Abstract
Endogenous membrane protein kinase activity and protein kinase substrates have been found in membrane fractions enriched in the acetylcholine receptor that were prepared from the electric organ of Torpedo californica. Phosphorylation of four polypeptides is stimulated 9-fold by K+. The specific cholinergic ligand, carbachol, inhibited phosphorylation of these four polypeptides by 72% in the presence of 1mM Na+ and 100 mM K+. The 65,000-dalton component of the acetylcholine receptor in the membrane fraction appears to be phosphorylated by the endogenous protein kinase. These results suggest that protein phosphorylation may play an important role in synaptic events at nicotinic cholinergic synapses.
Full text
PDF
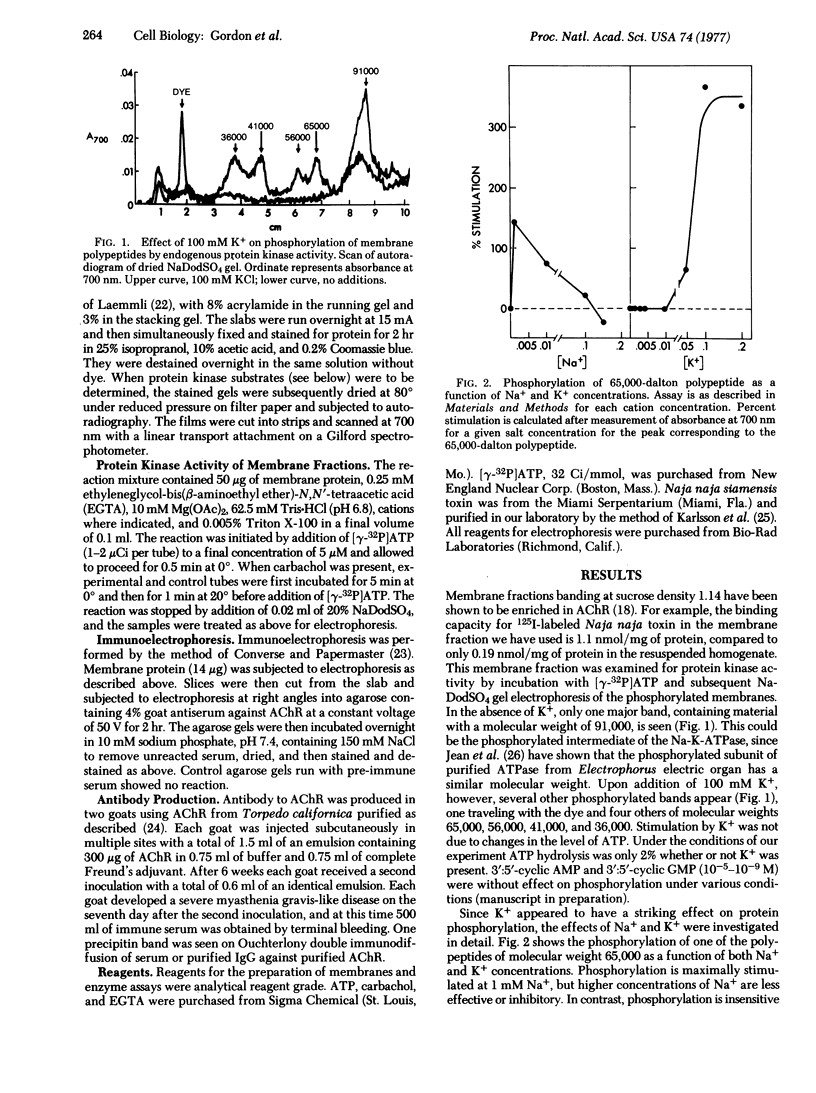
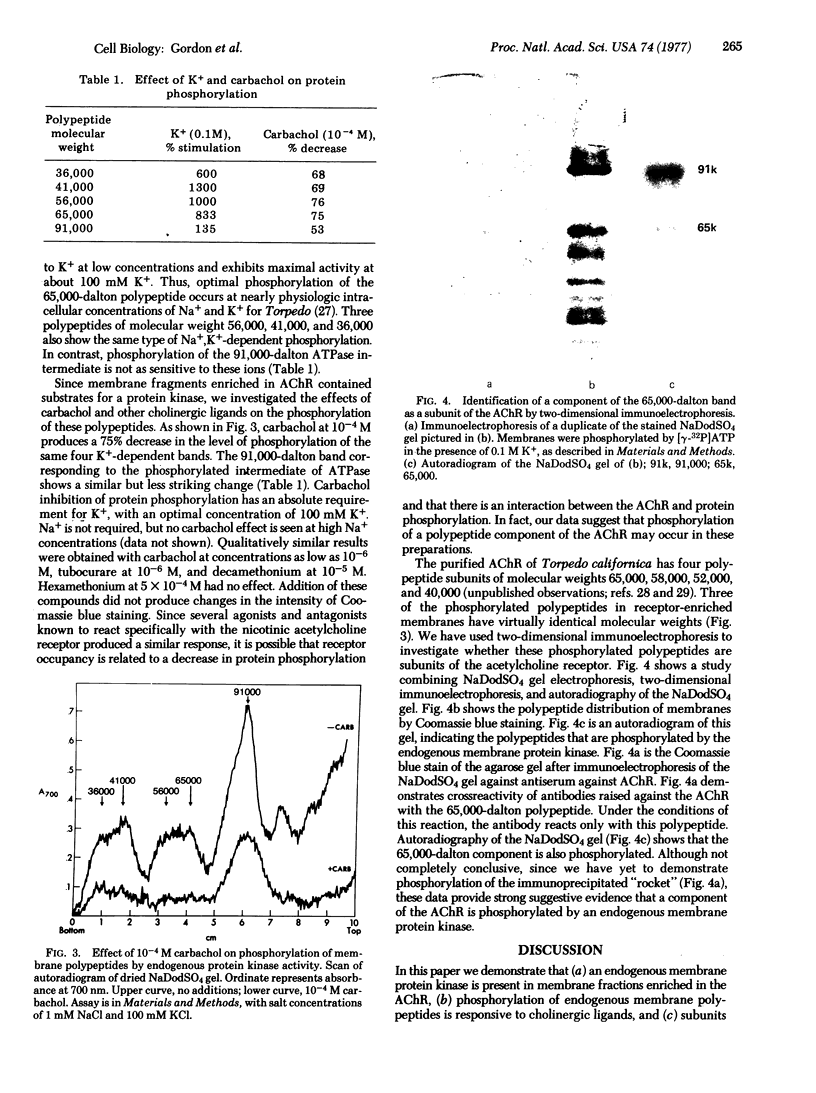


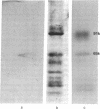
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew C. G., Almon R. R., Appel S. H. Macromolecular characterization of muscle membranes. Endogenous membrane kinase and phosphorylated protein substrate from normal and denervated muscle. J Biol Chem. 1975 May 25;250(10):3972–3980. [PubMed] [Google Scholar]
- Avruch J., Fairbanks G. Phosphorylation of endogenous substrates by erythrocyte membrane protein kinases. I. A monovalent cation-stimulated reaction. Biochemistry. 1974 Dec 31;13(27):5507–5514. doi: 10.1021/bi00724a009. [DOI] [PubMed] [Google Scholar]
- Casnellie J. E., Greengard P. Guanosine 3':5'-cyclic monophosphate-dependent phosphorylation of endogenous substrate proteins in membranes of mammalian smooth muscle. Proc Natl Acad Sci U S A. 1974 May;71(5):1891–1895. doi: 10.1073/pnas.71.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagas C., Esquibel M. A., Milhaud G. Action d'inhibiteurs de la phosphodiestérase sur la décharge électrique de l'électroplaque isolée de l'Electrophorus electricus (L. C R Acad Sci Hebd Seances Acad Sci D. 1972 Feb 28;274(9):1341–1344. [PubMed] [Google Scholar]
- Changeux J. P., Benedetti L., Bourgeois J. P., Brisson A., Cartaud J., Devaux P., Grünhagen H., Moreau M., Popot J. L., Sobel A. Some structural properties of the cholinergic receptor protein in its membrane environmental relevant to its function as a pharmacological receptor. Cold Spring Harb Symp Quant Biol. 1976;40:211–230. doi: 10.1101/sqb.1976.040.01.023. [DOI] [PubMed] [Google Scholar]
- Clement-Cormier Y. C., Kebabian J. W., Petzold G. L., Greengard P. Dopamine-sensitive adenylate cyclase in mammalian brain: a possible site of action of antipsychotic drugs. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1113–1117. doi: 10.1073/pnas.71.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Converse C. A., Papermaster D. S. Membrane protein analysis by two-dimensional immunoelectrophoresis. Science. 1975 Aug 8;189(4201):469–472. doi: 10.1126/science.1154021. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Walton K. G., Curran P. F., Greengard P. Regulation of phosphorylation of a specific protein in toad-bladder membrane by antidiuretic hormone and cyclic AMP, and its possible relationship to membrane permeability changes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):880–884. doi: 10.1073/pnas.70.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. R., Raftery M. A. Fractionation and partial characterization of membrane particles from Torpedo californica electroplax. Biochemistry. 1973 Sep 11;12(19):3593–3597. doi: 10.1021/bi00743a003. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Avruch J. Phosphorylation of endogenous substrates by erythrocyte membrane protein kinases. II. Cyclic adenosine monophosphate-stimulated reactions. Biochemistry. 1974 Dec 31;13(27):5514–5521. doi: 10.1021/bi00724a010. [DOI] [PubMed] [Google Scholar]
- Flanagan S. D., Barondes S. H., Taylor P. Affinity partitioning of membranes. Cholinergic receptor-containing membranes from Torpedo californica. J Biol Chem. 1976 Feb 10;251(3):858–865. [PubMed] [Google Scholar]
- Gordon A., Bandini G., Hucho F. Investigation of the Naja naja siamensis toxin binding site of the cholinergic receptor protein from Torpedo electric tissue. FEBS Lett. 1974 Oct 15;47(2):204–208. doi: 10.1016/0014-5793(74)81012-4. [DOI] [PubMed] [Google Scholar]
- Greengard P., McAfee D. A., Kebabian J. W. On the mechanism of action of cyclic AMP and its role in synaptic transmission. Adv Cyclic Nucleotide Res. 1972;1:337–355. [PubMed] [Google Scholar]
- Greengard P. Possible role for cyclic nucleotides and phosphorylated membrane proteins in postsynaptic actions of neurotransmitters. Nature. 1976 Mar 11;260(5547):101–108. doi: 10.1038/260101a0. [DOI] [PubMed] [Google Scholar]
- Hess G. P., Andrews J. P., Struve G. E., Goombs S. E. Acetylcholine-receptor-mediated ion flux in electroplax membrane preparations. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4371–4375. doi: 10.1073/pnas.72.11.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean D. H., Albers R. W., Koval G. J. Sodium-potassium-activated adenosine triphosphatase of electrophorus electric organ. X. Immunochemical properties of the Lubrol-solubilized enzume and its constituent polypeptides. J Biol Chem. 1975 Feb 10;250(3):1035–1040. [PubMed] [Google Scholar]
- Karlin A. The acetylcholine receptor: progress report. Life Sci. 1974 Apr 16;14(8):1385–1415. doi: 10.1016/0024-3205(74)90150-7. [DOI] [PubMed] [Google Scholar]
- Karlin A., Weill C. L., McNamee M. G., Valderrama R. Facets of the structures of acetylcholine receptors from Electrophorus and Torpedo. Cold Spring Harb Symp Quant Biol. 1976;40:203–210. doi: 10.1101/sqb.1976.040.01.022. [DOI] [PubMed] [Google Scholar]
- Karlsson E., Arnberg H., Eaker D. Isolation of the principal neurotoxins of two Naja naja subspecies. Eur J Biochem. 1971 Jul 15;21(1):1–16. doi: 10.1111/j.1432-1033.1971.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Greengard P. Dopamine-sensitive adenyl cyclase: possible role in synaptic transmission. Science. 1971 Dec 24;174(4016):1346–1349. doi: 10.1126/science.174.4016.1346. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Petzold G. L., Greengard P. Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the "dopamine receptor". Proc Natl Acad Sci U S A. 1972 Aug;69(8):2145–2149. doi: 10.1073/pnas.69.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McAfee D. A., Greengard P. Adenosine 3',5'-monophosphate: electrophysiological evidence for a role in synaptic transmission. Science. 1972 Oct;178(58):310–312. doi: 10.1126/science.178.4058.310. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Sealock R., Olsen R., Changeux J. P. Purification and properties of the cholinergic receptor protein from Electrophorus electricus electric tissue. Eur J Biochem. 1974 Jun 15;45(2):371–394. doi: 10.1111/j.1432-1033.1974.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Michaelson D. M., Raftery M. A. Purified acetylcholine receptor: its reconstitution to a chemically excitable membrane. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4768–4772. doi: 10.1073/pnas.71.12.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Rudolph S. A., Greengard P. Regulation of protein phosphorylation and membrane permeability by beta-adrenergic agents and cyclic adenosine 3':5'-monophosphate in the avian erythrocyte. J Biol Chem. 1974 Sep 10;249(17):5684–5687. [PubMed] [Google Scholar]
- SCHOFFENIELS E. Ion movements studied with single isolated electroplax. Ann N Y Acad Sci. 1959 Aug 28;81:285–306. doi: 10.1111/j.1749-6632.1959.tb49314.x. [DOI] [PubMed] [Google Scholar]
- Siggins G. R., Hoffer B. J., Bloom F. E. Studies on norepinephrine-containing afferents to Purkinje cells of rat cerebellum. 3. Evidence for mediation of norepinephrine effects by cyclic 3',5'-adenosine monophosphate. Brain Res. 1971 Feb 5;25(3):535–553. doi: 10.1016/0006-8993(71)90459-8. [DOI] [PubMed] [Google Scholar]
- Ueda T., Maeno H., Greengard P. Regulation of endogenous phosphorylation of specific proteins in synaptic membrane fractions from rat brain by adenosine 3':5'-monophosphate. J Biol Chem. 1973 Dec 10;248(23):8295–8305. [PubMed] [Google Scholar]
- Weight F. F., Petzold G., Greengard P. Guanosine 3',5'-monophosphate in sympathetic ganglia: increase assoicated with synaptic transmission. Science. 1974 Dec 6;186(4167):942–944. doi: 10.1126/science.186.4167.942. [DOI] [PubMed] [Google Scholar]
- Weller M., Rodnight R. Turnover of protein-bound phosphorylserine in membrane preparations from ox brain catalysed by intrinsic kinase and phosphatase activity. Biochem J. 1971 Sep;124(2):393–406. doi: 10.1042/bj1240393. [DOI] [PMC free article] [PubMed] [Google Scholar]