Abstract
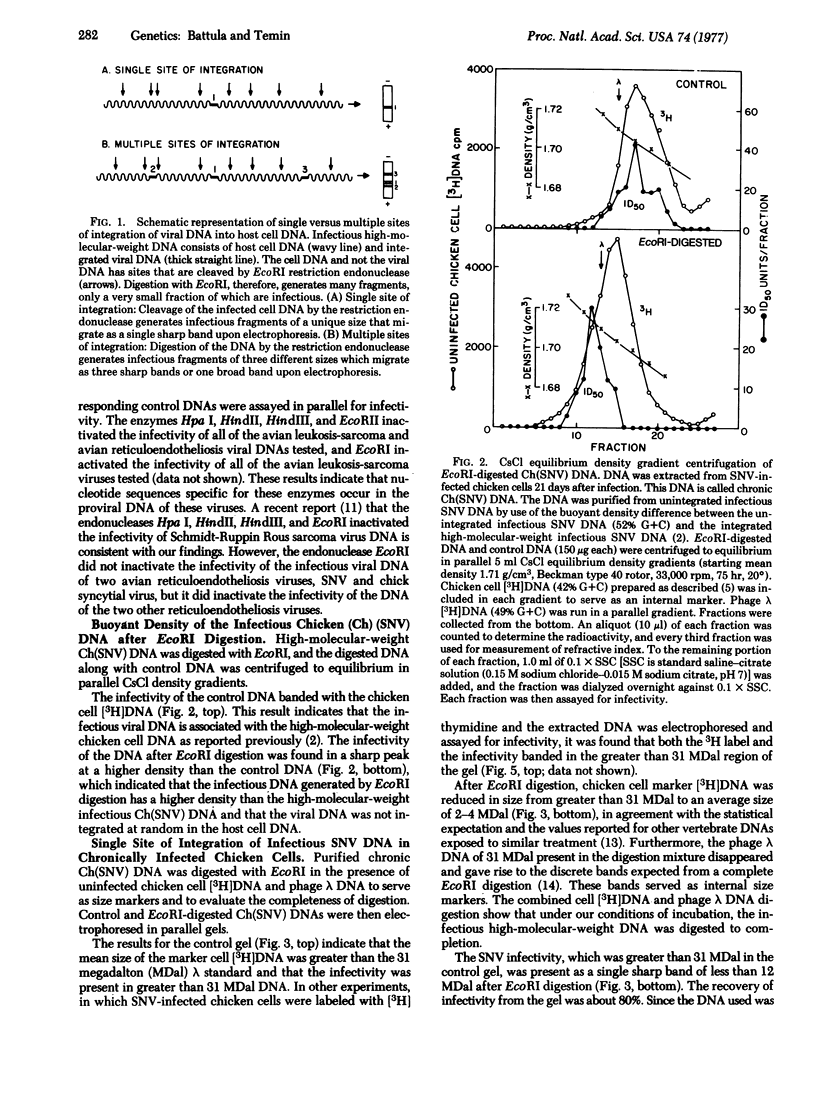
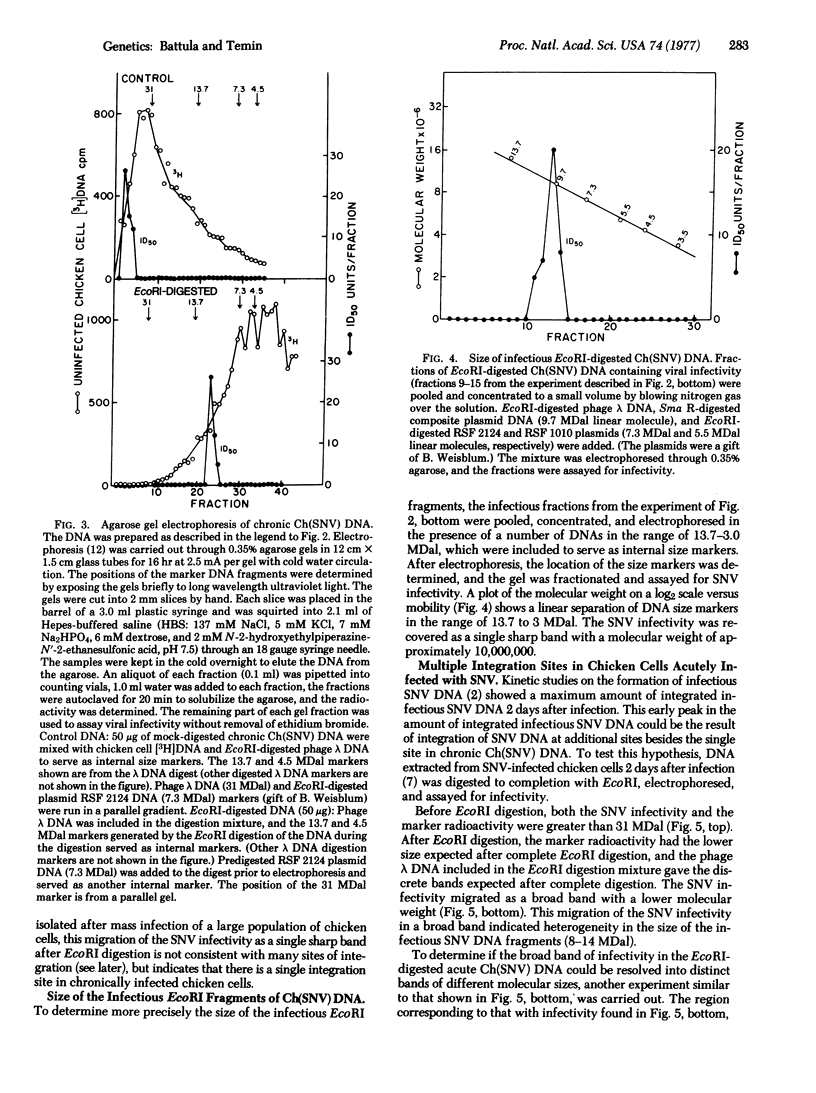
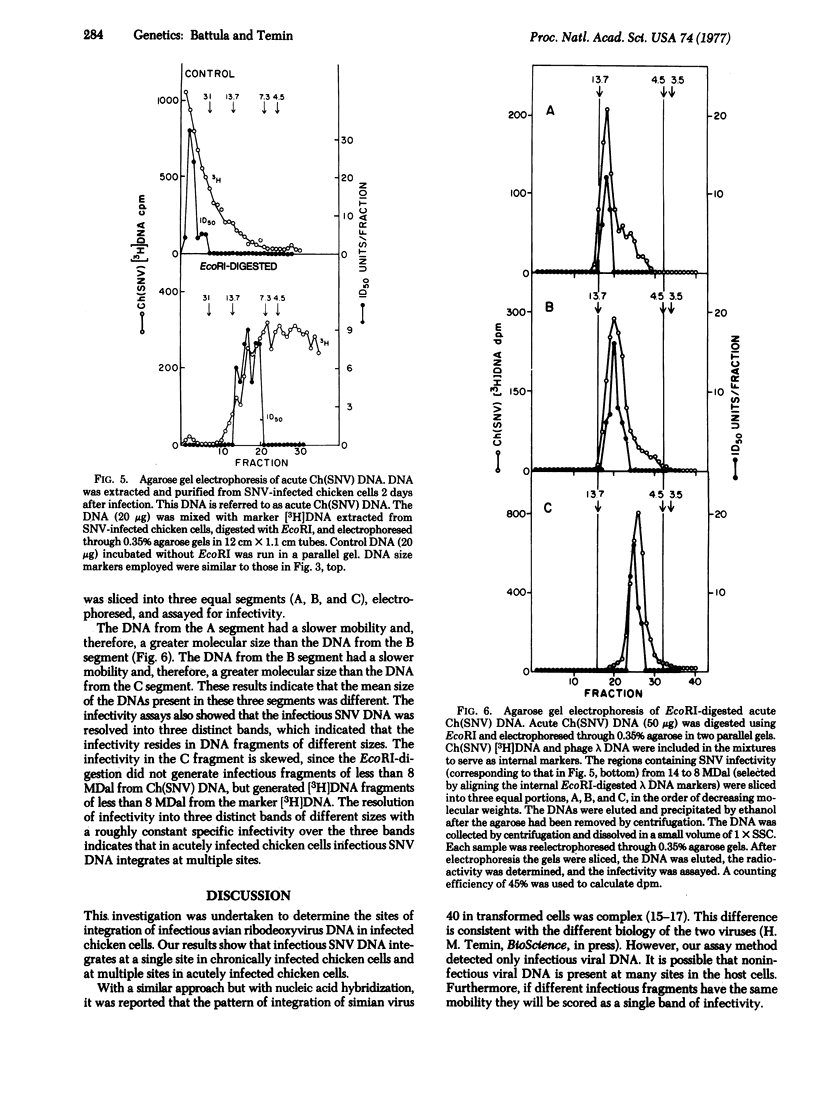
The infectious DNAs of a number of avian leukosis-sarcoma and reticuloendotheliosis viruses were digested with six nucleotide-specific restriction endonucleases, and the digests were tested for infectivity. All of the enzymes inactivated the viral infectivities except for EcoRI, which did not inactivate the infectivity of the DNA of two of the reticuloendotheliosis viruses, spleen necrosis and chick syncytial viruses. The infectious DNA of spleen necrosis virus after digestion with EcoRI had a buoyant density in CsCl solution greater than the density of the high-molecular-weight infectious viral DNA. The infectious EcoRI-digested spleen necrosis virus DNA from chronically infected chicken cells was uniform in size, 10 megadaltons, which indicated a single site of integration. The infectious EcoRI-digested spleen necrosis virus DNA from acutely infected cells was heterogeneous in size, ranging from 8-14 megadaltons, which indicated multiple sites of integration. These results are consistent with the hypothesis that cells that integrate infectious spleen necrosis virus DNA at a single site survive and multiply, whereas cells that integrate infectious viral DNA at additional sites either die or selectively lose or inactivate the DNA in the additional sites.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Jeppesen P. G., Katagiri K. J., Delius H. Mapping the DNA fragments produced by cleavage by lambda DNA with endonuclease RI. Nature. 1973 Jan 12;241(5385):120–123. doi: 10.1038/241120a0. [DOI] [PubMed] [Google Scholar]
- Botchan M., McKenna G., Sharp P. A. Cleavage of mouse DNA by a restriction enzyme as a clue to the arrangement of genes. Cold Spring Harb Symp Quant Biol. 1974;38:383–395. doi: 10.1101/sqb.1974.038.01.041. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Infectious rous sarcoma virus and reticuloendotheliosis virus DNAs. J Virol. 1974 Nov;14(5):1132–1141. doi: 10.1128/jvi.14.5.1132-1141.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Lack of infectivity of the endogenous avian leukosis virus-related genes in the DNA of uninfected chicken cells. J Virol. 1976 Feb;17(2):422–430. doi: 10.1128/jvi.17.2.422-430.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E., Temin H. M. Formation and structure of infectious DNA of spleen necrosis virus. J Virol. 1977 Jan;21(1):119–130. doi: 10.1128/jvi.21.1.119-130.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guntaka R. V., Mahy B. W., Bishop J. M., Varmus H. E. Ethidium bromide inhibits appearance of closed circular viral DNA and integration of virus-specific DNA in duck cells infected by avian sarcoma virus. Nature. 1975 Feb 13;253(5492):507–511. doi: 10.1038/253507a0. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Reticuloendotheliosis virus nucleic acid sequences in cellular DNA. J Virol. 1974 Nov;14(5):1179–1188. doi: 10.1128/jvi.14.5.1179-1188.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury A. T., Hanafusa H. Synethesis and integration of viral DNA in chicken cells at different time after infection with various multiplicities of avian oncornavirus. J Virol. 1976 May;18(2):383–400. doi: 10.1128/jvi.18.2.383-400.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecka H., Hillova J., Hill M. Effect of restriction endonucleases on infectivity of Rous sarcoma virus DNA. Nature. 1976 Jul 1;262(5563):72–74. doi: 10.1038/262072a0. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Dastoor M. N., Baluda M. A. Evidence for tandem integration of avian myeloblastosis virus DNA with endogenous provirus in leukemic chicken cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1749–1753. doi: 10.1073/pnas.73.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEMIN H. M. Mixed infection with two types of Rous sarcoma virus. Virology. 1961 Feb;13:158–163. doi: 10.1016/0042-6822(61)90049-6. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Kassner V. K. Replication of reticuloendotheliosis viruses in cell culture: acute infection. J Virol. 1974 Feb;13(2):291–297. doi: 10.1128/jvi.13.2.291-297.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling D. A., Temin H. M. High spontaneous mutation rate of an avian sarcoma virus. J Virol. 1975 Jan;17(1):74–84. doi: 10.1128/jvi.17.1.74-84.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



