Abstract
When Escherichia coli K-12 (λ) lysogens were infected with λ phages, genetic exchanges between phage and prophage occurred at low frequencies (less than 0.1% between the markers P3 and P80), but at frequencies above 1% if the infecting phages were first treated with the photosensitizing agent 4,5′,8-trimethylpsoralen and 360 nm light. Exchanges were induced by psoralen damage at about the same frequency in wild-type lysogens and in those carrying recB-, recC-, recF-, or lexA-, but at an intermediate frequency in a quadruple mutant carrying recB-recC-recF-sbcB-. Few if any exchanges were induced in lysogens carrying uvrA-, uvrB-, or recA-. The increase in the frequency of recombination was presumably due to the psoralen damage in the phage DNA molecules and the action of host cell repair and recombination enzymes. The production of crosslinks in the phage DNA by psoralen and 360 nm light was measured by sedimentation in alkali. It showed second-order kinetics indicative of a two-photon reaction. In contrast, first-order kinetics had been reported for monoadduct formation. Second-order kinetics, similar to those for crosslink production, were found for genetic exchanges in homoimmune crosses. Presumably, crosslinks, rather than monoadducts, cause most of the exchanges. Because the uvrA- gene product (UV-endonuclease) was required, it is likely that recombination was initiated by DNA molecules cut at crosslinks. This system has been used to show that after the crosslinked phage duplex has been cut, one or more of the subsequent steps—homologous pairing, cutting, and joining—require the recA+ gene product.
Keywords: recombination, crosslinks, excision, recA gene, recB gene
Full text
PDF
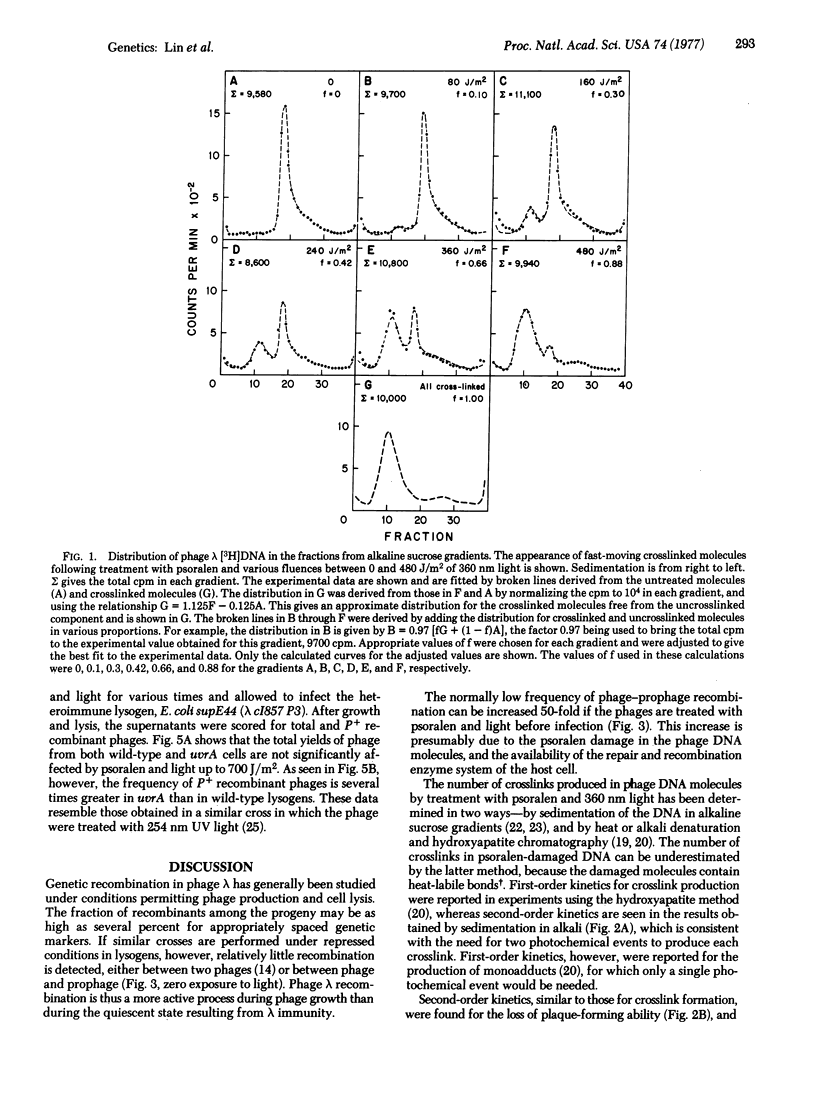

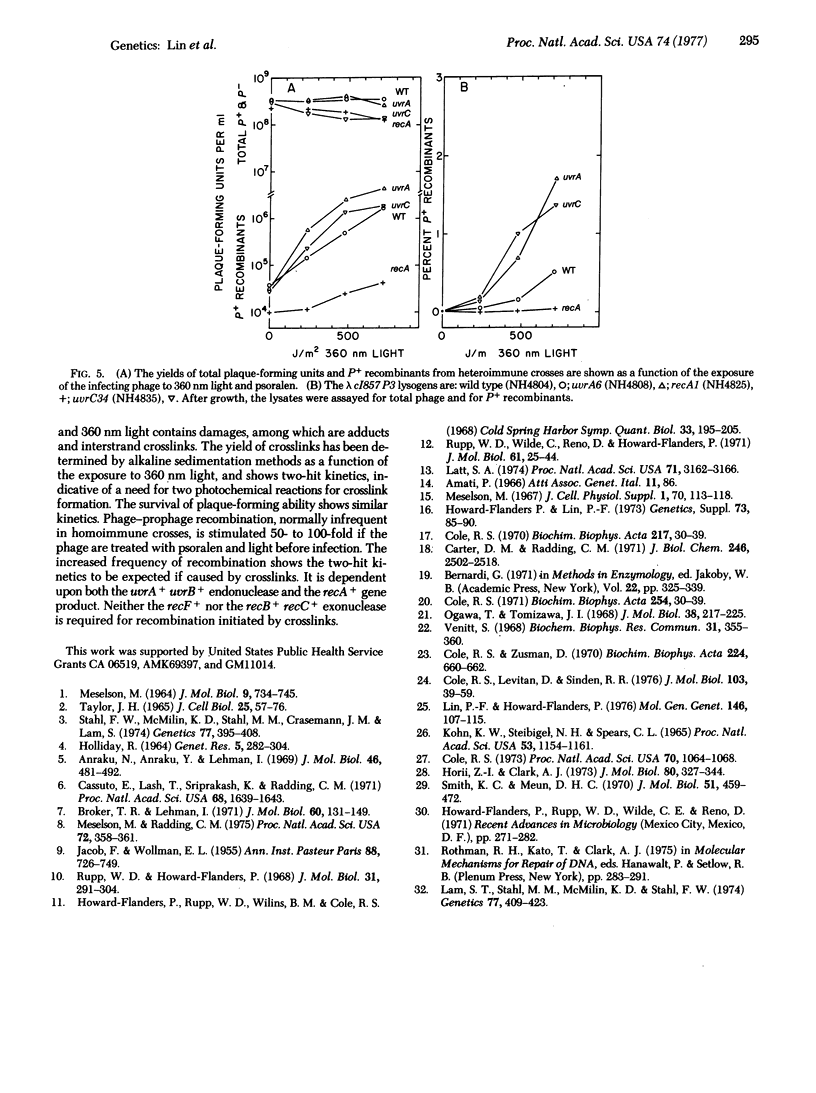
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku N., Anraku Y., Lehman I. R. Enzymic joining of polynucleotides. 8. Structure of hybrids of parental T4 DNA molecules. J Mol Biol. 1969 Dec 28;46(3):481–492. doi: 10.1016/0022-2836(69)90191-0. [DOI] [PubMed] [Google Scholar]
- Broker T. R., Lehman I. R. Branched DNA molecules: intermediates in T4 recombination. J Mol Biol. 1971 Aug 28;60(1):131–149. doi: 10.1016/0022-2836(71)90453-0. [DOI] [PubMed] [Google Scholar]
- Cassuto E., Lash T., Sriprakash K. S., Radding C. M. Role of exonuclease and beta protein of phage lambda in genetic recombination. V. Recombination of lambda DNA in vitro. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1639–1643. doi: 10.1073/pnas.68.7.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S., Levitan D., Sinden R. R. Removal of psoralen interstrand cross-links from DNA of Escherichia coli: mechanism and genetic control. J Mol Biol. 1976 May 5;103(1):39–59. doi: 10.1016/0022-2836(76)90051-6. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Psoralen monoadducts and interstrand cross-links in DNA. Biochim Biophys Acta. 1971 Nov 29;254(1):30–39. doi: 10.1016/0005-2787(71)90111-0. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Repair of DNA containing interstrand crosslinks in Escherichia coli: sequential excision and recombination. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1064–1068. doi: 10.1073/pnas.70.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S., Zusman D. Sedimentation properties of phage DNA molecules containing light-induced psoralen cross-links. Biochim Biophys Acta. 1970 Dec 14;224(2):660–662. doi: 10.1016/0005-2787(70)90607-6. [DOI] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Rupp W. D., Wilkins B. M., Cole R. S. DNA replication and recombination after UV irradiation. Cold Spring Harb Symp Quant Biol. 1968;33:195–207. doi: 10.1101/sqb.1968.033.01.023. [DOI] [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. L. Etude génétique d'un bactériophage tempéré d'Escherichia coli. III. Effet du rayonnement ultraviolet sur la recombinaison génétique. Ann Inst Pasteur (Paris) 1955 Jun;88(6):724–749. [PubMed] [Google Scholar]
- Kohn K. W., Steigbigel N. H., Spears C. L. Cross-linking and repair of DNA in sensitive and resistant strains of E. coli treated with nitrogen mustard. Proc Natl Acad Sci U S A. 1965 May;53(5):1154–1161. doi: 10.1073/pnas.53.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S. A. Sister chromatid exchanges, indices of human chromosome damage and repair: detection by fluorescence and induction by mitomycin C. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3162–3166. doi: 10.1073/pnas.71.8.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. F., Howard-Flanders P. Genetic exchanges caused by ultraviolet photoproducts in phage lambda DNA molecules: the role of DNA replication. Mol Gen Genet. 1976 Jul 23;146(2):107–115. doi: 10.1007/BF00268079. [DOI] [PubMed] [Google Scholar]
- MESELSON M. ON THE MECHANISM OF GENETIC RECOMBINATION BETWEEN DNA MOLECULES. J Mol Biol. 1964 Sep;9:734–745. doi: 10.1016/s0022-2836(64)80178-9. [DOI] [PubMed] [Google Scholar]
- McMilin K. D., Stahl M. M., Stahl F. W. Rec-mediated recombinational hot spot activity in bacteriophage lambda. I. Hot spot activity associated with spi-deletions and bio substitutions. Genetics. 1974 Jul;77(3):409–423. doi: 10.1093/genetics/77.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M. Reciprocal recombination in prophage lambda. J Cell Physiol. 1967 Oct;70(2 Suppl):113–118. doi: 10.1002/jcp.1040700409. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Tomizawa J. Replication of bacteriophage DNA. I. Replication of DNA of lambda phage defective in early functions. J Mol Biol. 1968 Dec 14;38(2):217–225. doi: 10.1016/0022-2836(68)90407-5. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Meun D. H. Repair of radiation-induced damage in Escherichia coli. I. Effect of rec mutations on post-replication repair of damage due to ultraviolet radiation. J Mol Biol. 1970 Aug;51(3):459–472. doi: 10.1016/0022-2836(70)90001-x. [DOI] [PubMed] [Google Scholar]
- Stahl F. W., McMilin K. D., Stahl M. M., Crasemann J. M., Lam S. The distribution of crossovers along unreplicated lambda bacteriophage chromosomes. Genetics. 1974 Jul;77(3):395–408. doi: 10.1093/genetics/77.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venitt S. Interstrand cross-links in the DNA of Escherichia coli B/r and Bs-1 and their removal by the resistant strain. Biochem Biophys Res Commun. 1968 May 10;31(3):355–360. doi: 10.1016/0006-291x(68)90483-x. [DOI] [PubMed] [Google Scholar]


