Abstract
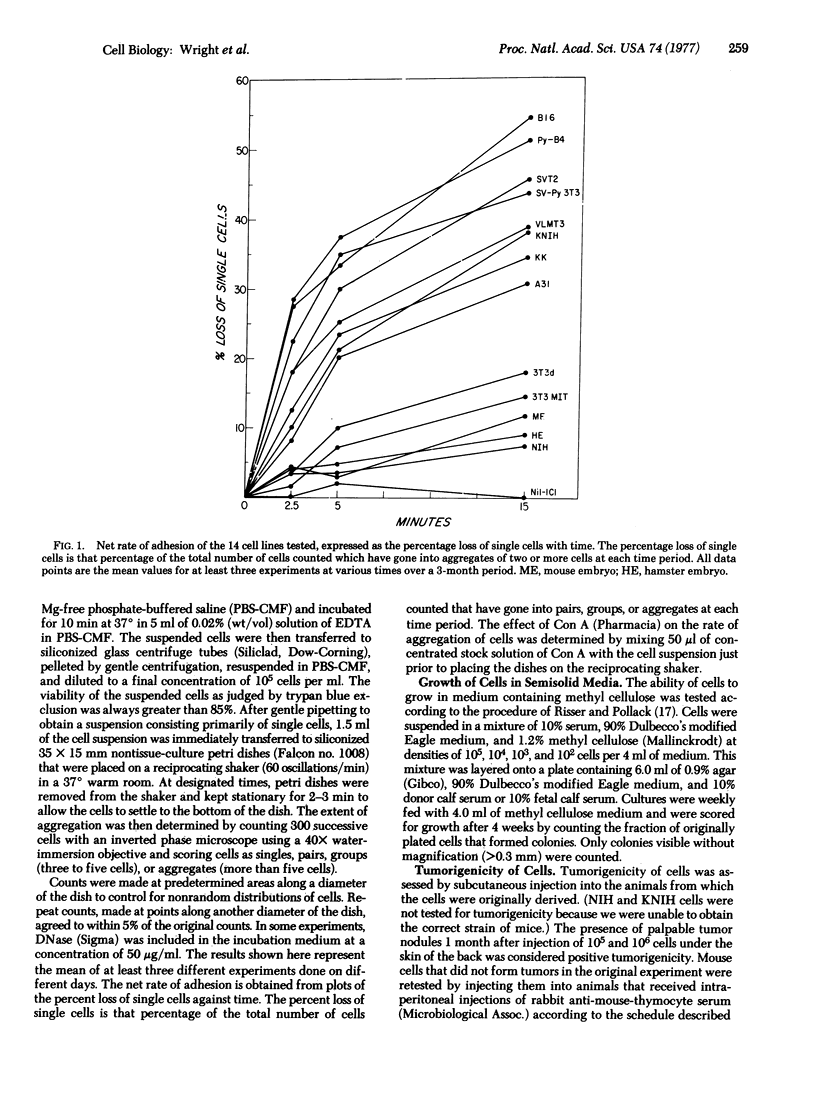
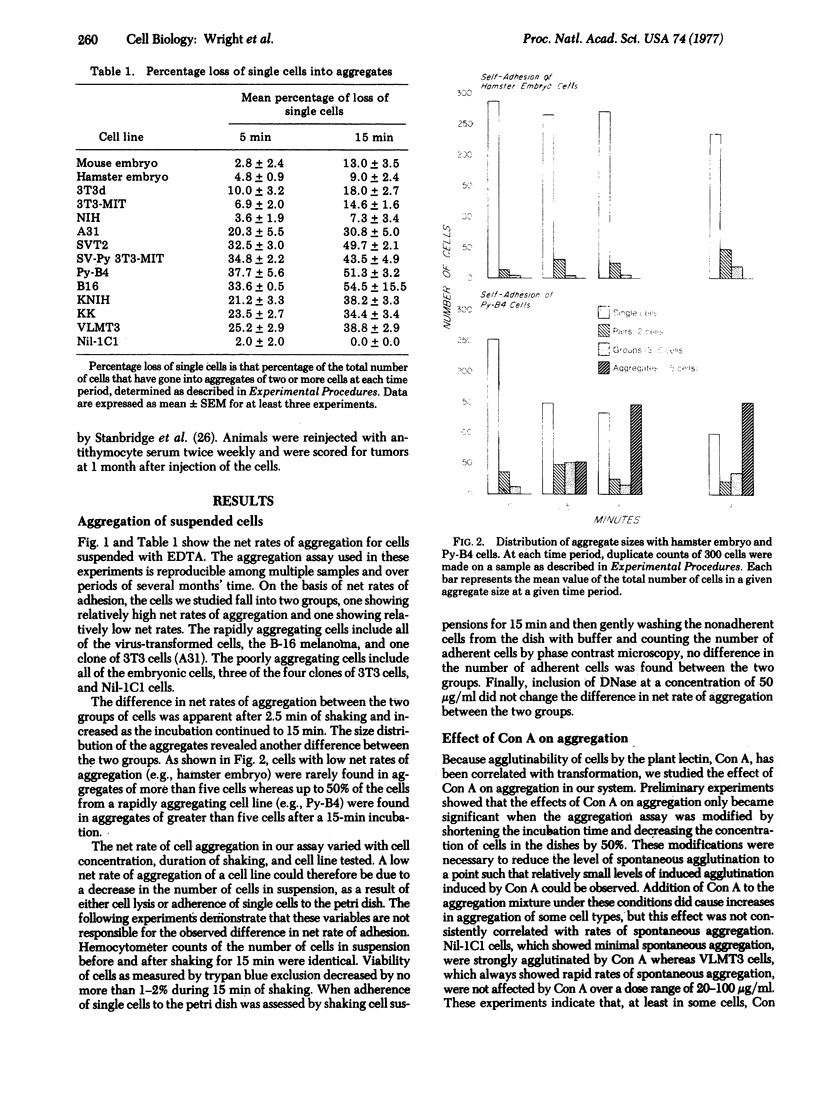
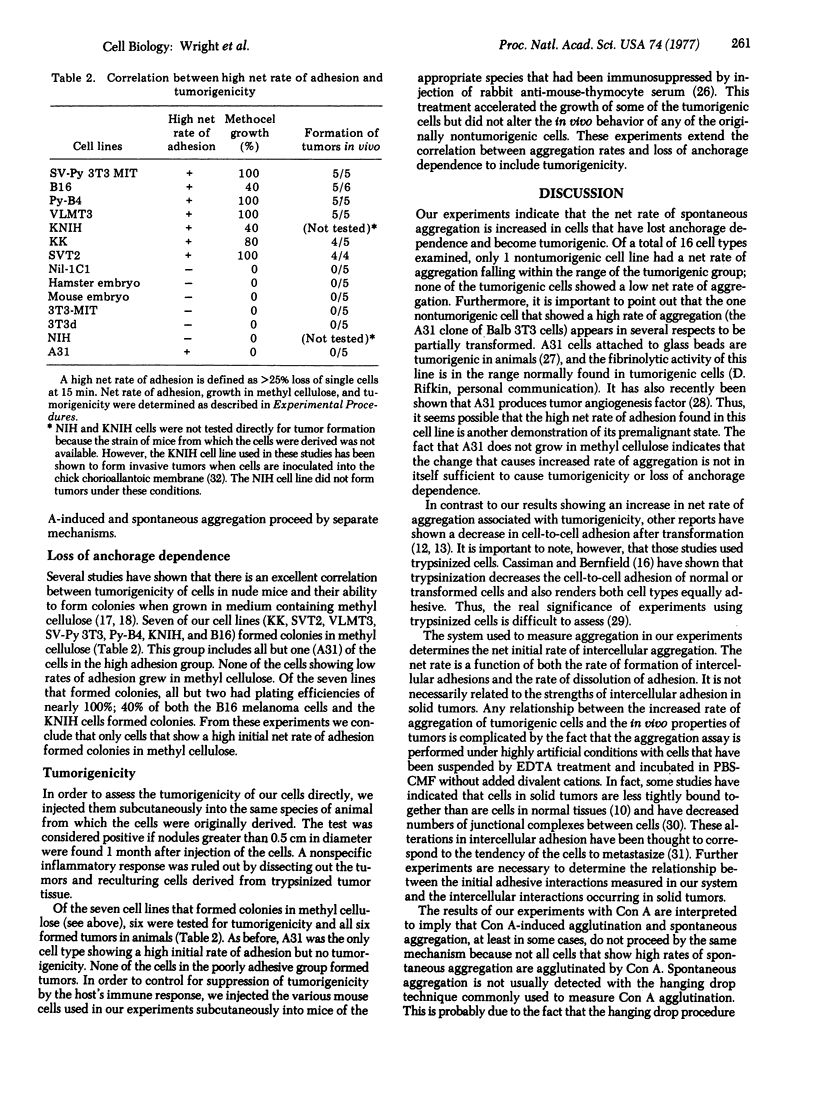
The net rate of spontaneous aggregation of cells suspended with EDTA was measured for various cell types including spontaneous transformants and cells transformed with DNA and RNA viruses. The anchorage dependence as determined by growth in methyl cellulose and the tumorigenicity in vivo were also determined. All cells that had lost their anchorage dependency and were tumorigenic showed a high net rate of spontaneous adhesion. A31 was the only nontransformed cell line to have a high net rate of adhesion. The net rate of spontaneous aggregation of cells is a quick and reliable index of tumorigenicity and offers a new approach to understanding the mechanisms of cell surface changes associated with transformation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERCROMBIE M., HEAYSMAN J. E. Observations on the social behaviour of cells in tissue culture. II. Monolayering of fibroblasts. Exp Cell Res. 1954 May;6(2):293–306. doi: 10.1016/0014-4827(54)90176-7. [DOI] [PubMed] [Google Scholar]
- Boone C. W. Malignant hemangioendotheliomas produced by subcutaneous inoculation of Balb/3T3 cells attached to glass beads. Science. 1975 Apr 4;188(4183):68–70. doi: 10.1126/science.1114343. [DOI] [PubMed] [Google Scholar]
- COMAN D. R. Adhesiveness and stickiness: two independent properties of the cell surface. Cancer Res. 1961 Nov;21:1436–1438. [PubMed] [Google Scholar]
- COMAN D. R. Mechanisms responsible for the origin and distribution of blood-borne tumor metastases: a review. Cancer Res. 1953 Jun;13(6):397–404. [PubMed] [Google Scholar]
- Cassiman J. J., Bernfield M. R. Transformation-induced alterations in fibroblast adhesion: masking by trypsin treatment. Exp Cell Res. 1975 Mar 1;91(1):31–35. doi: 10.1016/0014-4827(75)90137-8. [DOI] [PubMed] [Google Scholar]
- Coggin J. H., Jr, Anderson N. G. Cancer, differentiation and embryonic antigens: some central problems. Adv Cancer Res. 1974;19(0):105–165. doi: 10.1016/s0065-230x(08)60053-6. [DOI] [PubMed] [Google Scholar]
- Diamandopoulos G. T., Enders J. F. Comparison of the cytomorphologic characteristics of in vitro SV40-transformed hamster embryo cells with the histologic features of the neoplasms which they induce in the homologous host. Am J Pathol. 1966 Sep;49(3):397–417. [PMC free article] [PubMed] [Google Scholar]
- Dorsey J. K., Roth S. Adhesive specificity in normal and transformed mouse fibroblasts. Dev Biol. 1973 Aug;33(2):249–256. doi: 10.1016/0012-1606(73)90135-8. [DOI] [PubMed] [Google Scholar]
- Edwards J. G., Campbell J. A., Williams J. F. Transformation of polyoma virus affects adhesion of fibroblasts. Nat New Biol. 1971 Jun 2;231(22):147–148. doi: 10.1038/newbio231147a0. [DOI] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Halpern B., Pejsachowicz B., Febvre H. L., Barski G. Differences in patterns of aggregation of malignant and non-malignant mammalian cells. Nature. 1966 Jan 8;209(5019):157–159. doi: 10.1038/209157a0. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., Knighton D., Folkman J. Tumor angiogenesis activity in cells grown in tissue culture. Cancer Res. 1976 Jan;36(1):110–114. [PubMed] [Google Scholar]
- Levine E. M. Mycoplasma contamination of animal cell cultures: a simple, rapid detection method. Exp Cell Res. 1972 Sep;74(1):99–109. doi: 10.1016/0014-4827(72)90484-3. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Martz E., Steinberg M. S. Contact inhibition of what? An analytical review. J Cell Physiol. 1973 Feb;81(1):25–37. doi: 10.1002/jcp.1040810104. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Weinstein R. S. Carcinoma of the cervix: deficiency of nexus intercellular junctions. Science. 1969 Aug 8;165(3893):597–599. doi: 10.1126/science.165.3893.597. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Trans-membrane control of the receptors on normal and tumor cells. II. Surface changes associated with transformation and malignancy. Biochim Biophys Acta. 1976 Apr 30;458(1):1–72. doi: 10.1016/0304-419x(76)90014-7. [DOI] [PubMed] [Google Scholar]
- Old L. J., Boyse E. A., Stockert E. The G (Gross) leukemia antigen. Cancer Res. 1965 Jul;25(6):813–819. [PubMed] [Google Scholar]
- Sakiyama H., Gross S. K., Robbins P. W. Glycolipid synthesis in normal and virus-transformed hamster cell lines. Proc Natl Acad Sci U S A. 1972 Apr;69(4):872–876. doi: 10.1073/pnas.69.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C., Haudenschild C., Klagsbrun M. The chick chorioallantoic membrane as a model system for the study of tissue invasion by viral transformed cells. Cell. 1976 Jul;8(3):373–382. doi: 10.1016/0092-8674(76)90149-5. [DOI] [PubMed] [Google Scholar]
- Shields R., Pollock K. The adhesion of BHK and PyBHK cells to the substratum. Cell. 1974 Sep;3(1):31–38. doi: 10.1016/0092-8674(74)90034-8. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehan P., Friedman S. J. Regulation of Concanavalin A agglutination by the extracellular matrix. Exp Cell Res. 1975 May;92(2):350–360. doi: 10.1016/0014-4827(75)90389-4. [DOI] [PubMed] [Google Scholar]
- Stanbredge E. J., Boulger L. R., Franks C. R., Garrett J. A., Reeson D. E., Bishop D., Perkins F. T. Optimal conditions for the growth of malignant human and animal cell populations in immunosuppressed mice. Cancer Res. 1975 Aug;35(8):2203–2212. [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia S. S., MacMillan V. L. Acquisition of malignant properties by SV40-transformed mouse cells: relationship to type-C viral antigen expression. Intervirology. 1974;3(4):269–276. doi: 10.1159/000149763. [DOI] [PubMed] [Google Scholar]
- WEISS L. The effects of trypsin on the size, viability and dry mass of sarcoma 37 cells. Exp Cell Res. 1958 Feb;14(1):80–83. doi: 10.1016/0014-4827(58)90214-3. [DOI] [PubMed] [Google Scholar]