Abstract
Prepulse inhibition (PPI) of the startle reflex is disrupted in a number of developmental neuropsychiatric disorders, including Tourette syndrome (TS). This disruption is hypothesized to reflect abnormalities in sensorimotor gating. We applied whole-brain functional magnetic resonance imaging (fMRI) to elucidate the neural correlates of PPI in adult TS subjects using airpuff stimuli to the throat to elicit a tactile startle response. We used a cross-sectional, case-control study design and a blocked-design fMRI paradigm. There were 33 participants: 17 with TS and 16 healthy individuals. As a measure of PPI-related brain activity, we looked for differential cerebral activation to prepulse-plus-pulse stimuli versus activation to pulse-alone stimuli. In healthy subjects, PPI was associated with increased activity in multiple brain regions, of which activation in the left middle frontal gyrus in the healthy controls showed a significant linear correlation with the degree of PPI measured outside of the magnet. Group comparisons identified nine regions where brain activity during PPI differed significantly between TS and healthy subjects. Among the TS subjects, activation in the left caudate was significantly correlated with current tic severity as measured by the total score on the Yale Global Tic Severity Scale. Differential activation of the caudate nucleus associated with current tic severity is consistent with neuropathological data and suggests that portions of cortical-striatal circuits may modulate the severity of tic symptoms in adulthood.
Keywords: Prepulse inhibition, Tourette syndrome, Sensorimotor gating, Cortico-striatal circuits, Brain mechanisms, Adults
1. Introduction
Tourette syndrome (TS) is a childhood-onset neuropsychiatric disorder characterized by multiple motor tics and at least one phonic tic (Leckman, 2002,2012). Estimates of TS prevalence indicate prevalence in childhood on the order of 0.1% to 1.0% with a much lower prevalence in adulthood (Khalifa and von Knorring, 2003, 2006; Mol Debes et al., 2008; Scahill et al., 2007). The onset of TS is typically prepubertal, and boys are more commonly affected than girls. In both clinical and population-based samples, tic severity usually peaks between 8 and 12 years of age with many affected individuals showing a marked reduction in severity by the end of adolescence (Leckman et al., 1998; Bloch et al., 2006). Indeed, by the age of 20 years, tic severity is generally greatly reduced in a majority of individuals with TS (Leckman et al., 1998; Bloch et al., 2006).
Recent neuropathology studies have highlighted the loss of GABAergic and cholinergic interneurons in the caudate and putamen of individuals with TS (Kalanithi et al., 2005; Kataoka et al., 2010). Although structural and functional brain imaging studies have also implicated striatal involvement in the pathobiology and persistence of TS (Bloch et al., 2005), a number of other cortical and subcortical regions, including the hippocampus, have also been implicated (Peterson et al., 2007; Bansal et al., 2012). Indeed, multiple lines of evidence suggest that disturbances in the development of the sensorimotor portions of cortical–subcortical circuits likely predispose to the development TS, and that neuroplastic changes in the limbic and associative circuits may help to modulate the severity of symptom expression over the lifespan (Graybiel, 2008; Plessen et al., 2009; Leckman et al., 2010; Wang et al., 2011; Bansal et al., 2012). Hypothesized abnormalities in cortical–striatal circuits in TS have led to behavioral paradigms to detect inhibitory deficits, such as prepulse inhibition (PPI). PPI is a simple behavioral measure of inhibition of the startle blink reflex, referring to reduction in startle blink magnitude when a stimulus (prepulse) occurs 30–500 ms before a startle stimulus (Swerdlow et al., 2001). The prepulse is believed to activate automatic brain mechanisms that protect or “gate” the processing of that stimulus for a brief window of time. Animal studies show that PPI is mediated by brain stem circuits as well as forebrain circuits involving the prefrontal cortex, thalamus, hippocampus, amygdala, nucleus accumbens, striatum, ventral pallidum, and globus pallidus (Swerdlow et al., 2001, 2008). Although the neural substrates of PPI may vary to some degree between animals and humans (Swerdlow et al. 2008), imaging studies (Hazlett and Buchsbaum, 2001; Kumari et al., 2003, 2005; Campbell et al., 2007; Hazlett et al., 2008) support the involvement of similar brain regions in the modulation of human PPI.
In schizophrenia research PPI has played an important role in strategic efforts to develop and characterize new treatments as well as to identify valid endophenotypes (Postma et al., 2006; Swerdlow et al., 2008; Kumari et al., 2008; Greenwood et al., 2011). Similar opportunities may exist in efforts to understand and treat TS. Specifically, several studies have shown reduced PPI in TS subjects compared to control subjects (Smith and Lees, 1989; Castellanos et al., 1996; Swerdlow et al., 2001; Swerdlow, 2012).
We studied PPI in healthy controls and individuals with TS using functional magnetic resonance imaging (fMRI) to determine if a deficit in sensorimotor gating is a characteristic of TS in adulthood and if brain regions displaying differential activation to prepulse-plus-pulse vs. pulse-alone stimuli were associated with current levels of tic severity. Based on the available neuropathological and structural imaging studies, we were particularly interested in the possible association of current tic severity, PPI and differential BOLD responses in striatal and hippocampal regions.
2. Methods
2.1. Study subjects
Thirty-eight subjects were recruited to voluntarily participate in this fMRI study (18 TS and 20 healthy control subjects). There were 11 males and 7 female subjects with TS who ranged from 21 to 59, averaging 33.2 years of age. Healthy normal participants (12 male, 8 female) ranged from 21 to 44, averaging 30.4 years of age. The two groups did not differ significantly in age (p>0.2) or gender (p>0.2). We recruited persons with a lifetime diagnosis of TS and who were being followed in the Tic Disorder Specialty Clinic at the Yale Child Study Center in New Haven, CT, excluding persons who had an axis I disorder other than obsessive-compulsive disorder (OCD) or attention deficit hyperactivity disorder (ADHD) before the onset of TS (comorbid OCD: N=12, comorbid ADHD: N=9, both OCD and ADHD: N=7). We did not exclude individuals with a lifetime history of a mood disorder as long as the disorder was not currently a source of impairment (lifetime mood disorder: N=7). We recruited comparison subjects through advertisements on Craigslist <http://newhaven.craigslist.org/> and by word-of-mouth. We excluded those who reported a history of tic disorder, OCD, or ADHD, or who met diagnostic criteria for any axis I psychiatric disorder at the time of the Baseline assessment. Additional exclusionary criteria for both groups were a lifetime history of substance abuse or head trauma.
We administered the Schedule for Tourette and Other Behavioral Disorders (STOBD) (Pauls and Hurst, 1993), as well as clinical evaluations, to establish diagnoses through a consensus procedure of expert clinicians (Leckman et al., 1982). The STOBD includes the Schedule for Affective Disorders and Schizophrenia (SADS) (Endicott and Spitzer, 1978) for adults, as well as sections on TS and OCD. Ratings of current and worst-ever symptom severity of tic and obsessive–compulsive symptoms were obtained using the Yale Global Tic Severity Scale (YGTSS) (Leckman et al., 1989) and the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) (Goodman et al., 1989).
All subjects were screened for illicit drug abuse. Written informed consent was obtained at the time of the evaluation assessment. Compensation for participation was provided at both time points under the guidelines of the Yale University Human Investigation Committee. Data from four control subjects and one TS participant (who fell asleep) who startled on fewer than 36% pulse-alone trials during the psychophysiological assessment outside the scanner were excluded (see below). The demographic and clinical characteristics (including medication status) for these 33 subjects are presented in Table 1.
Table 1.
Demographic and clinical characteristics at baseline.
| Variable | Normal controls (N=16) | TS subjects (N=17)b | p-value |
|---|---|---|---|
| Age in years (SD) | 28.3 (7.4) | 32.5 (11.3) | 0.22 |
| Gender (% female) | 62.5% | 41.2% | 0.22 |
| Race (% Caucasian) | 85% | 94.2% | |
| Age of tic onset in yrs, mean (SD) | NA | 7.9 (3.6) | |
| Comorbid OCD lifetime, currenta (%) | NA | 64.7%, 29.4% | |
| Comorbid ADHD (%) | NA | 52.9% | |
| Comorbid OCD and ADHD (%) | NA | 41.2% | |
| Comorbid mood disorder lifetime (%) | NA | 41.2% | |
| Current symptom severityb | |||
| TS, mean (SD) | NA | 21.4 (11.9) | |
| OCD, mean (SD) | NA | 7.6 (7.6) | |
| Psychotropic medications | |||
| Alpha-2 agonists (%) | NA | 5.9% | |
| Neuroleptics (%) | NA | 11.8% | |
| Selective serotonin reuptake inhibitors (%) | NA | 41.2% | |
| Benzodiazepines (%) | NA | 17.6% |
TS=Tourette syndrome, OCD=obsessive–compulsive disorder, ADHD=attention deficit hyperactivity disorder; Mood disorder=a past history of major depressive disorder or depressive disorder not otherwise specified.
Subjects with a core on the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) > 16 at time of study were considered to have current comorbid OCD.
Total score of the current Yale Global Tic Severity Scale (YGTSS) and total score on the Yale-Brown Obsessive Compulsive Scale (Y-BOCS). However, only the YGTSS total tic severity score was used in analyzing the tactile startle and fMRI data (see text).
2.2. Startle task
A tactile (airpuff) startle paradigm in an fMRI setting similar to that used by Kumari et al. (2003) was employed to measure PPI. A block design with three conditions was used: pulse-alone, prepulse+pulse (PPI), and rest. The pulse-alone condition involved presentations of the pulse stimulus. The PPI condition involved presentation of the prepulse stimulus 100 ms before the pulse stimulus. Each condition was presented to the participant six times in 30-s blocks in pseudorandom order across two runs. The pulse-alone, PPI and rest blocks each occurred three times in run 1 and three times in run 2. Run 1 began with six pulse-alone stimuli (30 s). Each of the runs began with a 10-s resting baseline period. Within each 30-s block, six stimuli were presented with an inter-stimulus interval of 3 s to 6 s. Air puffs to the throat area were used as both pulse and prepulse. Stimulus presentation inside and outside the scanner was controlled by identical San Diego Instruments Human Startle Response Monitoring Systems (SR-Lab, San Diego Instruments, San Diego, CA, USA). The air-puff delivery system consisted of two cylinders of compressed breathable air (one for the pulse, one for the prepulse) and regulators for setting the psi level (pulse stimulus regulator range 0–240 psi; prepulse stimulus regulator range 0–30 psi). Solenoid-controlled valves and 10-m-long plastic tubes delivered the air. The pulse tube consisted of rigid nylon-11 material (6 mm I.D.). The prepulse tube consisted of flexible Tygon tubing (3 mm I.D.). To maintain comparable placement across participants, each tube was fastened to a customized protective ice hockey collar. Each participant wore the collar so that the air tubes were midline, over the larynx. Inside the collar, two parallel 1.5 cm×3 cm foam pads kept the tubing at 1.5 cm from the participant's larynx. Pulse-alone stimuli were 80 psi at the regulator and 40 ms in duration. Prepulses were 7 psi at the regulator and 20 ms in duration. Following Kumari et al. (2003), the startle responses were measured outside the fMRI setting. Since it was not possible to safely use electrodes to acquire electromyographic (EMG) activity during MRI scanning, the eye-blink reflex response was measured on the same day as the imaging scan, approximately 2.5 h post-scan. The startle assessment took place in a quiet, dimly lit room (60 W bulb). The individual sat in a comfortable chair with the EMG electrodes and tactile startle apparatus attached (Borelli et al., 2010).
Due to technical difficulties, one TS patient was scanned on a different day than his startle response measurement. In addition, three controls and one TS participant (who fell asleep) who startled on fewer than 36% pulse-alone trials during the psychophysiological assessment were excluded. In terms of cases, we had originally had 38 subjects. However, data from only 33 participants were included in the data analysis (17 with TS and 16 healthy individuals).
2.3. Startle and prepulse inhibition
Based on earlier studies, the eye-blink component of the startle response was assessed by recording the EMG activity of the left orbicularis oculi muscle via two miniature (contact area <4 mm) Ag/AgCl electrodes filled with Grass electrode cream (Blumenthal et al., 1996, 2004; Grillon et al., 1996). After preparation of the skin surface with Nuprep exfoliant and a cotton swab, one electrode was positioned below the eyelid in line with the pupil in forward gaze. A second electrode was placed ~1.5 cm lateral to the first. In addition, a ground (reference) electrode was placed on the inner side of the left forearm. The computer system recorded EMG activity for 1000 ms (sample interval 1 ms) from 200 ms prior to the onset of the startle stimulus. The amplification gain control for EMG signal was kept constant for all subjects. Recorded EMG activity was band-pass filtered, as recommended by SR-Lab. Analogue frequency filtering occurred before digitizing. The high-pass and low-pass cut-off frequencies were set at 30 Hz and 1 kHz respectively. A 60-Hz notch filter was used to eliminate the 60-Hz interference. The data were scored offline by the analytical program of this system for response amplitude (in arbitrary analog-to-digital units), and latencies to response peak (in ms). The scoring program contained a rolling average routine that smoothed the rectified EMG response. The latency to response peak was determined as the point of maximal amplitude that occurred within 120 ms from the startle stimulus. PPI calculation followed the guidelines proposed by Blumenthal et al. (1996). Average response to each stimulus condition was calculated across the entire session. Average response to the pulse-alone conditions was then subtracted from that in the PPI conditions, and this difference was divided by average response to the pulse-alone conditions. Ratios significantly less than zero indicate PPI.
2.4. Image acquisition
The fMRI data were acquired on a Siemens trio 3.0 T full-body scanner (Erlangen, Germany). Head movements were minimized with foam padding and surgical tape placed across participants' foreheads (sticky side up). Subjects were informed that the stimulus may cause them to blink and were instructed to avoid consciously altering their response.
First, high-resolution T1-weighted anatomical images were obtained (3D MPRAGE; TR=2530 ms; TE=3.66 ms; matrix size 256 × 256; 176 slices). Then, anatomical T1-weighted echo-planar images (spin-echo; TR=300 ms; TE=4 ms; matrix size 64 × 64; 30 axial slices; 3.125-mm in-plane resolution, 5-mm thick, no skip) were acquired to be coplanar with the functional scans for registration. The two functional runs, 400 s and 370 s in duration respectively, were acquired (echo planar T2*- weighted gradient-echo, TR=2000 ms, TE=30 ms, flip angle=80°, matrix size 64 × 64, 30 axial slices, 3.125-mm in-plane resolution; 5-mm thick, skip 0 mm) spanning the entire brain.
2.5. Image preprocessing
Image processing, analyses, and tests of statistical significance were performed using BrainVoyager QX version 2.07 software (Brain Innovation, Maastricht, The Netherlands). The functional data were preprocessed using slice scan time correction (cubic spline interpolation), three-dimensional motion correction (trilinear interpolation), spatial smoothing with a 4-mm Gaussian kernel, and temporal high-pass filtering (fast-Fourier-transform-based with a cutoff of 3 cycles/time course). Each individual functional data set underwent piecewise linear transformation into a proportional three-dimensional grid defined by Talairach and Tournoux (1988), and co-registered with the high-resolution 3D data set that was re-sampled to give 1 mm3 voxels. Estimated motion plots and cine loops were examined for each run to identify movements and eliminate runs in which participants displayed a deviation >3 mm in the estimated center of mass in any direction. As such, run 1 for one TS patient was excluded from further analysis.
2.6. PPI and the neural correlates of PPI
Our a priori hypothesis was that PPI and the associated neural correlates would differ in the TS versus the comparison subjects. First, we analyzed the group differences in PPI data collected outside of the magnet using a two-tailed t-test. Second, we examined the functional activation in the control group alone using a random effects general linear model (GLM) analysis contrasting the two experimental conditions: PPI and pulse alone. Third, a whole-brain Condition by Group analysis of variance (ANOVA) with random effects was used to assess group differences in functional activation. Two groups were used: healthy controls and subjects with TS. Three sets of conditions were used: PPI and pulse, pulse and rest, PPI and rest. Whole brain analysis used a voxel-wise threshold of p <0.01 to protect against Type 1 errors. Regions identified were then tested between groups using p value of <0.05.
To account for multiple comparisons, a cluster size threshold was computed using BrainVoyager's cluster level statistical threshold estimator plug-in to estimate cluster level false-positives. This algorithm uses Monte Carlo simulations to estimate the probability of clusters of a given size arising purely from chance adapted from Forman et al. (1995). After 5000 iterations and an alpha value of 0.05, a minimum cluster size of seven contiguous functional voxels was applied to all statistical maps.
A random effects covariate analysis with all subjects was used to check for effects of age and gender on regional brain activation for PPI vs. pulse contrast. Similarly, a random effects covariate analysis with subjects with TS was used to check for an effect of age, comorbid illnesses (OCD, ADHD, and a lifetime history of a mood disorder) and medications (relevant to >2 participants; SSRIs and benzodiazepines) on regional brain activation for the PPI vs. pulse contrast. Finally, we evaluated the association of regional brain activation (BOLD response) with tic (YGTSS total tic severity score, 0–50) and obsessive–compulsive symptom (YBOCS total score, 0–40) severity using simple linear regression analyses (p<0.05 significant). We also stratified the TS subjects according to their current total tic severity scale score into two groups: “mild or remitted” (n=6) vs. active (n=11). “Mild or remitted” TS cases are subjects whose total tic score on the Yale Global Tic Severity Scale was 0–12 for the 12 months prior to the study (Bloch et al., 2005).
3. Results
3.1. Prepulse inhibition
A normal level of PPI was observed in healthy controls (58.9%±28.5% reduction). As illustrated in Fig. 1, PPI was considerably lower in subjects with TS (39.9%±37.4%) compared to controls. However, this difference did not reach statistical significance (t=1.64, p=0.11). No association was observed between current or worst ever YGTSS total tic severity (0–50) and the degree of PPI (r2=0.04, p=0.47 and r2=0.07, p=0.29 respectively). Although there were no group differences in startle magnitude or latency, the TS subjects, on average, did have higher amplitudes and longer latencies (Supplemental Table S1).
Fig. 1.

Mean percent prepulse inhibition (PPI) in tactile startle in healthy controls and Tourette syndrome (TS) subjects. Healthy controls (N=16) show a greater reduction in PPI compared to subjects with Tourette syndrome (TS, N=17), p=0.11. Error bars represent standard error of the mean (SEM).
3.2. Neural correlates of PPI in healthy participants
A whole-brain random effects analysis of healthy participants revealed 18 distinct cortical and subcortical regions (p=0.01) where the BOLD signal was greater during PPI (pulse preceded by the prepulse) compared to the pulse alone (Table 2). An examination of the mean beta values of the pulse versus the PPI condition confirmed that 15 of these 18 differed significantly (p<0.05) in activity among healthy and TS groups (Fig. 2A and B) while three did not. For healthy controls, regression analysis demonstrated a significant linear relationship (r=0.51, r2=0.26, p<0.05) between PPI and activity in only one brain region—the left middle frontal gyrus (Fig. 3). BOLD activity in this region, however, did not correlate significantly with PPI for TS subjects.
Table 2.
Neural correlates of prepulse inhibition (PPI) in healthy control subjects. A whole-brain random effects analysis of healthy control subjects identified 18 regions of interest (anterior to posterior with cortical regions followed by subcortical regions). In each instance the BOLD signal was stronger during the PPI condition (prepulse stimuli 100 ms prior to the pulse stimuli) compared to the pulse alone condition.
| Brain region | Side | Number of voxels | Peak Talairach coordinates (in mm) |
Peak p | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Middle frontal gyrus* | Left | 286 | −42 | 5 | 52 | 0.000440 |
| Inferior medial frontal gyrus | Right | 279 | 3 | 29 | −11 | 0.000007 |
| Superior temporal gyrus | Right | 189 | 48 | 14 | −14 | 0.000501 |
| Paracentral lobule | Bilateral | 793 | 3 | −40 | 55 | 0.000012 |
| Inferior middle temporal gyrus | Left | 309 | −54 | −19 | −5 | 0.000027 |
| Superior temporal gyrus | Left | 751 | −48 | −43 | 10 | 0.000316 |
| Superior middle temporal gyrus | Left | 193 | −39 | −61 | 13 | 0.000113 |
| Inferior parietal lobule | Left | 905 | −48 | −43 | 22 | 0.000115 |
| Intraparietal sulcus | Right | 557 | 33 | −49 | 52 | 0.000123 |
| Cuneus/occipital | Right | 352 | 3 | −76 | 25 | 0.000594 |
| Cuneus/occipital | Left | 353 | −9 | −76 | 31 | 0.000740 |
| Inferior occipital gyrus | Left | 271 | −36 | −76 | −2 | 0.000156 |
| Insula | Right | 498 | 45 | −16 | 25 | 0.000765 |
| Insula | Left | 756 | −48 | −28 | 19 | 0.000060 |
| Anterior cingulate | Left | 359 | −9 | 35 | 25 | 0.000283 |
| Posterior cingulate | Left | 786 | −3 | −31 | 43 | 0.000001 |
| Parahippocampal gyrus | Left | 479 | −21 | −43 | 7 | 0.000008 |
| Corpus callosum | Bilateral | 517 | 6 | −37 | 16 | 0.000415 |
PPI-pulse contrast BOLD signal correlated with mean percent PPI measured by EMG (r=0.51, r2=0.26, p<0.05); see text.
Fig. 2.
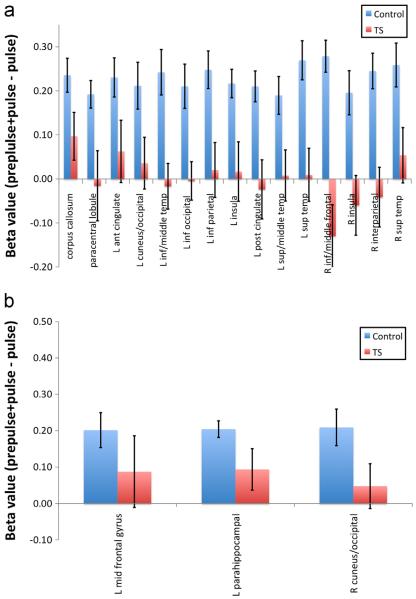
Functional brain region activation in healthy controls is associated with prepulse inhibition (PPI) in response to tactile startle. Whole-brain random effects analysis of healthy controls for PPI-pulse contrast (p<0.01, k=7) identified 18 regions of interest. Comparison of BOLD response of controls to Tourette syndrome (TS) subjects demonstrated 15 distinct brain regions which differed significantly (p<0.05) inactivity (A), while three regions did not (B). Error bars represent SEM. Co-varying for age and gender as well as co-occurring obsessive–compulsive disorder and attention deficit hyperactivity disorder and medication use across subjects with TS had no discernible effect on these findings (data not shown).
Fig. 3.
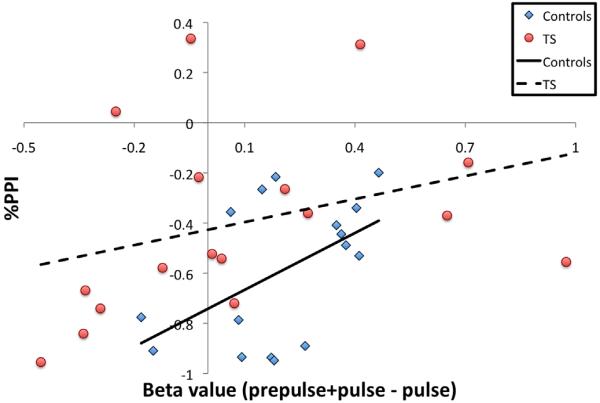
Degree of prepulse inhibition (PPI) is associated with a differential activation of the left middle frontal gyrus in healthy subjects. Regression analysis shows a significant linear relationship between the degree of PPI and the differential activation in a region within the left middle frontal gyrus (centered at x=−42; y=5; z=52; beta values [prepulse+pulse−pulse]) for healthy controls (r=0.51, r2=0.26, p<0.05), but not for subjects with TS (r=0.33, r2=0.11, p<0.05).
3.3. Differential PPI brain activation in healthy control and TS subjects
As shown in Fig. 4, a whole-brain Condition (PPI vs. pulse alone) by Group (healthy vs. TS subjects) ANOVA with random effects revealed nine distinct brain regions (p≤0.01) (see also Table 3 for additional information concerning localization and extent of activation in each of these regions). In most regions, the healthy controls exhibited increased activity in PPI compared to pulse alone with an opposite pattern of activity seen within the TS group (Fig. 5).
Fig. 4.
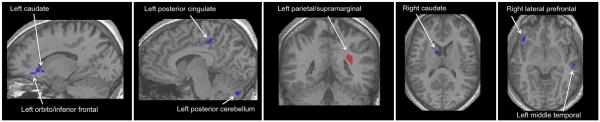
Differential PPI brain activation in healthy controls and Tourette syndrome (TS) subjects. An interaction analysis (po0.01, k=7, group [healthy controls, TS] by condition [PPI, pulse] revealed nine distinct neuroanatomical regions localized to cortical and subcortical regions (see text, Table 3, and Fig. 5 for more detail). In all brain regions with the exception of the left parietal/supramarginal cortex (red), TS individuals had significantly lower beta values (blue) than healthy individuals. Co-varying for age and gender as well as co-occurring obsessive–compulsive disorder and attention deficit hyperactivity disorder and medication use across subjects with TS had no discernible effect on these findings (data not shown). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Brain regions associated with differential activation contrasting the prepulse inhibition (PPI) condition with the startle (pulse) alone condition by diagnosis (healthy controls vs. Tourette syndrome [TS] subjects).* In all brain regions with the exception of the left parietal/supramarginal cortex, TS individuals had significantly lower beta values than healthy individuals (see Fig. 5). Brain regions are listed anterior to posterior with cortical regions followed by the cerebellum and caudate nuclei).
| Brain region | Side | Number of voxels | Peak Talairach coordinates (in mm) | Peak p | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Orbitofrontal/inferior frontal cortex | Left | 676 | −14 | 23 | −2 | 0.000028 |
| Lateral frontal cortex | Right | 326 | 36 | 32 | 13 | 0.000166 |
| Ventral lateral prefrontal cortex/anterior insula | Right | 312 | 39 | 29 | −6 | 0.000043 |
| Posterior cingulate cortex | Left | 352 | −3 | −31 | 40 | 0.000117 |
| Middle temporal gyrus | Left | 319 | −51 | −19 | −8 | 0.001158 |
| Parietal/supramarginal cortex | Left | 473 | −30 | −43 | 22 | 0.000327 |
| Posterior cerebellum | Left | 224 | −9 | −77 | −32 | 0.000730 |
| Caudate | Right | 189 | 7 | −1 | 13 | 0.000438 |
| Caudate | Left | 53 | −14 | 20 | −2 | 0.001005 |
Differences in activation after subtracting the mean beta values of the pulse condition from the PPI condition (all subjects, group [healthy controls, TS] by condition [PPI, pulse] interaction analysis [p<0.01, k=7]).
Fig. 5.
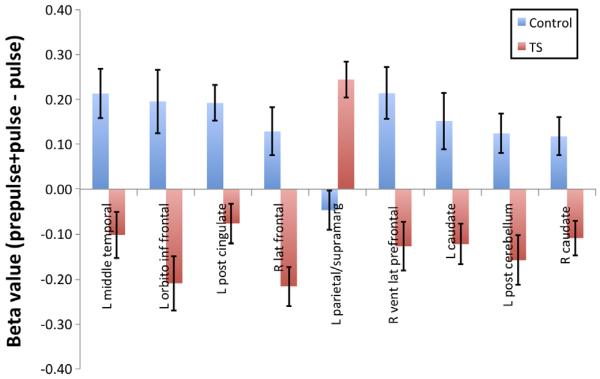
Differential brain region activation in healthy controls and Tourette syndrome (TS) subjects associated with prepulse inhibition (PPI) in response to tactile startle. Whole-brain random effects analysis of all subjects for PPI-pulse contrast (p<0.01, k=7) identified nine regions of interest. In all brain regions with the exception of the left parietal/supramarginal cortex, TS individuals had significantly lower beta values than healthy individuals. Error bars represent SEM. Covarying for age and gender as well as co-occurring obsessive–compulsive disorder and attention deficit hyperactivity disorder and medication use across subjects with TS had no discernible effect on these findings (data not shown).
3.4. Correlations with tic and OCD severity
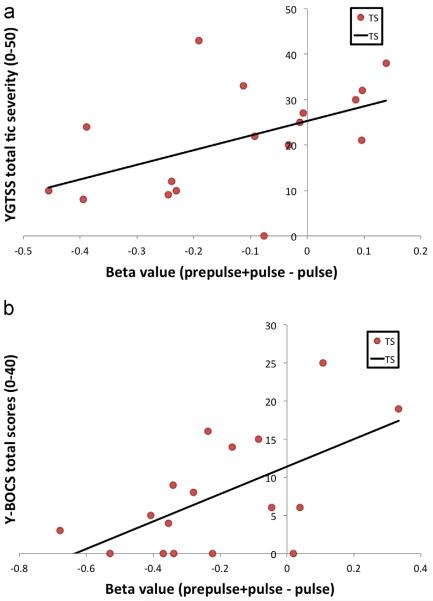
Among these nine regions, regression analyses demonstrated a significant positive linear relationship (p<0.05) between the current total tic severity score on the YGTSS in the TS population only with regard to the differential PPI BOLD activity (prepulse +pulse minus pulse alone) in the left caudate (r=0.50, r2=0.25, p<0.05) (Fig. 6A) while the right caudate approached significance (r=0.45, r2=0.20, p=0.07). No significant linear relationship was found between the worst ever tic severity score on the YGTSS and any of the nine ROIs. However, activity in the left posterior cingulate cortex approached significance (r=0.42, r2=0.18, p=0.09). In addition, a significant positive linear relationship was found between the severity of obsessive–compulsive symptoms measured with the YBOCS in the TS population and activity in the left orbito/inferior frontal cortex (r=0.59, r2=0.35, p<0.05) (Fig. 6B) while activity in the left caudate approached significance (r=0.43, r2=0.18, p=0.087).
Fig. 6.
Differential activation in the left caudate nucleus and the left orbito/inferior frontal cortex is associated with current tic and current obsessive–compulsive symptom severity, respectively (all subjects, group (controls, Tourette syndrome [TS]) by condition (PPI, pulse) interaction analysis [p<0.01, k=7]). Regression analysis shows a significant linear relationship (r=0.50, r2= 0.25, p<0.05) between the level of current tic severity* and activity in the left caudate (A), the level of current obsessive–compulsive symptom severity* and activity in the left orbito/inferior frontal cortex (r=0.59, r2=0.35, p<0.05) (B). Co-varying for age and gender as well as co-occurring obsessive–compulsive disorder and attention deficit hyperactivity disorder and medication use across subjects with TS had no discernible effect on these findings (data not shown). *YGTSS=Yale Global Tic Severity Scale; YBOCS=Yale-Brown Obsessive Compulsive Scale
In an effort to determine if our results were due to a differential response to the pulse-alone condition (pulse vs. rest) or the PPI condition (prepulse+pulse vs. rest), we also ran a set of post hoc analyses where we stratified the TS subjects based on their current total tic severity scale score into two groups: mild/remitted vs. active/moderate/severe. However, no differences were detected (see Supplemental materials).
4. Discussion
The patterns of BOLD activity seen in response to tactile startle stimuli (Table 2) in the healthy control subjects are largely congruent with earlier studies of PPI-related brain activity in healthy controls using either acoustic or tactile startle stimuli (Goldman et al., 2006; Campbell et al., 2007; Kumari et al., 2007). Although we were unable to monitor activity in the pontine midbrain nuclei, we did identify multiple (frontal, anterior cingulate, insular, temporal, and parietal) brain regions where the BOLD signal was significantly greater in the PPI condition compared to the pulse-alone condition. However, in contrast to these earlier studies, we did not detect significant activation in striatal, thalamic or hippocampal regions. The significance of the observed correlation between the BOLD response in left middle frontal gyrus and the degree of PPI (as measured outside of the magnet) is unclear as similar associations have not been reported in earlier studies using either acoustic or tactile startle (Goldman et al., 2006; Kumari et al., 2007). Specifically, Kumari et al. (2003) combined the data from six controls and seven schizophrenic patients and found significant associations between the BOLD response in the thalamus, nucleus accumbens, and inferior parietal lobe. In contrast, Goldman et al. (2006), using an event-related design in 14 healthy subjects, reported that the BOLD responses in anterior insula and cerebellum were linearly associated with the degree of PPI. Nevertheless, we did observe significant increases in the BOLD response in 18 brain regions in the healthy controls including the left and right insula and the left inferior parietal lobe. Numerous imaging studies have documented activations in these regions in association with eye blinking and eye blink inhibition in healthy controls as well as in individuals with TS (Dagenbach and Carr, 1994; Mazzone et al., 2010). We also note that in contrast to the fMRI studies of PPI-related brain activity, structural MRI studies using voxel-based morphometry have reported significant correlations between PPI of acoustic startle with grey matter volume in regions of the left frontal gyrus (Kumari et al., 2005; Hammer et al., 2013). The left dorsal premotor cortex region identified in the Hammer et al. study (centered at x=−21; y=3; z=72) overlaps with the left frontal region reported here (centered at x=−42; y=5; z=52). The healthy control subjects displayed greater tactile PPI than subjects with TS. Although the mean difference was not statistically significant (p=0.11), this trend supports the findings of earlier studies that have indicated that some individuals with TS have deficits in sensorimotor gating). The most compelling finding in the present study is that among the TS subjects there was a significant positive linear relationship between the current total tic severity score on the YGTSS and the PPI BOLD activity in the left caudate. If replicated, this suggests that activity in the caudate nucleus may be a critically important determinant of tic severity. The involvement of the caudate is consistent with earlier brain imaging and neuropathological studies (Peterson et al., 2003; Kalanithi et al., 2005; Leckman et al., 2006; Kataoka et al., 2010; Worbe et al., 2012) as well as earlier longitudinal follow-up studies where caudate volumes in childhood were found to be predictive of future tic severity in adulthood (Bloch et al., 2005). The caudate also appears to play a key role in the control of semi-voluntary behaviors including tics and eye blinks in subjects with TS (Peterson et al., 1998; Mazzone et al., 2010). There is also clear evidence that lesions to the striatum can disturb PPI in animal model systems (Kodsi and Swerdlow, 1995; Baldan Ramsey et al., 2011). It is also worth noting that although the data are largely anecdotal, virtually all TS subjects confirm that, when they are engaged in habitual behaviors that require focused attention and motor control, their tics largely disappear. These habitual behaviors are known to involve the caudate nuclei and their cortical connections (Graybiel, 2008).
In our exploratory analyses we also found a significant association between the current level of obsessive–compulsive symptom severity and differential activation in the left orbito/inferior frontal cortex during the PPI condition. Neuroimaging studies support the central role of frontal-basal ganglia–thalamic circuits in the pathophysiology of OCD. Converging lines of evidence have pointed to the abnormalities in this circuit, specifically involving orbital frontal cortex and anterior cingulate cortex, as well as the striatum and medial thalamus (Swedo et al., 1989; Rauch et al., 1994; Jenike et al., 1996; Schwartz et al., 1996; Saxena et al., 1999; Szeszko et al., 2008; Worbe et al., 2012). Although reduced PPI has been reported in OCD (Swerdlow et al., 1993; Hoenig et al., 2005; Hashimoto et al., 2008), this has not been a uniform finding (de Leeuw et al., 2010).
Finally, a closer examination of the neural activations seen during each of the three conditions (rest, pulse alone, and prepulse plus pulse) indicates that group differences are present in each of the conditions (see Supplemental materials, Tables 3S and 4S). For example, the healthy controls show widespread deactivation in response to pulse compared to rest, whereas the TS subjects show increased activity or close to zero activity. In contrast, the control subjects showed close to zero activity during PPI compared to rest, whereas TS subjects show marked activity. Of interest, a number of the regions identified in these analyses are known to be active during the period immediately prior to tic onset including the anterior cingulate and the insula (Bohlhalter et al., 2006; Hampson et al., 2009; Wang et al., 2011). If replicated, this would further strengthen the hypothesis that PPI and tic generation involve overlapping neural networks. In addition, two reports (Church et al., 2009; Worbe et al., 2012) describing immature and anomalous patterns of functional connectivity within the fronto-parietal network in adolescents with active TS are of relevance to the current findings. Indeed, several of the regions described in the Church et al. (2009) study show distinctive patterns of activation in both the active and remitted TS subjects who participated in the current study. This was particularly true in the interaction analysis of the pulse vs. the rest conditions (see Table 1S).
These initial findings provide limited support for two additional hypotheses. First, the neural networks involved in online adaptive control in the resting state may be altered in TS, perhaps due to the presence of atypical sensory inputs identified as premonitory urges in active TS cases. Alternatively, some of these interconnections may permit the failure of sensorimotor gating. Second, age-related improvements in tic severity including remission in some cases in TS, rather than simply being associated with an eventual maturation of these fronto-parietal circuits, may typically involve the development and reinforcement of anomalous patterns of functional connectivity. This perspective is consistent with the growing evidence that `learning' or the `adaptation' to atypical stimuli could alter patterns of inter-connectivity (Albert et al., 2009; Urner et al., 2013). The functional implications of the anomalous patterns of interconnectivity are unclear, but they may lead to unusual subjective sensory experiences of the sort described by individuals with TS (Bliss, 1980; Cohen and Leckman, 1992; Hollenbeck, 2001; Leckman et al., 1993). This unusual pattern of connectivity may also account for why tics largely disappear when a person with TS engages in goal directed behavior that requires focused attention and motor control including the competing responses used in habit reversal training (Piacentini et al., 2010). Future studies are needed to examine these hypotheses and alternative explanations in a larger cohort of well-characterized TS subjects. Additionally, both tactile as well as acoustic startle responses need to be assessed. Ideally, these studies would also include a more detailed analysis of the EMG data, given prior work on TS in this area (Schall et al., 1996). Based on these initial findings, future neuroimaging studies of individuals with TS whose symptoms have remitted or persist along with their unaffected siblings and typically developing individuals may permit a deeper understanding of the endophenotype of TS similar to earlier studies of OCD and autism spectrum disorders (Chamberlain et al., 2008; Kaiser et al., 2010). The use of mixed block/event-related designs may also be of value (Petersen and Dubis, 2012).
These initial findings must be interpreted in light of several limitations. First, considerable variability in PPI was observed outside of the magnet, particularly for the individuals with TS. This variability may have contributed to our failure to find a statistically significant difference in PPI across groups. This variability in PPI was also likely to contribute to variations in the patterns of neural responses seen in the BOLD response to specific conditions (rest, pulse alone, prepulse+pulse). Since we corrected and matched participants at the group level for head motion and excluded subjects who failed to show EMG and eye-blink responses to the startle stimuli outside the magnet, it is unlikely that the observed differences in BOLD activity are attributable to these confounding factors. Nonetheless, we cannot rule out other potential confounds such as background noise (associated with being in the scanner), the effects of trial repetition, and possible differences in pulse intensity. Future efforts at replication should include a large number of individuals with TS and healthy controls. The tactile startle responses also should be monitored directly while the subjects are in the magnet. A larger number of subjects will also permit a closer examination of the neural activations seen during rest, pulse alone, and prepulse plus pulse. Belluscio et al. (2011) have recently reported that TS subjects are particularly sensitive to faint sensory stimuli. Consequently, it will be important for future studies to examine the effects of faint tactile or acoustic stimuli (prepulse-alone condition). Indeed, until this question has been addressed, we will be unsure whether our findings reflect a deficit in sensorimotor gating or are simply due to the fact that TS subjects may be uniquely sensitive to faint sensory stimuli.
In addition, differences in age, gender (and menstrual cycle phase), co-occurring disorders and use of psychotropic medications could have influenced our results (Rahman et al., 2003; Jovanovic et al., 2004; Ashare et al., 2010; Holstein et al., 2013). Weighing against this possibility, however, is that co-varying for age and gender, as well as co-occurring OCD and ADHD and medication use, across subjects with TS had no discernible effects on our findings. In contrast, the small sample size precludes any definitive assessment of the potential impact of comorbid diagnoses or medications on these findings. However, the presence of a lifetime history of a mood disorder predicted significant activity in the left medial parietal cortex and the left medial paracentral and post-central gyri during the PPI-pulse condition (Table 3S). It is noteworthy that neither of these regions was determined to be a region of significant differential functional activity in healthy and TS subjects for the PPI condition in our previous analysis.
Future studies of PPI in animal models of TS have the potential to screen pharmaceutical agents for their potential therapeutic benefits (Swerdlow, 2012). For example, as reviewed by Swerdlow et al. (2006), PPI is regulated in part by norepinephrine (NE) and dopamine (DA) substrates that are neurochemically separable. Several of the agents that have been shown to have robust effects on PPI are used to treat TS. Given the recent genetic findings implicating another biogenic amine, histamine, in the pathobiology of TS (Ercan-Sencicek et al., 2010; Fernandez et al., 2012), it is intriguing that there are high levels of histamine in the striatum (Krusong et al., 2011) and that some histamine modulators can also affect the startle response (Baldan et al., 2012; Ligneau et al., 2007).
In our view, the results of this preliminary study support the hypothesis that at least some individuals with TS have sensor-imotor gating deficits. Comparable deficits in PPI and associated patterns of neural activations across TS adults, regardless of their level of current tic severity, suggest that sensorimotor gating deficits for some individuals remain even when their tics diminish. Differential patterns of activity that are correlated with current tic severity suggest that neuroplastic changes associated with lower levels of tic severity may be distinctive and vary markedly from patterns of activity seen in healthy individuals. Specifically, as presented in Fig. 6a, one might naturally expect that healthy controls would have lower beta values than the TS subjects with mild tic severity. However, the healthy controls have beta values higher than that seen in the severely affected TS subjects. The impact of these anomalous patterns of activity is unknown, but they may well contribute to a deeper understanding of the unique internal sensorimotor world that is Tourette's (Bliss, 1980; Hollenbeck, 2001).
Supplementary Material
Acknowledgements
The authors thank would also like to thank Neal R. Swerdlow for his encouragement to examine the neural substrates of Tourette syndrome and Veena Kumari for her assistance in developing the tactile startle paradigm used in this study.
Footnotes
Appendix A. Supporting information Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.pscychresns.2013.05.009.
References
- Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Current Biology. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Hawk LW, Jr., Shiels K, Rhodes JD, Pelham WE, Jr., Waxmonsky JG. Methylphenidate enhances prepulse inhibition during processing of task-relevant stimuli in attention-deficit/hyperactivity disorder. Psychophysiology. 2010;47(5):838–845. doi: 10.1111/j.1469-8986.2010.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldan LC, Williams KA, Gallezot JD, Crowley M, Pogorelov V, Anderson GM, Gorczyca R, Leventhal BL, Ohtsu H, Hughes Z, Krystal JH, Mayes LC, de Araujo I, Ding YS, State MW, Pittenger C. Histidine Decarboxylase Deficiency Produces Tourette Syndrome Phenomenology and Dopamine Dys-regulation in Humans and Mice. 2012 Annual Meeting of the American College of Neuropsychopharmacology; 2012. Abstract: W102. [Google Scholar]
- Baldan Ramsey LC, Xu M, Wood N, Pittenger C. Lesions of the dorsomedial striatum disrupt prepulse inhibition. Neuroscience. 2011;180:222–228. doi: 10.1016/j.neuroscience.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R, Staib LH, Laine AF, Hao X, Xu D, Liu J, Weissman M, Peterson BS. Anatomical brain images alone can accurately diagnose chronic neuropsychiatric illnesses. PLoS One. 2012;7(12):e50698. doi: 10.1371/journal.pone.0050698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluscio BA, Jin L, Watters V, Lee TH, Hallett M. Sensory sensitivity to external stimuli in Tourette syndrome patients. Movement Disorders. 2011;26:2538–2543. doi: 10.1002/mds.23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss J. Sensory experiences of Gilles de la Tourette syndrome. Archives of General Psychiatry. 1980;37:1343–1347. doi: 10.1001/archpsyc.1980.01780250029002. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65:1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Peterson BS, Scahill L, Otka J, Katsovich L, Zhang H, Leckman JF. Adulthood outcome of tic and obsessive–compulsive symptom severity in children with Tourette syndrome. Archives of Pediatrics and Adolescent Medicine. 2006;160:65–69. doi: 10.1001/archpedi.160.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal TD, Schicatano EJ, Chapman JG, Norris CM, Ergenzinger ER., Jr. Prepulse effects on magnitude estimation of startle-eliciting stimuli and startle responses. Perception and Psychophysics. 1996;58:73–80. doi: 10.3758/bf03205477. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Elden A, Flaten MA. A comparison of several methods used to quantify prepulse inhibition of eyeblink responding. Psychophysiology. 2004;41(2):326–332. doi: 10.1111/j.1469-8986.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, Wurzman R, Hallett M. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129:2029–2037. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- Borelli JL, Crowley MJ, David DH, Sbarra DA, Anderson GM, Mayes LC. Attachment and emotion in school-aged children. Emotion. 2010;10(4):475–485. doi: 10.1037/a0018490. [DOI] [PubMed] [Google Scholar]
- Campbell LE, Hughes M, Budd TW, Cooper G, Fulham WR, Karayanidis F, Hanlon MC, Stojanov W, Johnston P, Case V, Schall U. Primary and secondary neural networks of auditory prepulse inhibition: a functional magnetic resonance imaging study of sensorimotor gating of the human acoustic startle response. European Journal of Neuroscience. 2007;26:2327–2333. doi: 10.1111/j.1460-9568.2007.05858.x. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette's syndrome and ADHD: preliminary results. Biological Psychiatry. 1996;39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, Aitken M, Craig K, Owen AM, Bullmore ET, Robbins TW, Sahakian BJ. Orbitofrontal dysfunction in patients with obsessive–compulsive disorder and their unaffected relatives. Science. 2008;321:421–422. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- Church JA, Fair DA, Dosenbach NU, Cohen AL, Miezin FM, Petersen SE, Schlaggar BL. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain. 2009;132:225–238. doi: 10.1093/brain/awn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, Leckman JF. Sensory phenomena associated with Gilles de la Tourette's syndrome. Journal of Clinical Psychiatry. 1992;53:319–323. [PubMed] [Google Scholar]
- Dagenbach D, Carr TH, editors. Inhibitory Processes in Attention, Memory, and Language. Academic Press; San Diego: 1994. [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Archives of General Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Ercan-Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O'Roak BJ, Mason CE, Abbott T, Gupta A, King RA, Pauls DL, Tischfield JA, Heiman GA, Singer HS, Gilbert DL, Hoekstra PJ, Morgan TM, Loring E, Yasuno K, Fernandez T, Sanders S, Louvi A, Cho JH, Mane S, Colangelo CM, Biederer T, Lifton RP, Gunel M, State MW. L-histidine decarboxylase and Tourette's syndrome. The New England Journal of Medicine. 2010;362:1901–1908. doi: 10.1056/NEJMoa0907006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez TV, Sanders SJ, Yurkiewicz IR, Ercan-Sencicek AG, Kim YS, Fishman DO, Raubeson MJ, Song Y, Yasuno K, Ho WS, Bilguvar K, Glessner J, Chu SH, Leckman JF, King RA, Gilbert DL, Heiman GA, Tischfield JA, Hoekstra PJ, Devlin B, Hakonarson H, Mane SM, Gunel M, State MW. Rare copy number variants in tourette syndrome disrupt genes in histaminergic pathways and overlap with autism. Biological Psychiatry. 2012;71:392–402. doi: 10.1016/j.biopsych.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Heidinger L, Kulkarni K, Zhu DC, Chien A, McLaren DG, Shah J, Coffey CE, Jr., Sharif S, Chen E, Uftring SJ, Small SL, Solodkin A, Pilla RS. Changes in the amplitude and timing of the hemodynamic response associated with prepulse inhibition of acoustic startle. NeuroImage. 2006;32:1375–1384. doi: 10.1016/j.neuroimage.2006.04.228. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Archives of General Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annual Review of Neuroscience. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, Green MF, Gur RE, Gur RC, Hardiman G, Kelsoe JR, Leonard S, Light GA, Nuechterlein KH, Olincy A, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Freedman R, Braff DL. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. American Journal of Psychiatry. 2011;168(9):930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Southwick SM, Davis M, Charney DS. Baseline startle amplitude and prepulse inhibition in Vietnam veterans with posttraumatic stress disorder. Psychiatric Research. 1996;64(3):169–178. doi: 10.1016/s0165-1781(96)02942-3. [DOI] [PubMed] [Google Scholar]
- Hammer TB, Oranje B, Skimminge A, Aggernæs B, Ebdrup BH, Glenthøj B, Baaré W. Structural brain correlates of sensorimotor gating in antipsychotic-naive men with first-episode schizophrenia. Journal of Psychiatry and Neuroscience. 2013;38(1):34–42. doi: 10.1503/jpn.110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, King RA, Constable RT, Leckman JF. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biological Psychiatry. 2009;65:594–599. doi: 10.1016/j.biopsych.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Shimizu E, Koike K, Orita Y, Suzuki T, Kanahara N, Matsuzawa D, Fukami G, Miyatake R, Shinoda N, Fujisaki M, Shirayama Y, Hashimoto K, Iyo M. Deficits in auditory P50 inhibition in obsessive–compulsive disorder. Progress in Neuro-psychopharmacology and Biological Psychiatry. 2008;32:288–296. doi: 10.1016/j.pnpbp.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS. Sensorimotor gating deficits and hypofrontality in schizophrenia. Frontiers in Bioscience. 2001;6:D1069–D1072. doi: 10.2741/hazlett. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Zhang J, Newmark RE, Glanton CF, Zelmanova Y, Haznedar MM, Chu KW, Nenadic I, Kemether EM, Tang CY, New AS, Siever LJ. Frontal-striatal thalamic mediodorsal nucleus dysfunction in schizophrenia spectrum patients during sensorimotor gating. NeuroImage. 2008;42:1164–1177. doi: 10.1016/j.neuroimage.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenig K, Hochrein A, Quednow BB, Maier W, Wagner M. Impaired prepulse inhibition of acoustic startle in obsessive–compulsive disorder. Biological Psychiatry. 2005;57:1153–1158. doi: 10.1016/j.biopsych.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ. Insight and hindsight into Tourette syndrome. Advances in Neurology. 2001;85:363–367. [PubMed] [Google Scholar]
- Holstein DH, Vollenweider FX, Geyer MA, Csomor PA, Belser N, Eich D. Sensory and sensorimotor gating in adult attention-deficit/hyperactivity disorder (ADHD) Psychiatry Research. 2013;205(1–2):117–126. doi: 10.1016/j.psychres.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Jenike MA, Breiter HC, Baer L, Kennedy DN, Savage CR, Olivares MJ, O'Sullivan RL, Shera DM, Rauch SL, Keuthen N, Rosen BR, Caviness VS, Filipek PA. Cerebral structural abnormalities in obsessive–compulsive disorder. A quantitative morphometric magnetic resonance imaging study. Archives of General Psychiatry. 1996;53:625–632. doi: 10.1001/archpsyc.1996.01830070073011. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Szilagyi S, Chakravorty S, Fiallos AM, Lewison BJ, Parwani A, Schwartz MP, Gonzenbach S, Rotrosen JP, Duncan EJ. Menstrual cycle phase effects on prepulse inhibition of acoustic startle. Psychophysiology. 2004;41(3):401–406. doi: 10.1111/1469-8986.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Hudac CM, Shultz S, Lee SM, Cheung C, Berken AM, Deen B, Pitskel NB, Sugrue DR, Voos AC, Saulnier CA, Ventola P, Wolf JM, Klin A, Vander Wyk BC, Pelphrey KA. Neural signatures of autism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, Schwartz ML, Leckman JF, Vaccarino FM. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. Journal of Comparative Neurology. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifa N, von Knorring AL. Prevalence of tic disorders and Tourette syndrome in a Swedish school population. Developmental Medicine and Child Neurology. 2003;45:315–319. doi: 10.1017/s0012162203000598. [DOI] [PubMed] [Google Scholar]
- Khalifa N, von Knorring AL. Psychopathology in a Swedish population of school children with tic disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1346–1353. doi: 10.1097/01.chi.0000251210.98749.83. [DOI] [PubMed] [Google Scholar]
- Kodsi MH, Swerdlow NR. Ventral pallidal GABA-A receptors regulate prepulse inhibition of acoustic startle. Brain Research. 1995;684:26–35. doi: 10.1016/0006-8993(95)00372-w. [DOI] [PubMed] [Google Scholar]
- Krusong K, Ercan-Sencicek AG, Xu M, Ohtsu H, Anderson GM, State MW, Pittenger C. High levels of histidine decarboxylase in the striatum of mice and rats. Neuroscience Letters. 2011;495:110–114. doi: 10.1016/j.neulet.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Geyer MA, ffytche D, Soni W, Mitterschiffthaler MT, Vythelingum GN, Simmons A, Williams SC, Sharma T. Neural correlates of tactile prepulse inhibition: a functional MRI study in normal and schizophrenic subjects. Psychiatry Research. 2003;122:99–113. doi: 10.1016/s0925-4927(02)00123-3. [DOI] [PubMed] [Google Scholar]
- Kumari V, Antonova E, Zachariah E, Galea A, Aasen I, Ettinger U, Mitterschiffthaler MT, Sharma T. Structural brain correlates of prepulse inhibition of the acoustic startle response in healthy humans. NeuroImage. 2005;26(4):1052–1058. doi: 10.1016/j.neuroimage.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Kumari V, Antonova E, Geyer MA, Ffytche D, Williams SC, Sharma T. A fMRI investigation of startle gating deficits in schizophrenia patients treated with typical or atypical antipsychotics. International Journal of Neuropsycho-pharmacology. 2007;10:463–477. doi: 10.1017/S1461145706007139. [DOI] [PubMed] [Google Scholar]
- Kumari V, Fannon D, Geyer MA, Premkumar P, Antonova E, Simmons A, Kuipers E. Cortical grey matter volume and sensorimotor gating in schizophrenia. Cortex. 2008;44(9):1206–1214. doi: 10.1016/j.cortex.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF. Tourette's syndrome. Lancet. 2002;360:1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- Leckman JF. Tic disorders. British Medical Journal. 2012;344:d7659. doi: 10.1136/bmj.d7659. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Archives of General Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen DJ. The Yale Global tic severity scale: initial testing of a clinician-rated scale of tic severity. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Walker DE, Cohen DJ. Premonitory urges in Tourette's syndrome. American Journal of Psychiatry. 1993;150:98–102. doi: 10.1176/ajp.150.1.98. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Zhang H, Vitale A, Lahnin F, Lynch K, Bondi C, Kim YS, Peterson BS. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102:14–19. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Vaccarino FM, Kalanithi PS, Rothenberger A. Annotation: Tourette syndrome: a relentless drumbeat-driven by misguided brain oscillations. Journal of Child Psychology and Psychiatry. 2006;47:537–550. doi: 10.1111/j.1469-7610.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Bloch MH, Smith ME, Larabi D, Hampson M. Neurobiological substrates of Tourette's disorder. Journal of Child and Adolescent Psychopharmacology. 2010;20:237–247. doi: 10.1089/cap.2009.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligneau X, Landais L, Perrin D, Piriou J, Uguen M, Denis E, Robert P, Parmentier R, Anaclet C, Lin JS, Burban A, Arrang JM, Schwartz JC. Brain histamine and schizophrenia: potential therapeutic applications of H3-receptor inverse agonists studied with BF2.649. Biochemical Pharmacology. 2007;73:1215–1224. doi: 10.1016/j.bcp.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Mazzone L, Yu S, Blair C, Gunter BC, Wang Z, Marsh R, Peterson BS. An FMRI study of frontostriatal circuits during the inhibition of eye blinking in persons with Tourette syndrome. American Journal of Psychiatry. 2010;167:341–349. doi: 10.1176/appi.ajp.2009.08121831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol Debes NM, Hjalgrim H, Skov L. Validation of the presence of comorbidities in a Danish clinical cohort of children with Tourette syndrome. Journal of Child Neurology. 2008;23:1017–1027. doi: 10.1177/0883073808316370. [DOI] [PubMed] [Google Scholar]
- Pauls DL, Hurst C. Child Study Center. Yale University; New Haven, CT: 1993. Schedule for Tourette and Other Behavioral Disorders, Revised. [Google Scholar]
- Petersen SE, Dubis JW. The mixed block/event-related design. NeuroImage. 2012;62:1177–1184. doi: 10.1016/j.neuroimage.2011.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, Leckman JF, Gore JC. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Archives of General Psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, King RA, Leckman JF, Staib L. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Archives of General Psychiatry. 2003;60:415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Choi HA, Hao X, Amat JA, Zhu H, Whiteman R, Liu J, Xu D, Bansal R. Morphologic features of the amygdala and hippocampus in children and adults with Tourette syndrome. Archives of General Psychiatry. 2007;64(11):1281–1291. doi: 10.1001/archpsyc.64.11.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, Ginsburg GS, Deckersbach T, Dziura J, Levi-Pearl S, Walkup JT. Behavior therapy for children with Tourette disorder: a randomized controlled trial. Journal of the American Medical Association. 2010;303:1929–1937. doi: 10.1001/jama.2010.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessen KJ, Bansal R, Peterson BS. Imaging evidence for anatomical disturbances and neuroplastic compensation in persons with Tourette syndrome. Journal of Psychosomatic Research. 2009;67:559–573. doi: 10.1016/j.jpsychores.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P, Gray JA, Sharma T, Geyer M, Mehrotra R, Das M, Zachariah E, Hines M, Williams SC, Kumari V. A behavioural and functional neuroimaging investigation into the effects of nicotine on sensorimotor gating in healthy subjects and persons with schizophrenia. Psychopharmacology (Berlin) 2006;184:589–599. doi: 10.1007/s00213-006-0307-5. [DOI] [PubMed] [Google Scholar]
- Rahman Q, Kumari V, Wilson GD. Sexual orientation-related differences in prepulse inhibition of the human startle response. Behavioral Neuroscience. 2003;117(5):1096–1102. doi: 10.1037/0735-7044.117.5.1096. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, Fischman AJ. Regional cerebral blood flow measured during symptom provocation in obsessive–compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Archives of General Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Maidment KM, Dunkin JJ, Colgan M, Alborzian S, Phelps ME, Baxter LR., Jr. Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive–compulsive disorder. Neuropsychopharmacology. 1999;21:683–693. doi: 10.1016/S0893-133X(99)00082-2. [DOI] [PubMed] [Google Scholar]
- Scahill LBRH, Visser SN, Blumberg SJ. Prevalence of Diagnosed Tourette Syndrome in Persons Aged 6–17 Years—United States, 2007. Morbidity and Mortality Weekly Report. 2009;58:581–585. [PubMed] [Google Scholar]
- Schall U, Schon A, Zerbin D, Eggers C, Oades RD. Event-related potentials during an auditory discrimination with prepulse inhibition in patients with schizophrenia, obsessive–compulsive disorder and healthy subjects. International Journal of Neuroscience. 1996;84:15–33. doi: 10.3109/00207459608987247. [DOI] [PubMed] [Google Scholar]
- Schwartz JM, Stoessel PW, Baxter LR, Jr., Martin KM, Phelps ME. Systematic changes in cerebral glucose metabolic rate after successful behavior modification treatment of obsessive–compulsive disorder. Archives of General Psychiatry. 1996;53:109–113. doi: 10.1001/archpsyc.1996.01830020023004. [DOI] [PubMed] [Google Scholar]
- Smith SJ, Lees AJ. Abnormalities of the blink reflex in Gilles de la Tourette syndrome. Journal of Neurology, Neurosurgery, and Psychiatry. 1989;52:895–898. doi: 10.1136/jnnp.52.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedo SE, Schapiro MB, Grady CL, Cheslow DL, Leonard HL, Kumar A, Friedland R, Rapoport SI, Rapoport JL. Cerebral glucose metabolism in childhood-onset obsessive–compulsive disorder. Archives of General Psychiatry. 1989;46:518–523. doi: 10.1001/archpsyc.1989.01810060038007. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR. Update: Studies of prepulse inhibition of startle, with particular relevance to the pathophysiology or treatment of Tourette syndrome. Neuroscience and Bio-Behavioral Reviews 2012. 2012 doi: 10.1016/j.neubiorev.2012.09.002. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biological Psychiatry. 1993;33:298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Karban B, Ploum Y, Sharp R, Geyer MA, Eastvold A. Tactile prepuff inhibition of startle in children with Tourette's syndrome: in search of an “fMRI-friendly” startle paradigm. Biological Psychiatry. 2001;50:578–585. doi: 10.1016/s0006-3223(01)01164-7. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Bongiovanni MJ, Tochen L, Shoemaker JM. Separable noradrenergic and dopaminergic regulation of prepulse inhibition in rats: implications for predictive validity and Tourette syndrome. Psychopharmacology. 2006;186:246–254. doi: 10.1007/s00213-006-0374-7. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berlin) 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Christian C, Macmaster F, Lencz T, Mirza Y, Taormina SP, Easter P, Rose M, Michalopoulou GA, Rosenberg DR. Gray matter structural alterations in psychotropic drug-naive pediatric obsessive–compulsive disorder: an optimized voxel-based morphometry study. American Journal of Psychiatry. 2008;165:1299–1307. doi: 10.1176/appi.ajp.2008.08010033. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Georg Thieme, Stuttgart; New York: 1988. [Google Scholar]
- Urner M, Schwarzkopf DS, Friston K, Rees G. Early visual learning induces long-lasting connectivity changes during rest in the human brain. NeuroImage. 2013 doi: 10.1016/j.neuroimage.2013.03.050. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Maia TV, Marsh R, Colibazzi T, Gerber A, Peterson BS. The neural circuits that generate tics in Tourette's syndrome. American Journal of Psychiatry. 2011;168:1326–1337. doi: 10.1176/appi.ajp.2011.09111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbe Y, Malherbe C, Hartmann A, Pelegrini-Issac M, Messe A, Vidailhet M, Lehericy S, Benali H. Functional immaturity of cortico-basal ganglia networks in Gilles de la Tourette syndrome. Brain. 2012;135:1937–1946. doi: 10.1093/brain/aws056. [DOI] [PubMed] [Google Scholar]
- de Leeuw AS, Oranje B, van Megen HJ, Kemner C, Westenberg HG. Sensory gating and sensorimotor gating in medication-free obsessive–compulsive disorder patients. International Clinical Psychopharmacology. 2010;25:232–240. doi: 10.1097/yic.0b013e328338c4f0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.