Abstract
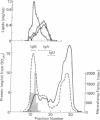
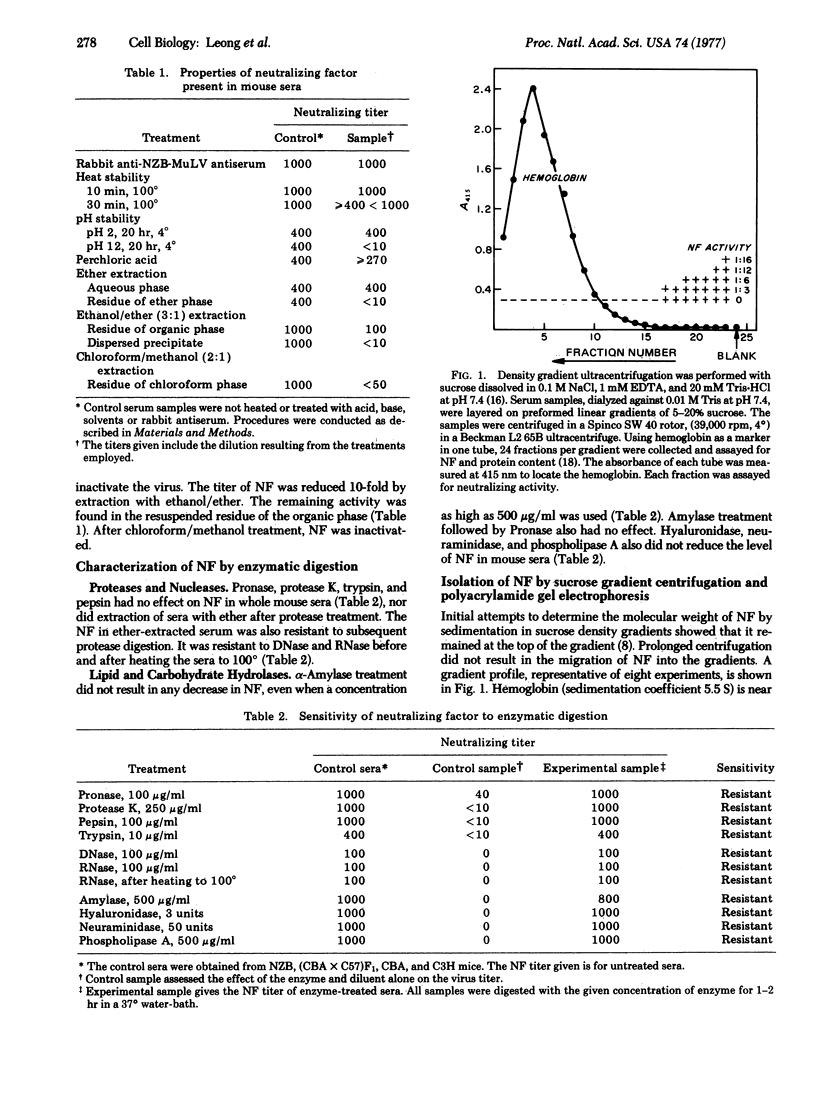
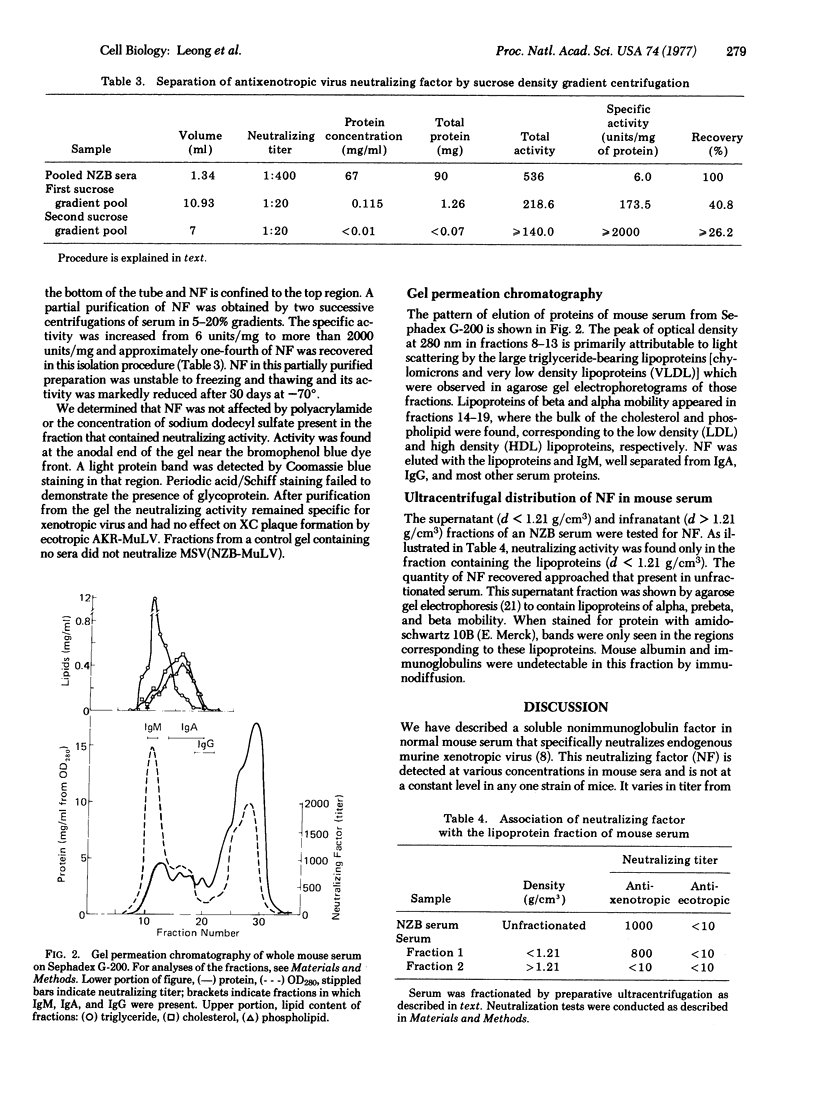
A soluble nonimmunoglobulin factor that specifically neutralizes mouse xenotropic C-type virus is found in normal mouse sera. It is stable from pH 2 to neutrality and resists ether extraction, freezing, or brief heating to 100 degrees. It can be separated from immunoglobulins by ultracentrifugation at a density of 1.21 g/cm3. Neutralizing activity is only found with the serum lipoproteins in the fraction with density less than 1.21 g/cm3.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Widespread natural occurrence of high titers of neutralizing antibodies to a specific class of endogenous mouse type-C virus. Proc Natl Acad Sci U S A. 1974 May;71(5):1957–1961. doi: 10.1073/pnas.71.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNFELD P., KELLEY T. F. PROTEOLYSIS OF HUMAN SERUM BETA-LIPOPROTEIN. J Biol Chem. 1964 Oct;239:3341–3346. [PubMed] [Google Scholar]
- Chisari F. V., Edgington T. S. Lymphocyte E rosette inhibitory factor: a regulatory serum lipoprotein. J Exp Med. 1975 Nov 1;142(5):1092–1107. doi: 10.1084/jem.142.5.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc-Nguyen H. Enhancing effect of diethylaminoethyl-dextran on the focus-forming titer of a murine sarcoma virus (Harvey strain). J Virol. 1968 Jun;2(6):643–644. doi: 10.1128/jvi.2.6.643-644.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Ihle J. N., Bolognesi D. P., Schäfer W. Inactivation of murine xenotropic oncornavirus by normal mouse sera is not immunoglobulin-mediated. Virology. 1976 May;71(1):346–351. doi: 10.1016/0042-6822(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Granda J. L., Scanu A. Solubilization and properties of the apoproteins of the very low- and low-density lipoproteins of human serum. Biochemistry. 1966 Oct;5(10):3301–3308. doi: 10.1021/bi00874a032. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino S., Stephenson J. R., Aaronson S. A. Radiommunoassays for the 70,000-molecular-weight glycoproteins of endogenous mouse type C viruses: viral antigen expression in normal mouse tissues and sera. J Virol. 1976 Jun;18(3):933–941. doi: 10.1128/jvi.18.3.933-941.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee J. C., Hanna M. G., Jr, Ihle J. N., Aaronson S. A. Autogenous immunity to endogenous RNA tumor virus: differential reactivities of immunoglobulins M and G to virus envelope antigens. J Virol. 1974 Oct;14(4):773–781. doi: 10.1128/jvi.14.4.773-781.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. A., Wilson C. B., Villano B. C., McConahey P. J., Dixon F. J. Endogenous oncornaviral gene expression in adult and fetal mice: quantitative, histologic, and physiologic studies of the major viral glycorprotein, gp70. J Exp Med. 1976 Jan 1;143(1):151–166. doi: 10.1084/jem.143.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Autoimmunity and neoplasia. The possible role of C-type viruses. Am J Clin Pathol. 1974 Aug;62(2):258–280. doi: 10.1093/ajcp/62.2.258. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Endogenous C-type viruses: double agents in natural life processes. Biomedicine. 1976 May;24(2):84–93. [PubMed] [Google Scholar]
- Levy J. A. Host range of murine xenotropic virus: replication in avian cells. Nature. 1975 Jan 10;253(5487):140–142. doi: 10.1038/253140a0. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Ihle J. N., Oleszko O., Barnes R. D. Virus-specific neutralization by a soluble non-immunoglobulin factor found naturally in normal mouse sera. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5071–5075. doi: 10.1073/pnas.72.12.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Kazan P., Varnier O., Kleiman H. Murine xenotropic type C viruses I. Distribution and further characterization of the virus in NZB mice. J Virol. 1975 Oct;16(4):844–853. doi: 10.1128/jvi.16.4.844-853.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic C-type viruses and autoimmune disease. J Rheumatol. 1975 Jun;2(2):135–148. [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Scanu A. M., Edelstein C. Solubility in aqueous solutions of ethanol of the small molecular weight peptides of the serum very low density and high density lipoproteins: relevance to the recovery problem during delipidation of serum lipoproteins. Anal Biochem. 1971 Dec;44(2):576–588. doi: 10.1016/0003-2697(71)90247-8. [DOI] [PubMed] [Google Scholar]
- Stewart C. P., Hendry E. B. The phospholipins of blood. Biochem J. 1935 Jul;29(7):1683–1689. doi: 10.1042/bj0291683. [DOI] [PMC free article] [PubMed] [Google Scholar]