Abstract
Recent investigations have expanded our knowledge of the regulatory bone marrow (BM) niche, which is critical in maintaining and directing hematopoietic stem cell (HSC) self-renewal and differentiation. Osteoblasts, mesenchymal stem cells (MSCs), and CXCL12-abundant reticular (CAR) cells are niche components in close association with HSCs and have been more clearly defined in immune cell function and homeostasis. Importantly, cellular inhabitants of the BM niche signal through G protein-coupled surface receptors (GPCRs) for various appropriate immune functions. In this article, recent literature on BM niche inhabitants (HSCs, osteoblasts, MSCs, CAR cells) and their GPCR mechanistic interactions are reviewed for better understanding of the BM cells involved in immune development, immunologic disease, and current immune reconstitution therapies.
Keywords: Bone marrow (BM), Endosteal niche, Perivascular niche, Hematopoietic stem cells (HSCs), Mesenchymal stem cells (MSCs), CXCL12-abundant reticular (CAR) cells, Osteoblasts, G protein-coupled receptors (GPCRs), G protein-coupled receptor kinase (GRK), Hematopoietic cell transplantation (HCT), Hematopoietic stem cell transplantation (HSCT), CXCL12, CXCR4, Sphingosine-1-phosphate (S1P), Sphingosine-1-phosphate receptor (S1PR), Parathyroid hormone (PTH), Parathyroid hormone receptor (PTH1R), Granulocyte colony-stimulating factor (G-CSF), AMD3100, FTY720, SEW2871
Introduction
Defining the bone marrow niche
In 1961, Till and McCulloch described the proliferative capacity in vivo of normal adult mouse bone marrow (BM) cells by counting gross nodules in the spleen of an irradiated recipient [1]. Till and McCulloch proposed that each spleen colony may be derived from one cell of “clonal origin” that microscopically resembles hematopoietic cells. This theory was later experimentally demonstrated in 1963 and is the foundation of the modern Colony Forming Unit Spleen assay (CFU-S) [2]. Five years later, Friedenstein et al. observed suspended BM cells could form colony fibroblasts from single cells [3]. Collectively, these studies strengthened the concept of proliferative hematopoietic [1, 2, 4, 5] and fibroblastic stromal cells [3, 6–11] residing in the BM. In 1978, Schofield proposed that stem cells associate with other cells to determine future behaviors, coining these stem cell interactions as a “niche” [12, 13]. Together, these observations and their aforementioned advancements established a foundation to propel future investigations that have since revealed the cellular complexity of niche inhabitants and interactions, critical to hematopoiesis.
Clinical and historical relevance: Transplantation
There has been extensive research exploring various hematopoietic cell transplantation methods and the importance of human leukocyte antigen (HLA) typing. These topics have been reviewed elsewhere and will not be the focus of this review. However, distinct and consistent terminology identifying each type of transplantation method is vital for communication and to move the field forward. Therefore, for this review, hematopoietic cell transplantation (HCT) will refer to the collection and transplantation of whole BM as a hematopoietic stem cell (HSC) source, whereas hematopoietic stem cell transplantation (HSCT) will refer to transplantation of either BM collection for HSC purification or peripheral blood collection for mobilized HSCs from BM. In addition, autologous (same donor and recipient) and allogeneic HSCT (same species, different donor and recipient) are two different graft or donor transplants that may play roles in the success of patient outcomes. Allogeneic transplantation may involve inadvertent transplantation of donor T lymphocytes along with beneficial HSCs from the peripheral blood, which can elicit graft-versus-host disease (GvHD), causing treatment complications. Further investigation into the complexity of the BM niche could contribute to the development of an improved transplantation model system that efficiently reconstitutes the immune system, reduces adverse effects to the patient, and alleviates disease.
The concept of HCT was developed in the 1950s by E. Donnall Thomas when his research investigations revealed human BM cell infusions could repopulate the BM and create new blood cells. Dr. Thomas performed the first successful marrow graft transplant in 1959 between monozygotic twins, of which one twin was diagnosed with refractory leukemia [14]. In 1968, Robert Good and colleagues performed the first successful non-malignancy HCT from a sibling to treat immune deficiency in an infant brother [15]. Dr. Thomas and colleagues then performed their first HCT using a HLA-matched sibling donor in 1969 [14]. HCT became standard of care over the next several decades as an approach to address multiple forms of malignant and non-malignant diseases [16]. Recently, an extensive global study involving investigations of 72 countries reported an increase in HCT from 46,563 in 2006 to 51,536 in 2008 [17], as an approach to treat malignancies, as well as immune deficiency, autoimmunity and hereditary diseases [16, 18, 19].
Further advancements in BM niche investigations and transplantation studies have revealed the importance of specific proliferative cell populations—the BM stem cells. Research efforts began to focus on the stem cell populations of HCT, which created an HSC selection transplantation model. HSCT commonly involves an administration of a stimulating factor that releases BM HSCs into the blood to ease the collection for transplantation use. However, HSCT is a high cost, specialized procedure that is still associated with significant morbidity and mortality [20], including GvHD when allogeneic donors are used. HSCT is also associated with variable patient immune reconstitution outcomes due to multiple factors, such as HLA matching, major histocompatibility (MHC) region variations, and genetic factors that may affect immune responses [21]. Interestingly, it has been shown that transplantation of mobilized HSCs in peripheral blood fuels immune reconstitution more efficiently than HSC from the BM [22], allowing for faster hematopoietic recovery, shorter hospital stays, and similar early survival outcomes [23]. Recent findings in a worldwide study show peripheral blood was used as a source for stem cells in 98 % of autologous transplants and 64 % of allogeneic transplants, whereas BM was used as a source of stem cells in 2 % autologous transplants and 26 % allogeneic transplants [17].
HCT is utilized to treat multiple forms of cancer and hereditary diseases, while specialized HSCT is also a potential treatment under continuous refinement. Interestingly, Jansen et al. in 2005 suggested that specific diseases and their stages may direct the sources of cells for transplantation (ie: HCT vs. HSCT). Patients with “good-prognostic” leukemia may more readily benefit from HCT, whereas the preferred therapy for patients with high-risk disease may be HSCT from mobilized HSCs [22]. This suggests that transplant therapy may differ in approach and stem cell source depending on the disease and its prognosis. For example, clinical studies by Mancardi investigated HCT (with no specific stem cell selection) for treatment of an autoimmune disease, multiple sclerosis, which showed promising results of decreased relapses and active lesion load by MRI [24, 25]. Whereas recent clinical trials are investigating utilization of HSCT for treatment of another autoimmune disease, systemic sclerosis (SSc)/scleroderma, clinically characterized as an excessive accumulation of collagen in skin and organs resulting from vasculopathy, inflammation, and fibrosis with few therapeutic options [26, 27]. HSCT results show improved skin and functional abilities as well as long-term survival; however, the benefit of HSCT in comparison to the risk of adverse effects and HSCT-related mortality (6–10 %) is still under critical evaluation [27].
There is also research investigating the use of mesenchymal stem cells (MSCs) in over 200 small clinical trials for therapeutic effects on hematopoietic malignancies, GvHD, bone repair, myocardial infarction, Alzheimer’s/Parkinson’s, liver cirrhosis, and several autoimmune diseases [28]. It has been expected that infusion of allogeneic MSCs would initiate an immune response, but interestingly MSC transplantation to our knowledge has shown no adverse events [28], suggesting they may be immune-privileged. In pre-clinical non-human primate studies, co-transplantation of MSCs improved engraftment of HSCs after autologous HCT [29]. However, precise identification of cellular and molecular interactions among MSCs and HSCs remains relatively undefined.
Extensive research efforts for technological and biological advancements have been put forth to better define and understand the BM niche and stem cells involved in immune development, immunologic disease, and current immune reconstitution therapies. The overall goal of this article is to define regions of the BM niche, describe key BM inhabitants and their mechanistic interactions, and address how targeting such inhabitants or interactions may serve as therapeutic strategies.
Key inhabitants of the bone marrow niche
Hematopoietic stem cells
HSCs are the most primitive cells within the BM that give rise to myeloid lineage-derived blood cells (neutrophils, monocytes, basophils, eosinophils, erythrocytes, and megakaryocytes), and lymphoid lineage-derived immune cells (natural killer, NK, cells and T/B lymphocytes). HSCs are predominantly found in the BM, though a limited number may also be found in the peripheral blood. HSCs have the ability to migrate into the peripheral blood during stress responses, such as during inflammation or bleeding [30, 31]. Thus, the self-renewal, multipotency, and mobility properties of HSCs can be utilized for therapeutic applications.
Irving Weissman and colleagues described the phenotypic characteristics of undifferentiated human HSCs as having a lack of markers indicative of specific lineage commitment, termed lineage marker negative (Lin−). Human Lin− HSCS have additional surface expression of markers CD34, CD90/Thy1, yet low to negative expression of CD38 and CD45RA [32]. Mouse Lin− HSCs were defined as Sca-1+, c-Kit+, Slamf1+, and with negative expression of CD34 and Flk-2 [32]. Some HSCs are thought to remain dormant to preserve a long-term self-renewal population pool in the BM niche, whereas other HSCs actively self-renew to aid in the maintenance of hematopoiesis [33]. In specific, dormant Lin− Sca1+ c-Kit+, CD150+CD48−CD34− HSCs may give rise to the more active CD34+ HSCs [34].
Osteoblasts
Osteoblasts are responsible for bone formation via synthesis, deposition, and mineralization of extracellular matrix and reside at the endosteum and medullary cavity interface within the BM. Osteoblasts have been shown to support immature HSC expansion in vitro and secrete multiple factors to regulate HSC activity [35, 36]. In addition, increased number of osteoblasts, via constitutively activated parathyroid hormone (PTH) receptor, correlated with an increased number of HSCs in vivo [37]. A simultaneous study reported an increase in spindle-shaped N-cadherin+CD45− osteoblastic (SNO) cells lining the bone surface correlated with an increase in HSCs and proposed long-term HSCs associate with SNO cells [38]. Regulation of HSC proliferation and function by osteoblasts in a subregion of the BM niche was further shown in studies that investigated the removal of osteoblasts, which correlated with reduced BM cellularity and 3- to 10-fold reductions in HSCs [39]. Although collective data support osteoblasts as key components in the BM niche, specific interactions between HSCs and progenitor cells remain an area of heavy investigation.
Mesenchymal stem cells
BM-derived MSCs are “stromal-like” cells that are multipotent, self-renewing precursor cells that can differentiate into adipocytes, osteocytes, and chondrocytes [40]. Studies have focused on characterization of these MSC subpopulations with the hope to better define and understand their biological activity and therapeutic potentials.
Evaluation of BM MSC cell markers have been reviewed for both human and mouse [28], as various MSC phenotypes have been reported. Human CD146+CD45− [41] and mouse Nestin+CD45−CD31− MSCs [42] demonstrate stem cell activity by CFU-fibroblast sphere formations and in vivo self-renewal. A recent investigation utilized an extensive flow cytometry screening panel to show mouse MSCs highly express 13 markers [43], in which 3 novel MSC markers were identified to differentiate between distinct subtypes of MSCs. CD200 was characteristic for osteogenic MSCs; SSEA4 and CD140a were expressed on adipogenic progenitors [43]. Similarly, an extensive screening of human MSCs markers were investigated from 53 donors of diverse age and sex. Results showed younger donors had increased expression of 7 markers, including CD146; younger female donors possessed a more frequent and rapidly proliferative clonogenic MSC population; and mesodermal differentiation was unaffected by donor age or gender [44]. Overall, these investigations touch upon the MSC phenotypes within the BM niche. With continued research, a better understanding and utilization of defined MSC subpopulations and their functional properties, as well as potential donor-related effects, may enhance stem cell therapy patient outcomes.
CXCL12-abundant reticular (CAR) cells
In 2006, work from the Nagasawa laboratory identified a unique, small population of reticular cells with long processes that were uniformly dispersed in mouse BM. These reticular cells secreted high levels of CXCL12, and thus were termed CXCL12-abundant reticular (CAR) cells. CAR cells surround the sinusoidal endothelial cells [45, 46] in association with CD150+CD48−CD41− HSCs, early B cell precursors and plasma cells [47–50], plasmacytoid dendritic cells [51] and NK cells [52] in the perivascular niche. CAR cells express VCAM-1, CD44, and PDGFRα/β and do not express surface makers Sca1, CD45, or CD31 [47]. The human equivalent of mouse CAR cells are currently thought to be the subendothelial cells with CD146 expression [41].
Disruption of the CAR cell population alters the steady state and HSC populations within the BM niche. Selective depletion of CAR cells reduced the adipogenic and osteogenic differentiation ability of BM cells, reduced the size and number of HSCs, and reduced B cell and erythroid progenitors, but no immediate effects on osteoblasts and endothelial cells were observed [53]. These and other data further confirm CAR cells may be required for proliferation and maintenance of HSCs, B cells, and erythroid progenitor cells [47, 53]. Interestingly, studies by Visnjic et al. and Zhu et al. of osteoblastic lineage cell depletion also demonstrated a reduction in both erythroid progenitors and B cells [39, 54]. Therefore, while depletion of niche components has been shown to alter steady state BM niches, the specific cells, interactions, and locations of immune development may need to be further defined within the BM niche.
In summary, collective data suggest HSCs, osteoblasts, MSCs, and CAR cells participate in the intricate complexity of BM niche interactions during immune cell development and may serve as vital components for clinical therapeutic strategies.
Functional regions of the bone marrow niche
Endosteal and perivascular niche
Immune cell development takes place within a regulatory BM niche that has been conceptually divided into two main functional regions, the endosteal and perivascular niche. HSCs, MSCs [28], CAR cells [46], and osteoblasts [37] have all been implicated as key components in these functional regions. The endosteal niche resides at the endosteum and medullary cavity interface within the BM where historically osteoblasts are thought to sustain HSC quiescence, whereas the microenvironment of sinusoidal vessels surrounded by endothelial cells and subendothelial stromal cells comprise the perivascular BM niche. It is thought that HSCs preferentially associate with the perivascular region [55] due to nearby stromal CAR cells, which readily secrete a key chemoattractant chemokine CXCL12. HSCs and CAR cells are nestled within the perivascular niche with a self-renewing MSC population identified by the presence of an intermediate filament protein, Nestin [56]. Nestin-positive MSCs have been linked to influence HSC migration and maintenance [42].
A vexing question lingers as to whether particular immune cells or certain developmental stages are specific to specialized compartments, as opposed to sharing one common BM niche. This was highlighted in two recent and simultaneously released studies. Ding and Morrison demonstrated the diverse effects on various HSC or progenitor cell populations during controlled CXCL12 deletion studies [57]. Selective CXCL12 deletions in osteoblasts within the endosteal niche showed depletion of only early lymphoid progenitors. In contrast, depletion of CXCL12 in perivascular niche endothelial and perivascular stromal cells demonstrated depletion of HSCs. Depletion of perivascular stromal cells also depleted specific progenitors and forced egression outside the BM. This study suggests distinctive stem and progenitor cell subsets reside in specific BM niches. Specifically, early lymphoid progenitors [57] and long-lived, dormant HSCs [58] may more readily occupy the endosteal niche, whereas the perivascular niche may more readily house active HSCs [58]. Greenbaum et al. also reported CXCL12 deletion studies and concluded similar findings of osteoblastic and CAR cells support B-lymphoid progenitor cells and retain hematopoietic progenitor cells (HPCs), whereas the perivascular niche supports HSCs [59].
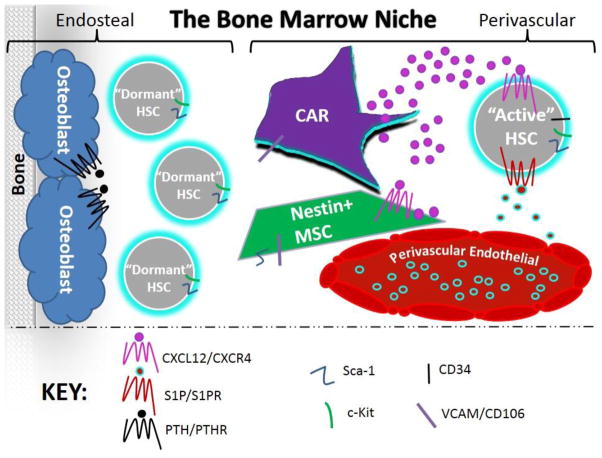
Interesting new data implicate megakaryocytes as an additional component of the HSC niche that may regulate HSC proliferation through cytokine release of IGF-1 and IGFBP-3 [60]. Neuronal/glial cells, adipocytes, osteoclasts, and monocytes/macrophages have also newly demonstrated roles in the BM niche, though these have been reviewed elsewhere and are beyond the scope of this particular review [61]. In summary, recent data strengthen the concept of distinct functional regions within the BM that house specific developmental stages of immune cell development (Fig. 1), and additional subregions may be defined by future studies.
Figure 1. The bone marrow niche.
Long-lived, dormant hematopoietic stem cells (HSCs) associate with osteoblasts to sustain quiescence in the endosteal niche and serve as the primitive cell “reservoir” within the bone marrow (BM). HSCs that reside within the perivascular niche associate with Nestin-positive mesenchymal stem cells (MSCs) and CXCL12-abundant reticular (CAR) cells that secrete CXCL12 to influence HSC maintenance and activity by either retaining cells within the BM or allowing cells into circulation, which may be impacted by the balance and regulation of CXCL12/CXCR4 and S1P/S1P1R.
G protein-coupled receptor (GPCR) molecular interactions within the bone marrow niche
Overview
G protein-coupled receptors (GPCRs) consist of seven transmembrane domains that elicit intracellular signaling upon extracellular ligand stimulation. Ligand and GPCR interactions force conformational change and elicit activation of guanine nucleotide binding proteins, referred to as G proteins [62]. G proteins (α,β/γ) dissociate from the GPCR and initiate downstream signaling [62]. GPCRs may also signal G-protein independent pathways, including negative regulation of receptor expression by G protein-coupled receptor kinases (GRKs) [62]. GPCRs encompass 3–5 % of the human genome and play vital roles in various physiological functions, serving as ideal therapeutic targets. In fact, 20–50 % of market pharmaceuticals focus on GPCR targeting [40].
One area of GPCR signaling is to elicit fundamental processes for immune development and function [40]. Without these interactions, cellular survival, proliferation, differentiation, or trafficking may be severely compromised. This review focuses on three particular GPCRs that have been implicated in BM immune cell function: PTH-receptor, CXCR4, and sphingosine-1-phosphate receptor (S1PR).
PTH/PTH-receptor signaling
Parathyroid hormone (PTH) is released from parathyroid glands and binds PTH-receptor (PTH1R), a G protein-coupled receptor on cells of the osteoblast lineage, whereby mechanisms have been extensively reviewed [63]. The Calvi laboratory has highlighted the importance of PTH in the BM niche as PTH/PTH1R ligation elicits HSC expansion in vitro and in vivo with increased survival after HCT during pre-clinical trials [37, 64]. In addition, patients with hyperparathyroidism produce excess PTH and have been shown to have an increase in HSC mobilization [65, 66]. However, when PTH signaling was constitutively activated in osteocytes or fully differentiated osteoblastic cells, the number of HSCs and function remained unchanged, suggesting that kinetics of PTH stimulation are critical [65, 67].
CXCL12/CXCR4 signaling
There are 4 distinct classes of chemokines and receptors, defined by their structural terminal cysteine residues that determine function and ligand-receptor binding: CC, CXC, XC, and CX3C chemokines, which bind to their respective CCR, CXCR, XCR, and CX3CR receptors with redundancy and promiscuity [68]. CXCR4 is a unique chemokine receptor since it exclusively binds CXCL12 [69]. CXCR4 is expressed on HSCs, mature leukocyte subsets including monocytes, neutrophils, T-lymphocytes, and B-lymphocytes [70], and a small subset of MSCs [71, 72]. CXCR4 ligation of CXCL12 is a vital signaling mechanism for immune cellular development in the BM niche by eliciting a signaling cascade to regulate leukocyte survival, proliferation, chemotaxis, and egress from the BM [73]. In leukocytes, CXCR4 signaling is negatively regulated by phosphorylation of its C-terminus tail via a G protein-coupled receptor kinase (GRK). GRKs trigger GPCR receptor internalization by recruiting β-arrestin and uncoupling the receptor from G-proteins [74].
If the CXCL12/CXCR4 signaling cascade is dysregulated, it may lead to abnormal receptor desensitization and internalization causing a gain-of-function in CXCR4, now well characterized as WHIM syndrome (Warts, Hypogammaglobulinemia, Infection, and Myelokathexis). In WHIM, excessive signaling through CXCL12/CXCR4 leads to retention of leukocytes in the BM (myelokathexis) and fewer circulating mature leukocytes in the blood necessary to protect the individual from infection [75, 76]. GRK-3 appears to have selective regulation of CXCR4 demonstrated both in vitro biochemically and in vivo biologically with clinical patients [76, 77]. Recent work by Tarrant et al. has confirmed that GRK-3 deficiency in mice impairs CXCL12/CXCR4 responses and demonstrates myelokathexis and hypogammaglobulinemia mirrored in WHIM syndrome [78]. GRK-3-deficient mice specifically demonstrate hypercellularity of the BM with retention of GR-1hi CD11bhi myeloid cells [78]. The GRK-3-deficient mouse model represents a clinical immunological disease manifestation of a subset of WHIM syndrome and allows an ideal model system to further investigate the inhabitants and interactions of hematopoiesis in the BM niche.
S1P/S1PR signaling
Sphingosine-1-phosphate (S1P) ligands are active phospholipids thought to be produced by mature red blood cells [79, 80], endothelial cells [81–83], and platelets [84]. S1P has vast physiological roles, one of which is a chemoattractant for HSCs. S1P is expressed with low levels in the BM and tissues and high levels in peripheral blood to thus stimulate HSC trafficking [85], in which the S1P levels are controlled by S1P lyase, an enzyme ubiquitously expressed that irreversibly degrades S1P [86]. HSCs express a subset of GPCR sphingosine-1-phosphate receptors 1–5 (S1PR1–5) that bind S1P ligands. S1PR-1 has been shown to be expressed on immature and committed progenitor mouse BM cells, whereas S1P3 and S1P5 are expressed mostly on immature progenitors, and S1P2 and S1P4 are expressed on committed progenitors [87, 88].
S1P has also been shown to be involved in MSC recruitment and differentiation, mediated through Rho/ERK signaling [89]. A recent novel finding by Zhang et al. implicates S1P/S1PR signaling axis as a vital BM mechanism to also direct elongation of megakaryocytic pro-platelet extensions into BM sinusoids, creating shedding of platelets [90]. This novel finding implicates S1P/S1PR signaling in the BM niche as a master regulator of thrombopoiesis, and suggests the S1P/S1PR interaction may be a potential therapeutic target for patients with thrombocytopenia. In fact, dysregulation of S1P correlates with progression of various diseases, such as cancer, atherosclerosis, diabetes, and osteoporosis [91]. Partial deletion or inhibition of S1P lyase has also been implicated as a potential therapeutic target for autoimmune disease, such as rheumatoid arthritis and multiple sclerosis, since reduction in S1P lyase activity correlated with decreased numbers of circulating lymphocytes [86, 91–93].
While the vast physiological roles of S1PR in the BM niche are progressively becoming more defined, the signaling mechanisms are still under active investigation. Recent findings suggest G protein-coupled receptor kinase-2 (GRK-2) down-regulates S1PR-1 by desensitization to initiate lymphocytes egress from the circulation into tissues [94]. Moreover, signaling through S1PR-1 and S1PR-3 has been shown to stimulate proliferation in a β-arrestin-dependent manner in the embryonic stem cell population [95]. These exciting investigations emphasize the importance of S1PR1-5 in physiological function, particularly within stem cell and progenitor populations, though a better understanding of the mechanisms may provide enhanced potential therapeutic targets.
Cross-talk between CXCL12/CXCR4 and S1P/S1PR
Recent work has implicated a cross-talk mechanism between CXCL12 and S1P that may regulate HSC migration, development, and bone remodeling [87]. Decreasing chemo-attractant CXCL12 in the BM and increasing S1P in the blood may control how quiescent HSCs shift to a migratory and differentiating phenotype. A pre-clinical study by Golan et al. has shown that disruption of S1P signaling decreased HSC and progenitor cell egress by inhibition of CXCL12 release. S1P has been shown to induce CXCL12 secretion from BM stromal cells (Nestin+ MSCs) in a reactive oxygen species (ROS) signaling-dependent manner. CXCL12 is then released into the circulation to further elicit cell mobilization. Increased S1P levels in the BM have also been shown to increase ROS production in HSCs, suggesting S1P/S1PR signaling mechanism that regulates progenitor cell egress via ROS signaling in HSCs and MSCs with enhanced CXCL12 secretion [87, 96].
Bone marrow niche therapeutic targeting strategies
Retention signaling
The BM hematopoietic system is typically in a steady state of HSC maintenance with low-level release of HSCs from the BM into the circulation, serving as a sustaining role to replenish the immune system. HSCs are more readily released from the BM upon hematopoietic stresses, such as exercise or infection [97]. By disrupting “retention signals” with stimulating factors in the BM niche, studies have revealed that HSCs are retained within the BM in part by GPCR signaling, chemotactic gradients, and cellular interactions. Well-studied retention signals include CXCL12/CXCR4 signaling interactions (described above) and VCAM1 (on MSCs [43] and CAR cells [47])/VLA-4 (on HSCs) adhesive interactions; however, there is also evidence supporting S1P/S1PR interactions, all of which have been recently reviewed [98]. Taken together, these signaling interactions between HSCs and other BM niche cells have been utilized as targets of current stem cell therapeutics to disrupt retention and force HSC egress from the BM to rapidly fuel immune reconstitution.
Granulocyte colony-stimulating factor (G-CSF) treatment
Administration of G-CSF analogues (also known as Neupogen or filgrastim) has been shown to increase BM cellularity in combination with enhanced egress of mature and immature BM cells, including HSCs [97]. Therefore, G-CSF has been used clinically for HSC mobilization [97, 99]. It has been suggested that G-CSF partially acts as an HSC mobilizer by inducing protease activity in the niche, which cleaves VCAM, thus disrupting a vital retention mechanism [98, 100]. G-CSF has also been shown to down-regulate and cleave CXCL12, which affects CXCR4 ligation for retention signaling [98, 101]. However, due to further studies, a more complex model of G-CSF mechanism has emerged that suggests the protease-dependent and protease-independent decrease in CXCL12 mRNA and protein expression in the BM may elicit HSC mobilization [102]. Interestingly, administration of G-CSF has also been shown to increase S1P signaling in the BM in a P13-kinase-dependent manner [87], suggesting that further studies are warranted to better understand the seemingly complex mechanism of G-CSF treatment.
GPCR antagonist therapies
AMD3100
Plerixafor, also more commonly known as AMD3100, is used as a CXCR4 antagonist that blocks the CXCL12/CXCR4 signaling mechanism within the BM niche, forcing egress of HSCs into the periphery [103]. A phase III clinical trial study reported that participants who were treated with HSC mobilization by AMD3100 and G-CSF had a significantly higher CD34+ cell count than those who were mobilized with just G-CSF alone [104]. The FDA has since approved AMD3100 for use in combination with G-CSF for mobilization of HSCs in adults, specifically with non-Hodgkin’s lymphoma [105] or multiple myeloma [106], for immune reconstitution.
AMD3100 has also been implicated in cancer therapeutics for reduction of tumor proliferation, impaired cell survival, and induction of differentiation, particularly in acute myeloid leukemia human cells [107]. Kang et al. demonstrated AMD3100 treatment post-HSCT significantly improved animal survival, enhanced donor cell engraftment, and supported recovery of myeloid and lymphoid lineages [108]. To date, there are 69 clinical trials worldwide investigating AMD3100 treatment on various malignancies. There are currently 12 clinical trials investigating AMD3100 for use in treatment of non-malignant diseases, and 20 clinical studies investigating AMD3100 in other transplantation models (Table 1).
Table 1.
Clinical trial investigations utilizing AMD3100
| Clinical investigations of AMD3100 | No. of studies | Key references |
|---|---|---|
|
| ||
| Non-malignant diseases | ||
| Immune deficiency | 3 | |
| Myelokathexis/neutropenia | 1 | McDermott 2011 [119] |
| WHIM syndrome | 1 | Dale 2011 [109] |
| Severe combined immunodeficiency | 1 | |
|
| ||
| Autoimmune disease | 1 | Song 2010a [120] |
| Scleroderma | 1 | Makino 2013a [121] |
|
| ||
| Hereditary diseases | 3 | |
| Beta-thalassemia | 1 | Yannaki 2012 [122] |
| Fanconi anemia | 2 | Pulliam 2008a [123] |
|
| ||
| Other | 5 | |
| Myelodysplastic syndrome | 1 | |
| Diabetic ulcer | 1 | Nishimura 2012a [124] |
| Renal impairment | 1 | MacFarland 2010 [125] |
| COPD/cystic fibrosis/pulmonary fibrosis | 1 | |
| End-stage liver fisease | 1 | |
|
| ||
| Malignant diseases | ||
| Hematological neoplasm/malignancy | 66 | Uy 2012 [126] Devine 2004 [103] DiPersio 2009 [104, 127] Cashen 2008 [128] Hubel 2012 [129] Attolico 2012 [130] Maziarz 2013 [105] |
| Acute myeloid leukemia | 11 | |
| Plasma cell/multiple myeloma (MM) | 13 | |
| Lymphoma | ||
| Non-Hodgkin’s | 9 | |
| Hodgkin’s | 2 | |
| Unspecified | 3 | |
| Chronic lymphocytic leukemia and/or small | 2 | |
| lymphocytic lymphoma (unspecified) and MM | 9 | |
| Non-Hodgkin’s lymphoma and MM | 7 | |
| leukemia and myelodysplastic syndrome | 5 | |
| Unspecified | 5 | |
|
| ||
| Solid tumor neoplasm/malignancy | ||
| Glioma | 3 | Rubin 2003a [131] |
| Ewings sarcoma/soft tissue | 2 | Redjal 2006a [132] |
| Sarcoma/neuroblastoma | 1 | Vives 2012a [133] |
|
| ||
| Transplantation | 20 | |
| Autologous/allogeneic stem cell Transplantation | 9 | |
| Mobilization (healthy donor) | 9 | Devine 2008 [134] |
| Mastocytosis (mast cell release) | 1 | Shepherd 2006 [135] |
| Safety/efficacy subcutaneous administration | 1 | |
Classified by condition-descriptions from ClinicalTrials.gov. Reference list is not exhaustive. References include published clinical trial results, if currently available
Indicates pre-clinical reference.
In immune deficiency, AMD3100 has been investigated in phase I clinical trials as a CXCR4 antagonist for the treatment of WHIM syndrome. Treatment of WHIM patients demonstrated leukocytosis and restored neutrophil and B lymphocyte numbers into the circulation [109]. In summary, AMD3100 holds therapeutic potential for the treatment of malignancies and hereditary diseases, including immune deficiency (Table 1).
GPCR agonist therapies
PTH treatment
PTH signaling has been investigated as a potential therapy to facilitate stem cell mobilization and engraftment. PTH administration for stem cell mobilization has been explored in phase I clinical trials as a follow-up to pre-clinical studies demonstrating enhanced survival with PTH administered after transplantation [110]. In 20 patients, PTH was well tolerated with no dose-limiting toxicities and showed adequate CD34+ stem cell mobilization [110]. Although further phase II clinical trials of 13 patients showed engraftment, 38.5 % of patients experienced GvHD leading to four deaths and premature study termination [111]. To our knowledge, there are no current on-going clinical trials evaluating a potential therapeutic model of PTH in stem cell mobilization.
FTY720 and SEW2871 treatments
In addition to synergistic interactions between CXCR4 and G-CSF signaling, cross-talk pathways between CXCR4 and S1PR have also been shown to enhance HSCs migratory response [88, 112]. Thus, targeting CXCR4, S1PR, and cross-talk of CXCR4 and S1PR signaling mechanisms in the BM niche may serve as therapeutic strategies.
Treatment using the S1P1R agonist FTY720 (fingolimod) binds and initiates receptor internalization and degradation and leads to S1P/S1PR signaling suppression. As a result, FTY720 treatment reduces the number of HSCs in peripheral blood [113] and leads to retention of T [114, 115] and B lymphocytes [116, 117] in pre-clinical mouse studies. Golan et al. reported that FTY720 treatment reduced HSC mobilization into the circulation through S1P1R desensitization and inhibition of CXCL12 secretion from mesenchymal stromal cells. These data suggest cross-talk between S1P and CXCL12 to direct MSC and HSC mobilization [96]. Therefore, these collective data strongly suggest that S1P/S1PR molecular signaling, as well as CXCL12/CXCR4 interactions, are vital for HSC, progenitor, and mature cell mobilization and egress from the BM.
Combination therapy of CXCR4 antagonists with S1PR agonists have been investigated as possible therapeutic targets. When AMD3100 treatment was accompanied with S1PR agonist SEW2871, which recycles the S1PR receptor, HSC and progenitor mobilization was significantly enhanced. In contrast, SEW2871 alone had no effect on increasing HSC mobilization into the blood [118]. Therefore, combination therapy of AMD3100 and SEW2871 showed synergistic therapeutic benefits in pre-clinical models [118], but SEW2871 remains to be tested in humans [85]. However, when AMD3100 was administered with another S1PR agonist, FTY720 (fingolimod), which internalizes and degrades S1PR, immune mobilization was suppressed [96]. Therefore, while agonist FTY720 has been more extensively studied and already clinically used (Table 2), it typically sequesters cells for limited BM release by S1PR degradation and may not be best suited in combination with AMD3100 for immune reconstitution therapy.
Table 2.
Clinical trial investigations utilizing FTY720
| Clinical trial investigations of FTY720 | No. of Studies | Key references |
|---|---|---|
|
| ||
| Autoimmune and inflammatory fiseases | 46 | Kappos 2006 [136] |
| Multiple sclerosis | Kappos 2010 [137] | |
| Primary progressive | 2 | Comi 2010 [138] |
| Relapsing, remitting | 22 | O’Connor 2010 [139] |
| Non-specified | 18 | |
| Chronic inflammatory demyelinating polyradiculoneuropathy | 1 | Kurose 2000a [140] Commodaro 2009a [141] Copland 2012a [142] Zhang 2009a [143] Sawicka 2003a [144] |
| Uveitis | 1 | |
| Acute demyelinating optic neuritis | 1 | |
| Asthma | 1 | |
|
| ||
| Neurological disease/disorder | 2 | |
| Amyotrophic lateral sclerosis | 1 | ClinicalTrials.gov |
| Schizophrenia | 1 | |
|
| ||
| Kidney Disease | 11 | |
| Transplantation | 10 | Budde 2002 [145] |
| Renal insufficiency | 1 | |
|
| ||
| Other | 1 | |
| Cardiac/pulmonary function | 1 | Schmouder 2012 [146] |
Classified by condition-descriptions from ClinicalTrials.gov. Reference list is not exhaustive. References include published clinical trial results, if currently available
Indicates pre-clinical reference.
Conclusions
Past and recent studies of the BM have collectively provided a greater understanding of the molecular interactions among the vital inhabitants of the niche. Applications of this knowledge have enhanced therapeutics in the clinical setting for transplantation of malignancy and non-malignant diseases of autoimmunity and immune deficiency. Future investigations of mechanistic GPCR intracellular signaling and utilization of receptor agonist/antagonist interactions in the BM niche are warranted. With further investigations and collaborative studies, the field of stem cell therapy will continue to advance toward the treatment of human disease.
Acknowledgments
Teresa K. Tarrant has received NIH grant support from R03AR059286 and K01AI091863.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Jaime M. Brozowski, Matthew J. Billard, and Teresa K. Tarrant declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.McCulloch EA, Till JE. The radiation sensitivity of normal mouse bone marrow cells, determined by quantitative marrow transplantation into irradiated mice. Radiat Res. 1960;13:115–25. [PubMed] [Google Scholar]
- 2.Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–4. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein AJ, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230–47. [PubMed] [Google Scholar]
- 4.McCulloch EA, Till JE. Proliferation of Hemopoietic Colony-Forming Cells Transplanted into Irradiated Mice. Radiat Res. 1964;22:383–97. [PubMed] [Google Scholar]
- 5.Till JE, Mc CE. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–22. [PubMed] [Google Scholar]
- 6.Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327–59. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- 7.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 8.Friedenstein AJ, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331–40. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Friedenstein AJ, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2(2):83–92. [PubMed] [Google Scholar]
- 10.Friedenstein AJ, et al. Origin of bone marrow stromal mechanocytes in radiochimeras and heterotopic transplants. Exp Hematol. 1978;6(5):440–4. [PubMed] [Google Scholar]
- 11.Luria EA, Panasyuk AF, Friedenstein AY. Fibroblast colony formation from monolayer cultures of blood cells. Transfusion. 1971;11(6):345–9. doi: 10.1111/j.1537-2995.1971.tb04426.x. [DOI] [PubMed] [Google Scholar]
- 12.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 13.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25. [PubMed] [Google Scholar]
- 14.Thomas ED. The Nobel Lectures in Immunology. The Nobel Prize for Physiology or Medicine, 1990. Bone marrow transplantation--past, present and future. Scand J Immunol. 1994;39(4):339–45. doi: 10.1111/j.1365-3083.1994.tb03383.x. [DOI] [PubMed] [Google Scholar]
- 15.Gatti RA, et al. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2(7583):1366–9. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- 16.Griffith LM, et al. Target populations in allogeneic hematopoietic cell transplantation for autoimmune diseases--a workshop accompanying: cellular therapy for treatment of autoimmune diseases, basic science and clinical studies, including new developments in hematopoietic and mesenchymal stem cell therapy. Biol Blood Marrow Transplant. 2006;12(6):688–90. doi: 10.1016/j.bbmt.2006.02.007. [DOI] [PubMed] [Google Scholar]
- ••17.Gratwohl A, et al. Quantitative and qualitative differences in use and trends of hematopoietic stem cell transplantation: a Global Observational Study. Haematologica. 2013;98(8):1282–90. doi: 10.3324/haematol.2012.076349. Extensive report on worldwide analysis of HCT and HSCT during a 3-year period: 2006–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armitage JO. Bone marrow transplantation. N Engl J Med. 1994;330(12):827–38. doi: 10.1056/NEJM199403243301206. [DOI] [PubMed] [Google Scholar]
- 19.Atkins HL, et al. Autologous hematopoietic stem cell transplantation for autoimmune disease--is it now ready for prime time? Biol Blood Marrow Transplant. 2012;18(1 Suppl):S177–83. doi: 10.1016/j.bbmt.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gratwohl A, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303(16):1617–24. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersdorf EW. The major histocompatibility complex: a model for understanding graft-versus-host disease. Blood. 2013;122(11):1863–72. doi: 10.1182/blood-2013-05-355982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansen J, et al. Transplantation of hematopoietic stem cells from the peripheral blood. J Cell Mol Med. 2005;9(1):37–50. doi: 10.1111/j.1582-4934.2005.tb00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavletic ZS, et al. Hematopoietic recovery after allogeneic blood stem-cell transplantation compared with bone marrow transplantation in patients with hematologic malignancies. J Clin Oncol. 1997;15(4):1608–16. doi: 10.1200/JCO.1997.15.4.1608. [DOI] [PubMed] [Google Scholar]
- 24.Mancardi G, Saccardi R. Autologous haematopoietic stem-cell transplantation in multiple sclerosis. Lancet Neurol. 2008;7(7):626–36. doi: 10.1016/S1474-4422(08)70138-8. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan KM, Muraro P, Tyndall A. Hematopoietic cell transplantation for autoimmune disease: updates from Europe and the United States. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S48–56. doi: 10.1016/j.bbmt.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milanetti F, et al. Autologous hematopoietic stem cell transplantation for systemic sclerosis. Curr Stem Cell Res Ther. 2011;6(1):16–28. doi: 10.2174/157488811794480663. [DOI] [PubMed] [Google Scholar]
- 27.Naraghi K, van Laar JM. Update on stem cell transplantation for systemic sclerosis: recent trial results. Curr Rheumatol Rep. 2013;15(5):326. doi: 10.1007/s11926-013-0326-2. [DOI] [PubMed] [Google Scholar]
- ••28.Frenette PS, et al. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285–316. doi: 10.1146/annurev-immunol-032712-095919. Vast review on defining MSCs and discussing their role in the BM niche, involvement in immune function, and therapeutic applications. [DOI] [PubMed] [Google Scholar]
- 29.Masuda S, et al. Cotransplantation with MSCs improves engraftment of HSCs after autologous intra-bone marrow transplantation in nonhuman primates. Exp Hematol. 2009;37(10):1250–1257 e1. doi: 10.1016/j.exphem.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Cheshier SH, Prohaska SS, Weissman IL. The effect of bleeding on hematopoietic stem cell cycling and self-renewal. Stem Cells Dev. 2007;16(5):707–17. doi: 10.1089/scd.2007.0017. [DOI] [PubMed] [Google Scholar]
- 31.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32(2):57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seita J, I, Weissman L. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2(6):640–53. doi: 10.1002/wsbm.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trumpp A, Essers M, Wilson A. Awakening dormant haematopoietic stem cells. Nat Rev Immunol. 2010;10(3):201–9. doi: 10.1038/nri2726. [DOI] [PubMed] [Google Scholar]
- 34.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–29. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 35.Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood. 1996;87(2):518–24. [PubMed] [Google Scholar]
- 36.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179(5):1677–82. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 39.Visnjic D, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–64. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- ••40.Doze VA, Perez DM. GPCRs in stem cell function. Prog Mol Biol Transl Sci. 2013;115:175–216. doi: 10.1016/B978-0-12-394587-7.00005-1. Thorough review on various G protein-coupled receptors and embryonic, induced pluripotent, cancer, and adult stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sacchetti B, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 42.Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rostovskaya M, Anastassiadis K. Differential expression of surface markers in mouse bone marrow mesenchymal stromal cell subpopulations with distinct lineage commitment. PLoS One. 2012;7(12):e51221. doi: 10.1371/journal.pone.0051221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siegel G, et al. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146. doi: 10.1186/1741-7015-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tokoyoda K, et al. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20(6):707–18. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Sugiyama T, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 47.Sugiyama T, Nagasawa T. Bone marrow niches for hematopoietic stem cells and immune cells. Inflamm Allergy Drug Targets. 2012;11(3):201–6. doi: 10.2174/187152812800392689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A. 1994;91(6):2305–9. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagasawa T, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 50.Nagasawa T. The chemokine CXCL12 and regulation of HSC and B lymphocyte development in the bone marrow niche. Adv Exp Med Biol. 2007;602:69–75. doi: 10.1007/978-0-387-72009-8_9. [DOI] [PubMed] [Google Scholar]
- 51.Kohara H, et al. Development of plasmacytoid dendritic cells in bone marrow stromal cell niches requires CXCL12-CXCR4 chemokine signaling. Blood. 2007;110(13):4153–60. doi: 10.1182/blood-2007-04-084210. [DOI] [PubMed] [Google Scholar]
- 52.Noda M, et al. CXCL12-CXCR4 chemokine signaling is essential for NK-cell development in adult mice. Blood. 2011;117(2):451–8. doi: 10.1182/blood-2010-04-277897. [DOI] [PubMed] [Google Scholar]
- 53.Omatsu Y, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33(3):387–99. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 54.Zhu J, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109(9):3706–12. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 55.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 56.Pinho S, et al. PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210(7):1351–67. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •57.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. doi: 10.1038/nature11885. Demonstrates that different stem and progenitor cells occupy distinct BM niches—simultaneous study; see Greenbaum et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsu YC, Fuchs E. A family business: stem cell progeny join the niche to regulate homeostasis. Nat Rev Mol Cell Biol. 2012;13(2):103–14. doi: 10.1038/nrm3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •59.Greenbaum A, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–30. doi: 10.1038/nature11926. Demonstrates different stem and progenitor cells occupy distinct BM niches— simultaneous study; see Ding et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heazlewood SY, et al. Megakaryocytes co-localise with hemopoietic stem cells and release cytokines that up-regulate stem cell proliferation. Stem Cell Res. 2013;11(2):782–92. doi: 10.1016/j.scr.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Calvi LM, Link DC. Cellular Complexity of the Bone Marrow Hematopoietic Stem Cell Niche. Calcif Tissue Int. 2013 doi: 10.1007/s00223-013-9805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DeWire SM, et al. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 63.Goltzman D. Studies on the mechanisms of the skeletal anabolic action of endogenous and exogenous parathyroid hormone. Arch Biochem Biophys. 2008;473(2):218–24. doi: 10.1016/j.abb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Calvi LM. Osteoblastic activation in the hematopoietic stem cell niche. Ann N Y Acad Sci. 2006;1068:477–88. doi: 10.1196/annals.1346.021. [DOI] [PubMed] [Google Scholar]
- 65.Smith JN, Calvi LM. Concise review: Current concepts in bone marrow microenvironmental regulation of hematopoietic stem and progenitor cells. Stem Cells. 2013;31(6):1044–50. doi: 10.1002/stem.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brunner S, et al. Primary hyperparathyroidism is associated with increased circulating bone marrow-derived progenitor cells. Am J Physiol Endocrinol Metab. 2007;293(6):E1670–5. doi: 10.1152/ajpendo.00287.2007. [DOI] [PubMed] [Google Scholar]
- 67.Calvi LM, et al. Osteoblastic expansion induced by parathyroid hormone receptor signaling in murine osteocytes is not sufficient to increase hematopoietic stem cells. Blood. 2012;119(11):2489–99. doi: 10.1182/blood-2011-06-360933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chemokine/chemokine receptor nomenclature. Cytokine. 2003;21(1):48–9. doi: 10.1016/s1043-4666(02)00493-3. [DOI] [PubMed] [Google Scholar]
- 69.Vila-Coro AJ, et al. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. Faseb J. 1999;13(13):1699–710. [PubMed] [Google Scholar]
- 70.Forster R, et al. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J Immunol. 1998;160(3):1522–31. [PubMed] [Google Scholar]
- 71.Wynn RF, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104(9):2643–5. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 72.Sordi V, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106(2):419–27. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 73.Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34(8):967–75. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 74.Premont RT, Inglese J, Lefkowitz RJ. Protein kinases that phosphorylate activated G protein-coupled receptors. Faseb J. 1995;9(2):175–82. doi: 10.1096/fasebj.9.2.7781920. [DOI] [PubMed] [Google Scholar]
- 75.Balabanian K, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105(6):2449–57. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- 76.Balabanian K, et al. Leukocyte analysis from WHIM syndrome patients reveals a pivotal role for GRK3 in CXCR4 signaling. J Clin Invest. 2008;118(3):1074–84. doi: 10.1172/JCI33187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mueller W, et al. Hierarchical organization of multi-site phosphorylation at the CXCR4 C terminus. PLoS One. 8(5):e64975. doi: 10.1371/journal.pone.0064975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tarrant TK, et al. G protein-coupled receptor kinase-3-deficient mice exhibit WHIM syndrome features and attenuated inflammatory responses. J Leukoc Biol. doi: 10.1189/jlb.0213097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pappu R, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316(5822):295–8. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 80.Ito K, et al. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem Biophys Res Commun. 2007;357(1):212–7. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- 81.Venkataraman K, et al. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397(3):461–71. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hisano Y, et al. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One. 2012;7(6):e38941. doi: 10.1371/journal.pone.0038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fukuhara S, et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest. 2012;122(4):1416–26. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yatomi Y, et al. Sphingosine 1-phosphate: synthesis and release. Prostaglandins. 2001;64(1–4):107–122. doi: 10.1016/s0090-6980(01)00103-4. [DOI] [PubMed] [Google Scholar]
- 85.Bendall LJ, Basnett J. Role of sphingosine 1-phosphate in trafficking and mobilization of hematopoietic stem cells. Curr Opin Hematol. 2013;20(4):281–8. doi: 10.1097/MOH.0b013e3283606090. [DOI] [PubMed] [Google Scholar]
- 86.Billich A, et al. Partial deficiency of sphingosine-1-phosphate lyase confers protection in experimental autoimmune encephalomyelitis. PLoS One. 2013;8(3):e59630. doi: 10.1371/journal.pone.0059630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •87.Golan K, Lapidot Tsvee KO. Dynamic cross talk between S1P and CXCL12 regulates hamatopoietic stem cells migration, development, and bone remodeling. Pharmaceuticals. 2013;6(9):1145–1169. doi: 10.3390/ph6091145. Review that highlights the concept of synchronized S1P/S1PR and CXCL12/CXCR4 interactions in HSC mobilization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kimura T, et al. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood. 2004;103(12):4478–86. doi: 10.1182/blood-2003-03-0875. [DOI] [PubMed] [Google Scholar]
- 89.Meriane M, et al. Cooperation of matrix metalloproteinases with the RhoA/Rho kinase and mitogen-activated protein kinase kinase-1/extracellular signal-regulated kinase signaling pathways is required for the sphingosine-1-phosphate-induced mobilization of marrow-derived stromal cells. Stem Cells. 2006;24(11):2557–65. doi: 10.1634/stemcells.2006-0209. [DOI] [PubMed] [Google Scholar]
- 90.Zhang L, et al. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J Exp Med. 2012;209(12):2165–81. doi: 10.1084/jem.20121090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maceyka M, et al. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22(1):50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bagdanoff JT, et al. Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime (LX2931) and (1R,2S,3R)-1-(2-(isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932) J Med Chem. 2010;53(24):8650–62. doi: 10.1021/jm101183p. [DOI] [PubMed] [Google Scholar]
- 93.Bagdanoff JT, et al. Inhibition of sphingosine-1-phosphate lyase for the treatment of autoimmune disorders. J Med Chem. 2009;52(13):3941–53. doi: 10.1021/jm900278w. [DOI] [PubMed] [Google Scholar]
- 94.Arnon TI, et al. GRK2-dependent S1PR1 desensitization is required for lymphocytes to overcome their attraction to blood. Science. 2011;333(6051):1898–903. doi: 10.1126/science.1208248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ryu JM, et al. Sphingosine-1-phosphate-induced Flk-1 transactivation stimulates mouse embryonic stem cell proliferation through S1P/S1P-dependent beta-arrestin/c-Src pathways. Stem Cell Res. 2013;12(1):69–85. doi: 10.1016/j.scr.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 96.Golan K, et al. S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood. 2012;119(11):2478–88. doi: 10.1182/blood-2011-06-358614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27(1):24–31. doi: 10.1038/leu.2012.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Motabi IH, DiPersio JF. Advances in stem cell mobilization. Blood Rev. 2012;26(6):267–78. doi: 10.1016/j.blre.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 99.Arcese W, De Angelis G, Cerretti R. Granulocyte-mobilized bone marrow. Curr Opin Hematol. 2012;19(6):448–53. doi: 10.1097/MOH.0b013e32835903ab. [DOI] [PubMed] [Google Scholar]
- 100.Levesque JP, et al. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98(5):1289–97. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 101.Levesque JP, et al. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111(2):187–96. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Levesque JP, et al. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004;104(1):65–72. doi: 10.1182/blood-2003-05-1589. [DOI] [PubMed] [Google Scholar]
- 103.Devine SM, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22(6):1095–102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 104.DiPersio JF, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27(28):4767–73. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 105.Maziarz RT, et al. Plerixafor plus granulocyte colony-stimulating factor improves the mobilization of hematopoietic stem cells in patients with non-Hodgkin lymphoma and low circulating peripheral blood CD34+ cells. Biol Blood Marrow Transplant. 2013;19(4):670–5. doi: 10.1016/j.bbmt.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 106.Sison EA, Brown P. The bone marrow microenvironment and leukemia: biology and therapeutic targeting. Expert Rev Hematol. 2011;4(3):271–83. doi: 10.1586/ehm.11.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tavor S, et al. The CXCR4 antagonist AMD3100 impairs survival of human AML cells and induces their differentiation. Leukemia. 2008;22(12):2151–5158. doi: 10.1038/leu.2008.238. [DOI] [PubMed] [Google Scholar]
- 108.Kang Y, et al. Selective enhancement of donor hematopoietic cell engraftment by the CXCR4 antagonist AMD3100 in a mouse transplantation model. PLoS One. 2010;5(6):e11316. doi: 10.1371/journal.pone.0011316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dale DC, et al. The CXCR4 antagonist plerixafor is a potential therapy for myelokathexis, WHIM syndrome. Blood. 2011;118(18):4963–6. doi: 10.1182/blood-2011-06-360586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ballen KK, et al. Phase I trial of parathyroid hormone to facilitate stem cell mobilization. Biol Blood Marrow Transplant. 2007;13(7):838–43. doi: 10.1016/j.bbmt.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 111.Ballen K, et al. Phase II trial of parathyroid hormone after double umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2012;18(12):1851–8. doi: 10.1016/j.bbmt.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Whetton AD, et al. Lysophospholipids synergistically promote primitive hematopoietic cell chemotaxis via a mechanism involving Vav 1. Blood. 2003;102(8):2798–802. doi: 10.1182/blood-2002-12-3635. [DOI] [PubMed] [Google Scholar]
- 113.Massberg S, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131(5):994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Halin C, et al. The S1P-analog FTY720 differentially modulates T-cell homing via HEV: T-cell-expressed S1P1 amplifies integrin activation in peripheral lymph nodes but not in Peyer patches. Blood. 2005;106(4):1314–22. doi: 10.1182/blood-2004-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maeda Y, et al. Sphingosine 1-phosphate receptor type 1 regulates egress of mature T cells from mouse bone marrow. Int Immunol. 2010;22(6):515–25. doi: 10.1093/intimm/dxq036. [DOI] [PubMed] [Google Scholar]
- 116.Pereira JP, Xu Y, Cyster JG. A role for S1P and S1P1 in immature-B cell egress from mouse bone marrow. PLoS One. 2010;5(2):e9277. doi: 10.1371/journal.pone.0009277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Allende ML, et al. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J Biol Chem. 2011;286(9):7348–58. doi: 10.1074/jbc.M110.171819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Juarez JG, et al. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood. 2012;119(3):707–16. doi: 10.1182/blood-2011-04-348904. [DOI] [PubMed] [Google Scholar]
- 119.McDermott DH, et al. The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome. Blood. 118(18):4957–62. doi: 10.1182/blood-2011-07-368084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Song JS, et al. Inhibitory effect of CXC chemokine receptor 4 antagonist AMD3100 on bleomycin induced murine pulmonary fibrosis. Exp Mol Med. 42(6):465–72. doi: 10.3858/emm.2010.42.6.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Makino H, et al. Antifibrotic effects of CXCR4 antagonist in bleomycin-induced pulmonary fibrosis in mice. J Med Invest. 60(1–2):127–37. doi: 10.2152/jmi.60.127. [DOI] [PubMed] [Google Scholar]
- 122.Yannaki E, et al. Hematopoietic stem cell mobilization for gene therapy of adult patients with severe beta-thalassemia: results of clinical trials using G-CSF or plerixafor in splenectomized and nonsplenectomized subjects. Mol Ther. 20(1):230–8. doi: 10.1038/mt.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pulliam AC, et al. AMD3100 synergizes with G-CSF to mobilize repopulating stem cells in Fanconi anemia knockout mice. Exp Hematol. 2008;36(9):1084–90. doi: 10.1016/j.exphem.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nishimura Y, et al. CXCR4 antagonist AMD3100 accelerates impaired wound healing in diabetic mice. J Invest Dermatol. 132(3 Pt 1):711–20. doi: 10.1038/jid.2011.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.MacFarland R, et al. A pharmacokinetic study of plerixafor in subjects with varying degrees of renal impairment. Biol Blood Marrow Transplant. 16(1):95–101. doi: 10.1016/j.bbmt.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 126.Uy GL, et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 119(17):3917–24. doi: 10.1182/blood-2011-10-383406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.DiPersio JF, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–6. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 128.Cashen A, et al. A phase II study of plerixafor (AMD3100) plus G-CSF for autologous hematopoietic progenitor cell mobilization in patients with Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14(11):1253–61. doi: 10.1016/j.bbmt.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 129.Hubel K, et al. European data on stem cell mobilization with plerixafor in non-Hodgkin’s lymphoma, Hodgkin’s lymphoma and multiple myeloma patients. A subgroup analysis of the European Consortium of stem cell mobilization. Bone Marrow Transplant. 47(8):1046–50. doi: 10.1038/bmt.2011.216. [DOI] [PubMed] [Google Scholar]
- 130.Attolico I, et al. Plerixafor added to chemotherapy plus G-CSF is safe and allows adequate PBSC collection in predicted poor mobilizer patients with multiple myeloma or lymphoma. Biol Blood Marrow Transplant. 18(2):241–9. doi: 10.1016/j.bbmt.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 131.Rubin JB, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003;100(23):13513–8. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Redjal N, et al. CXCR4 inhibition synergizes with cytotoxic chemotherapy in gliomas. Clin Cancer Res. 2006;12(22):6765–71. doi: 10.1158/1078-0432.CCR-06-1372. [DOI] [PubMed] [Google Scholar]
- 133.Vives S, et al. Plerixafor plus G-CSF in combination with chemotherapy for stem cell mobilization in a pediatric patient with Ewing’s sarcoma. J Clin Apher. 27(5):260–2. doi: 10.1002/jca.21234. [DOI] [PubMed] [Google Scholar]
- 134.Devine SM, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112(4):990–8. doi: 10.1182/blood-2007-12-130179. [DOI] [PubMed] [Google Scholar]
- 135.Shepherd RM, et al. Angiogenic cells can be rapidly mobilized and efficiently harvested from the blood following treatment with AMD3100. Blood. 2006;108(12):3662–7. doi: 10.1182/blood-2006-06-030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kappos L, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355(11):1124–40. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 137.Kappos L, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 362(5):387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 138.Comi G, et al. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3-year results. Mult Scler. 16(2):197–207. doi: 10.1177/1352458509357065. [DOI] [PubMed] [Google Scholar]
- 139.O’Connor P, et al. Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a phase II extension study. Neurology. 2009;72(1):73–9. doi: 10.1212/01.wnl.0000338569.32367.3d. [DOI] [PubMed] [Google Scholar]
- 140.Kurose S, et al. Effects of FTY720, a novel immunosuppressant, on experimental autoimmune uveoretinitis in rats. Exp Eye Res. 2000;70(1):7–15. doi: 10.1006/exer.1999.0777. [DOI] [PubMed] [Google Scholar]
- 141.Commodaro AG, et al. Evaluation of experimental autoimmune uveitis in mice treated with FTY720. Invest Ophthalmol Vis Sci. 51(5):2568–74. doi: 10.1167/iovs.09-4769. [DOI] [PubMed] [Google Scholar]
- 142.Copland DA, et al. Therapeutic dosing of fingolimod (FTY720) prevents cell infiltration, rapidly suppresses ocular inflammation, and maintains the blood-ocular barrier. Am J Pathol. 180(2):672–81. doi: 10.1016/j.ajpath.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang Z, et al. Distribution of Foxp3(+) T-regulatory cells in experimental autoimmune neuritis rats. Exp Neurol. 2009;216(1):75–82. doi: 10.1016/j.expneurol.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 144.Sawicka E, et al. Inhibition of Th1- and Th2-mediated airway inflammation by the sphingosine 1-phosphate receptor agonist FTY720. J Immunol. 2003;171(11):6206–14. doi: 10.4049/jimmunol.171.11.6206. [DOI] [PubMed] [Google Scholar]
- 145.Budde K, et al. First human trial of FTY720, a novel immunomodulator, in stable renal transplant patients. J Am Soc Nephrol. 2002;13(4):1073–83. doi: 10.1681/ASN.V1341073. [DOI] [PubMed] [Google Scholar]
- 146.Schmouder R, Hariry S, David OJ. Placebo-controlled study of the effects of fingolimod on cardiac rate and rhythm and pulmonary function in healthy volunteers. Eur J Clin Pharmacol. 68(4):355–62. doi: 10.1007/s00228-011-1146-9. [DOI] [PubMed] [Google Scholar]