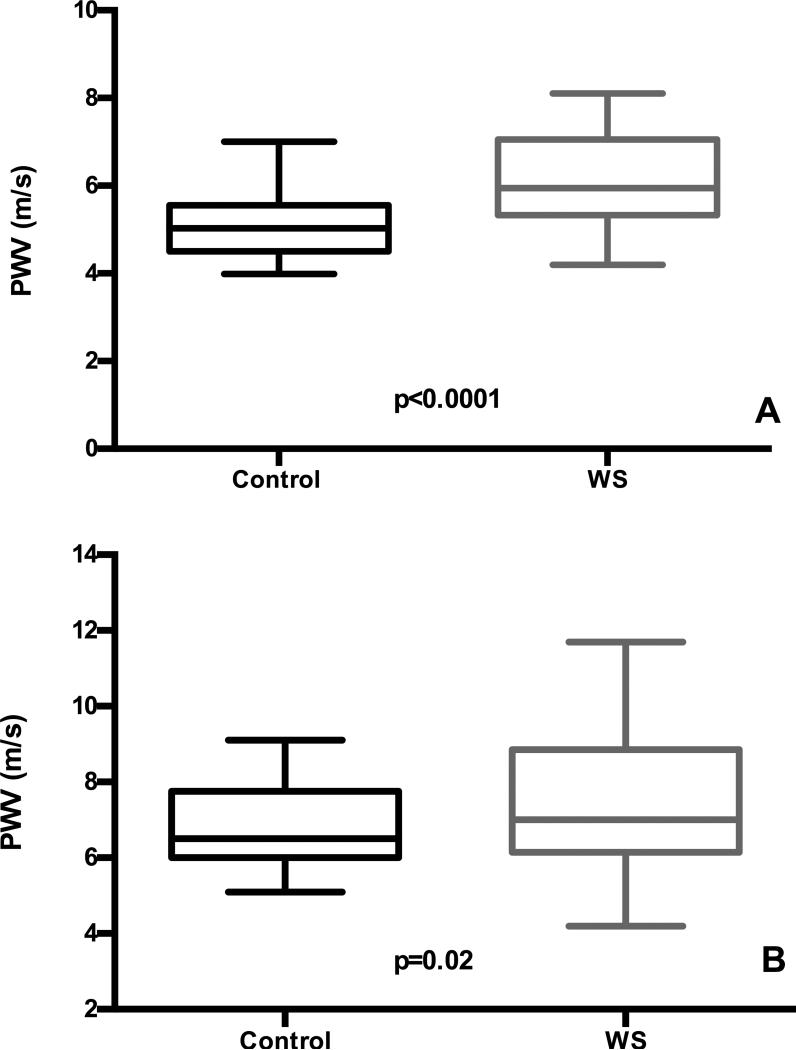
PWV from the 77 WS individuals were compared to pediatric and adult controls. When the pediatric subset were compared in paired t-tests, those with WS were found, on average, to have significantly higher PWV (6.1 ±1.0 m/s (mean ± SD, WS) 5.1 ± 0.8m/s, (mean ± SD, control) (p<0.0001), Figure 1A. Increased PWV was obvious in even the youngest WS participants (See Figure S3A for raw WS data plotted against healthy population control means (derived from 14, 18)). In adults, paired t-tests again showed higher PWV in WS adults (7.5 ± 1.8 m/s, WS) vs. controls (6.9 ± 1.1 m/s, controls) (p=0.02), Figure 1B. PWV normally increases with age 7, 18. To evaluate for possible differential effects of aging on PWV in WS, regression was performed using the full WS data set and matched controls (n=77 each). This analysis showed higher PWVs in WS participants across the whole age distribution (p < 0.0001 for elevation) with no statistical difference in the rate of PWV increase with age compared to controls (p=NS for slope, Figure S3B).
Figure 1. Pediatric cases and adults with WS have higher PWV than matched controls.
PWV was measured in WS pediatric cases (n=36, aged 7.6-21.2 years) and adults (n=41, aged 21.5-62.4 years). When compared in paired t-tests, youth with WS were found, on average, to have significantly higher PWV (Panel A). The WS adults were compared to those with a similar compilation of hypertension, diabetes, anti-hypertension medication use and BMI in addition to matched age and gender, and also had higher PWV (Panel B). Controls are black and WS participants are gray. The box shows the standard deviation with the mean PWV marked inside the box. The whiskers on the boxes depict the extreme reach of the PWV's measured.
PWV: WS clinical phenotypes associated with vascular stiffness
We sought to determine whether hypertension and diabetes, previously linked to increased vascular stiffness in non-Williams cohorts7, 23, 24, were associated with higher PWV in WS. Regression analyses showed no significant relationship between PWV and hypertension (treated and untreated, Figure 2A) or diabetes (Figure S4). We did, however, see relative protection from increased PWV in WS individuals taking anti-hypertensives (p=0.001, Figure 2B). Of note, 58% (21/36) of those with PWV more than 1m/s higher than the population mean for age and gender14, 18 had no history of hypertension, while 36% (4/11) of those with reported untreated hypertension did not show elevated PWV.
Decreased NCF1 gene copy number protects from vascular stiffness in WS
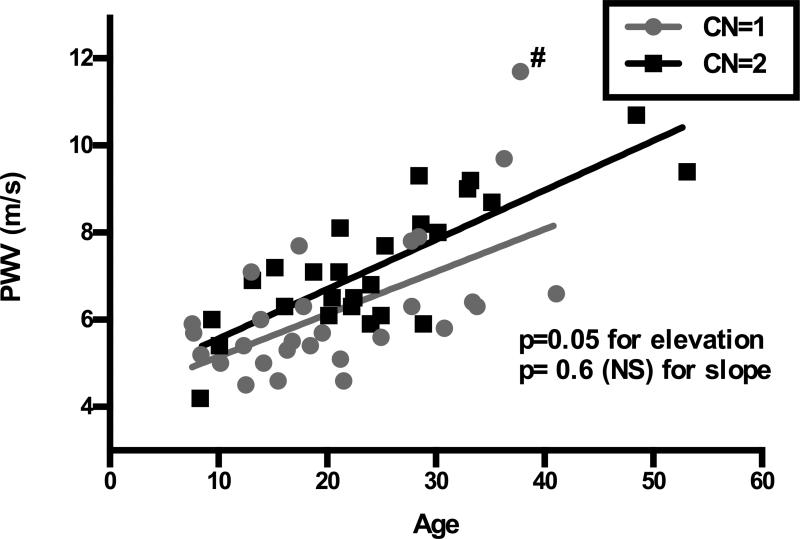
We investigated whether NCF1 copy number was also associated with changes in vascular stiffness, as predicted by quantitative trait analysis in the Eln+/− mouse25. Regression analysis showed higher PWVs in WS participants who had ≥2 copies of NCF1 rather than one (p=0.05 for elevation, Figure 3). There was no statistical difference in the rate of PWV increase with age (p=NS for slope) in individuals with CN=1 or 2 in this analysis. Interestingly, removal of the single participant with the highest PWV (denoted #) increases the difference in PWV observed between CN=1 and CN=2 individuals (p= 0.005 for elevation) with a trend toward better protection from increasing PWV with older age (p for slope improves from 0.6 to 0.1 (Figure S5).
Figure 3. NCF1 copy number modifies vascular disease severity in WS.
Regression analysis is used to assess the effect of NCF1 copy number on PWV (n=55). Those with NCF1 CN≥2 (black) have higher PWV than those with CN=1 (gray) (p=0.05 for elevation), but there is no difference in the increase in stiffness over time in this cohort (p=NS for slope). The (#) denotes the one CN=1 outlier.
NCF1 gene copy number and hypertension in WS
To determine whether or not deletion of NCF1 affected hypertension in our cohort, we compared the frequency of hypertension between WS individuals with 1 vs. ≥ 2 NCF1 genes. In our total cohort of 99 WS individuals in whom both NCF1 copy number and hypertension status were known, a single copy of NCF1 was associated with protection from hypertension (p= 0.03, Figure S6). This association persisted even when restricting the analysis to those <18 year of age (p=0.04, data not shown) where the WS related component of hypertension may be more dominant. These results confirm the previously reported protective association for reduced NCF1 copy number and risk of hypertension in WS8.
Discussion
In a developing blood vessel, collagen is responsible for tensile strength while elastin provides recoil capability. When the elastin gene is mutated or deleted, the resulting elastin insufficiency leads to multiple cardiovascular abnormalities. Most consistently described in these populations are focal arterial stenoses and hypertension 5, 6, 26, 27. However, the severity of the vascular features in WS is variable. The frequency of WS-associated vascular symptoms in WS-SAVE is similar to previous reports 6, 28, with history of SVAS in 55% and hypertension in 40%. Importantly, only 25% of the cohort reported using anti-hypertensive medication for blood pressure control. Decision to treat was made by each individual's primary medical team and may reflect overall population under-treatment or possible hesitancy to treat hypertension in syndromic individuals in whom high blood pressure measurements are often felt to represent anxiety in the medical setting. Interestingly, studies into this question have shown a paucity of true “white coat hypertension” in this population28.
Previous investigations of vascular stiffness in WS yielded contradictory results, ranging from increased arterial stiffness29, 30 to normal or even paradoxically reduced values31, 32 in small studies consisting of 3 to 29 participants. Consistent with these reports and with other cardiovascular features in WS, our larger analysis found considerable inter-person PWV variability. However, even with this variability stiffer vessels than matched controls. As in the Eln+/− mouse, this stiffness is apparent from the earliest ages studied20, 33, and progresses with increasing age at the same rate as controls, suggesting that elastin insufficiency causes early onset, and possibly congenital, arterial stiffness. Eln+/− mice generally become hypertensive in the neonatal period, a process suggested to be a physiologic response to the altered vascular mechanics brought on by elastin insufficiency21. In many of our cases, vascular stiffness was present without hypertension and conversely, in some, hypertension was present without stiffness, suggesting the two features may be related but independent effects of elastin insufficiency. For some of the studied individuals, increased pulse wave velocity is the only known cardiovascular manifestation of the disorder. The development of hypertension in WS is likely multifactorial with influences due to vascular stiffness, complex effects of genetic background and other features of WS like renal artery stenosis and small for gestational age birth28.
Our data show that vessels of pediatric WS participants are more uniformly stiff than matched controls. Greater variability is seen in older WS participants, with evidence of protection in those on anti-hypertension medications. The mean PWV observed in pediatric WS participants is 6.1 m/s, approaching that seen in pre-dialysis youth with end stage renal disease (6.6 m/s, 11) and youth with type 2 diabetes (6.4 m/s, 12) (both with similar age demographics). Interestingly, in the ESRD study, no improvement resulted from hemodialysis, suggesting a final common pathway to these conditions ending in destruction of elastic matrices and lasting alteration of the biomechanical properties of the vessel wall. The fact that we did not see a primary “hypertension by PWV” effect in regression analysis may result from an admixture of untreated hypertensive individuals (who generally have higher PWVs) with treated hypertensive WS participants (in whom our data show lower PWVs). This mixing may ultimately act to “normalize” the PWV for the hypertensive group causing it to appear similar to the non-hypertensive subset. Further subgroup analysis, however, was underpowered. Anti-hypertension medication use showed greater protection in older WS individuals, possibly suggesting cumulative effects of longer treatment duration, however longitudinal evaluation is needed to confirm this.
Since vascular stiffness is strongly associated with negative cardiovascular consequences such as myocardial infarction and stroke1, further investigation into the predictive power of PWV for clinical outcome in individuals with WS is warranted. In our study, 48 participants were surveyed about their stroke history and 3/48 (6%), aged 22, 32, and 32, reported a history of stroke. Age adjusted stroke prevalence for control individuals 18-45 years old is 0.6-0.7%34, compared to 12% (3/25) when we consider only the subset of WS participants in this demographic. In 2/3 WS participants with stroke, we obtained successful PWV measurements and both were elevated (+0.6 m/s and +1.4 m/s relative to age/gender matched controls) even though both participants were receiving anti-hypertension medications at the time of PWV measurement. Currently, the best estimate of risk of sudden death in WS is 25-100 fold increased relative to the general population10. It is likely that this risk does not apply equally to all individuals with WS and the use of PWV may enable individualized risk assessment. Full details about the nature of the strokes in these participants are not available and further investigation into this area is warranted.
Because of its status as a variably deleted gene in WS, NCF1 copy number was investigated and found to be a significant modifier of arterial stiffness in WS. Data from the current cohort also confirms the reduced risk of hypertension associated with NCF1 CN=1 originally described by DelCampo et al8. While our study found generally lower PWV's in WS participants with only one copy of NCF1 relative to those with ≥2, initial analysis revealed no difference in the rate of age-associated increase in PWV between the two groups. However, when a single outlier is removed, a trend toward progressive protection with advancing age is identified in those with CN=1. As NCF1 is a member of the NADPH oxidase (NOX) family and is involved in the generation of reactive oxygen species in tissues35, its association with severity of vascular stiffness suggests a role for chronic oxidative stress in the pathology of vascular stiffness in WS. Additional studies with focused recruitment of older WS individuals, while difficult, may help clarify this intriguing finding.
Limitations to our study include the possibility that, due to the topic of the study, parents of individuals with significant vascular disease were more interested in participating. However, our study includes many subjects with no previously known vascular features of WS (who, in fact, have high PWV). Second, challenges inherent to WS limited the number of successful PWV studies we were able to obtain. Individuals with WS have an average IQ of 55 and are often anxious, making it difficult to obtain resting measurements in younger children with WS. High BMI, in combination with these features, additionally complicated PWV acquisition in some adults. Reassuringly, phenotypes of participants on whom we failed to obtain PWV trended toward more severe vascular disease with higher rates of hypertension and stenosis seen in this group. These findings suggest that it is unlikely that the data loss excluded a milder cohort that might skew the PWV results. Finally, our studies are merely a single snapshot in time. To investigate the interaction between vascular stiffness and hypertension in elastin insufficiency additional larger prospective studies are needed to document the natural evolution of these phenotypes.
Perspectives
This study successfully evaluated vascular stiffness in the largest cohort of WS individuals reported to date and demonstrates that individuals with WS, regardless of their blood pressure, are at risk for increased aortic stiffness, a well-established surrogate for adverse cardiovascular outcomes in many disease processes. This increase in vascular stiffness is caused by elastin insufficiency, as evidenced by Eln+/− mouse studies20-22 and importantly, is modified by anti-hypertensive use and NCF1 copy number. PWV differences are detectable at the earliest ages studied and continue into adulthood, making Williams syndrome and elastin insufficiency useful models in which to study the long-term effects of chronic and early onset vascular stiffness. Over all, this study suggests that: 1) monitoring of vascular stiffness is warranted in WS; 2) anti-hypertensive treatment might protect against vascular stiffness and consequent adverse cardiovascular events in individuals with WS, even without overt hypertension. Consequently, at a minimum, anti-hypertensives should be considered in all WS individuals with hypertension; and finally, 3) novel medications, aimed at the NOX pathway, should be investigated as potential modulators of vascular disease severity in this condition with utility potentially determined by NCF1 copy number. To generate formal treatment guidelines, larger prospective studies are needed to quantify the effects of anti-hypertensive drug choice and duration in treating arterial stiffness in WS. In addition, more work is needed to better understand the prognostic relevance of early onset vascular stiffness in those with WS, with special attention given to organ systems known to be adversely effected in aging adults with vascular stiffness, such as the kidneys, heart, and brain. Finally, medication by NCF1 copy number studies are needed to determine which patients may most benefit from pharmacological intervention.
Supplementary Material
Novelty and Significance.
What is new? The WS-SAVE study is the largest multi-institutional study of vascular stiffness in Williams syndrome to date, revealing increased pulse wave velocity in individuals with this rare condition.
What is relevant? Increased pulse wave velocity was identified in even the youngest Williams participants, suggesting that vascular stiffness is of very early, if not congenital, onset. Stiffness appears to be independent of hypertension in WS, making vascular stiffness a new primary WS phenotype. Protection against increasing stiffness is afforded by anti-hypertensive medication and also by deletion of NCF1, an NADPH oxidase component and gene variably deleted in WS.
Summary: Elastin insufficiency causes increased arterial stiffness, a phenotype associated with multiple negative cardiovascular outcomes complicating WS. Elevated pulse wave velocity is seen less often in those with WS deletions also removing the NCF1 gene and in those on anti-hypertension therapy. Our findings suggest the need for enhanced evaluation and anticipatory treatment of vascular disease in Willams syndrome.
Acknowledgements
The authors wish to thank the Williams Syndrome Association and the families who participated in the WS-SAVE study. We gratefully acknowledge the assistance of Dr. Chengsheng Zhang in clarifying the copy number status of genes within the Williams region for select patients; Dr. Mark Johnson for review of Williams echocardiograms; and Dr. Richard Feinn for recommendations regarding statistical tests. We thank Dr. Carmel McEniery for sharing updated normative data from the ACCT14 and Dr. Mark Levin for his helpful review of the manuscript.
Grant support:
Funding was provided to Dr. Kozel by the Children's Discovery Institute of Washington University and St. Louis Children's Hospital. In addition, Dr. Kozel received funding for the study through her appointment as a scholar of the Child Health Research Center in Developmental Biology (NIH K12-HD01487) and the Genetic Basis of Inflammatory Airway Disease (NIH K12-HL089968). Original pediatric control studies were supported by the Hungarian National Research Fund, OTKA 100909.
Footnotes
Conflicts of Interest: None
References
- 1.Franklin SS. Beyond blood pressure: Arterial stiffness as a new biomarker of cardiovascular disease. J Am Soc Hypertens. 2008;2:140–151. doi: 10.1016/j.jash.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Kozel BA, Mecham RP, Rosenbloom J. Elastin. In: Mecham RP, editor. The extracellular matrix, an overview. Springer; 2011. [Google Scholar]
- 3.Curran ME, Atkinson DL, Ewart AK, Morris CA, Leppert MF, Keating MT. The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis. Cell. 1993;73:159–168. doi: 10.1016/0092-8674(93)90168-p. [DOI] [PubMed] [Google Scholar]
- 4.Metcalfe K, Rucka AK, Smoot L, Hofstadler G, Tuzler G, McKeown P, Siu V, Rauch A, Dean J, Dennis N, Ellis I, Reardon W, Cytrynbaum C, Osborne L, Yates JR, Read AP, Donnai D, Tassabehji M. Elastin: Mutational spectrum in supravalvular aortic stenosis. Eur J Hum Genet. 2000;8:955–963. doi: 10.1038/sj.ejhg.5200564. [DOI] [PubMed] [Google Scholar]
- 5.Urban Z, Michels VV, Thibodeau SN, Davis EC, Bonnefont JP, Munnich A, Eyskens B, Gewillig M, Devriendt K, Boyd CD. Isolated supravalvular aortic stenosis: Functional haploinsufficiency of the elastin gene as a result of nonsense-mediated decay. Hum Genet. 2000;106:577–588. doi: 10.1007/s004390000285. [DOI] [PubMed] [Google Scholar]
- 6.Pober BR, Johnson M, Urban Z. Mechanisms and treatment of cardiovascular disease in williams-beuren syndrome. J Clin Invest. 2008;118:1606–1615. doi: 10.1172/JCI35309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutouyrie P, Vermeersch SJ. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. European heart journal. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Campo M, Antonell A, Magano LF, Munoz FJ, Flores R, Bayes M, Perez Jurado LA. Hemizygosity at the ncf1 gene in patients with williams-beuren syndrome decreases their risk of hypertension. Am J Hum Genet. 2006;78:533–542. doi: 10.1086/501073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bird LM, Billman GF, Lacro RV, Spicer RL, Jariwala LK, Hoyme HE, Zamora-Salinas R, Morris C, Viskochil D, Frikke MJ, Jones MC. Sudden death in williams syndrome: Report of ten cases. J Pediatr. 1996;129:926–931. doi: 10.1016/s0022-3476(96)70042-2. [DOI] [PubMed] [Google Scholar]
- 10.Wessel A, Gravenhorst V, Buchhorn R, Gosch A, Partsch CJ, Pankau R. Risk of sudden death in the williams-beuren syndrome. Am J Med Genet A. 2004;127A:234–237. doi: 10.1002/ajmg.a.30012. [DOI] [PubMed] [Google Scholar]
- 11.Covic A, Mardare N, Gusbeth-Tatomir P, Brumaru O, Gavrilovici C, Munteanu M, Prisada O, Goldsmith DJ. Increased arterial stiffness in children on haemodialysis. Nephrol Dial Transplant. 2006;21:729–735. doi: 10.1093/ndt/gfi196. [DOI] [PubMed] [Google Scholar]
- 12.Wadwa RP, Urbina EM, Anderson AM, Hamman RF, Dolan LM, Rodriguez BL, Daniels SR, Dabelea D. Measures of arterial stiffness in youth with type 1 and type 2. diabetes: The search for diabetes in youth study. Diabetes Care. 2010;33:881–886. doi: 10.2337/dc09-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kis E, Cseprekal O, Kerti A, Salvi P, Benetos A, Tisler A, Szabo A, Tulassay T, Reusz GS. Measurement of pulse wave velocity in children and young adults: A comparative study using three different devices. Hypertens Res. 2011;34:1197–1202. doi: 10.1038/hr.2011.103. [DOI] [PubMed] [Google Scholar]
- 14.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: Differential effects on wave reflection and aortic pulse wave velocity: The anglo-cardiff collaborative trial (acct). J Am Coll Cardiol. 2005;46:1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 15.Pober BR. Williams-beuren syndrome. N Engl J Med. 2010;362:239–252. doi: 10.1056/NEJMra0903074. [DOI] [PubMed] [Google Scholar]
- 16.Roesler J, Curnutte JT, Rae J, Barrett D, Patino P, Chanock SJ, Goerlach A. Recombination events between the p47-phox gene and its highly homologous pseudogenes are the main cause of autosomal recessive chronic granulomatousdisease. Blood. 2000;95:2150–2156. [PubMed] [Google Scholar]
- 17.Heyworth PG, Noack D, Cross AR. Identification of a novel ncf-1 (p47-phox) pseudogene not containing the signature gt deletion: Significance for a47 degrees chronic granulomatous disease carrier detection. Blood. 2002;100:1845–1851. doi: 10.1182/blood-2002-03-0861. [DOI] [PubMed] [Google Scholar]
- 18.Reusz GS, Cseprekal O, Temmar M, Kis E, Cherif AB, Thaleb A, Fekete A, Szabo AJ, Benetos A, Salvi P. Reference values of pulse wave velocity in healthy children and teenagers. Hypertension. 2010;56:217–224. doi: 10.1161/HYPERTENSIONAHA.110.152686. [DOI] [PubMed] [Google Scholar]
- 19.Shahin Y, Khan JA, Chetter I. Angiotensin converting enzyme inhibitors effect on arterial stiffness and wave reflections: A meta-analysis and meta-regression of randomised controlled trials. Atherosclerosis. 2012;221:18–33. doi: 10.1016/j.atherosclerosis.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Wagenseil JE, Ciliberto CH, Knutsen RH, Levy MA, Kovacs A, Mecham RP. Reduced vessel elasticity alters cardiovascular structure and function in newborn mice. Circ Res. 2009;104:1217–1224. doi: 10.1161/CIRCRESAHA.108.192054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B, Mecham RP. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest. 2003;112:1419–1428. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pezet M, Jacob MP, Escoubet B, Gheduzzi D, Tillet E, Perret P, Huber P, Quaglino D, Vranckx R, Li DY, Starcher B, Boyle WA, Mecham RP, Faury G. Elastin haploinsufficiency induces alternative aging processes in the aorta. Rejuvenation Res. 2008;11:97–112. doi: 10.1089/rej.2007.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: A pathway to cardiovascular disease. Diabetologia. 2008;51:527–539. doi: 10.1007/s00125-007-0918-3. [DOI] [PubMed] [Google Scholar]
- 24.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA : the journal of the American Medical Association. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozel BA, Knutsen RH, Ye L, Ciliberto CH, Broekelmann TJ, Mecham RP. Genetic modifiers of cardiovascular phenotype caused by elastin haploinsufficiency act by extrinsic noncomplementation. J Biol Chem. 2011;286:44926–44936. doi: 10.1074/jbc.M111.274779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eronen M, Peippo M, Hiippala A, Raatikka M, Arvio M, Johansson R, Kahkonen M. Cardiovascular manifestations in 75 patients with williams syndrome. J Med Genet. 2002;39:554–558. doi: 10.1136/jmg.39.8.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park S, Seo EJ, Yoo HW, Kim Y. Novel mutations in the human elastin gene (eln)causing isolated supravalvular aortic stenosis. Int J Mol Med. 2006;18:329–332. [PubMed] [Google Scholar]
- 28.Broder K, Reinhardt E, Ahern J, Lifton R, Tamborlane W, Pober B. Elevated ambulatory blood pressure in 20 subjects with williams syndrome. Am J Med Genet. 1999;83:356–360. doi: 10.1002/(sici)1096-8628(19990423)83:5<356::aid-ajmg2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.Bassareo PP, Mercuro G. Increased arterial stiffness in children with Williams syndrome and normal blood pressure. Blood Press Monit. 2010;15:257–261. doi: 10.1097/MBP.0b013e32833e4f7d. [DOI] [PubMed] [Google Scholar]
- 30.Salaymeh KJ, Banerjee A. Evaluation of arterial stiffness in children with Williams syndrome: Does it play a role in evolving hypertension? Am Heart J. 2001;142:549–555. doi: 10.1067/mhj.2001.116763. [DOI] [PubMed] [Google Scholar]
- 31.Aggoun Y, Sidi D, Levy BI, Lyonnet S, Kachaner J, Bonnet D. Mechanical properties of the common carotid artery in williams syndrome. Heart. 2000;84:290–293. doi: 10.1136/heart.84.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacolley P, Boutouyrie P, Glukhova M, Daniel Lamaziere JM, Plouin PF, Bruneval P, Vuong P, Corvol P, Laurent S. Disruption of the elastin gene in adult Williams syndrome is accompanied by a paradoxical reduction in arterial stiffness. Clin Sci(Lond) 2002;103:21–29. doi: 10.1042/cs1030021. [DOI] [PubMed] [Google Scholar]
- 33.Le VP, Knutsen RH, Mecham RP, Wagenseil JE. Decreased aortic diameter and compliance precedes blood pressure increases in postnatal development of elastin-insufficientmice. Am J Physiol Heart Circ Physiol. 2011;301:H221–229. doi: 10.1152/ajpheart.00119.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang J, Shaw K, George M. Prevalence of stroke – united states, 2006–2010. Morbidity and Mortality Weekly Report (MMWR) 2012;61:379–382. [PubMed] [Google Scholar]
- 35.Lassegue B, Griendling KK. Nadph oxidases: Functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.