Abstract
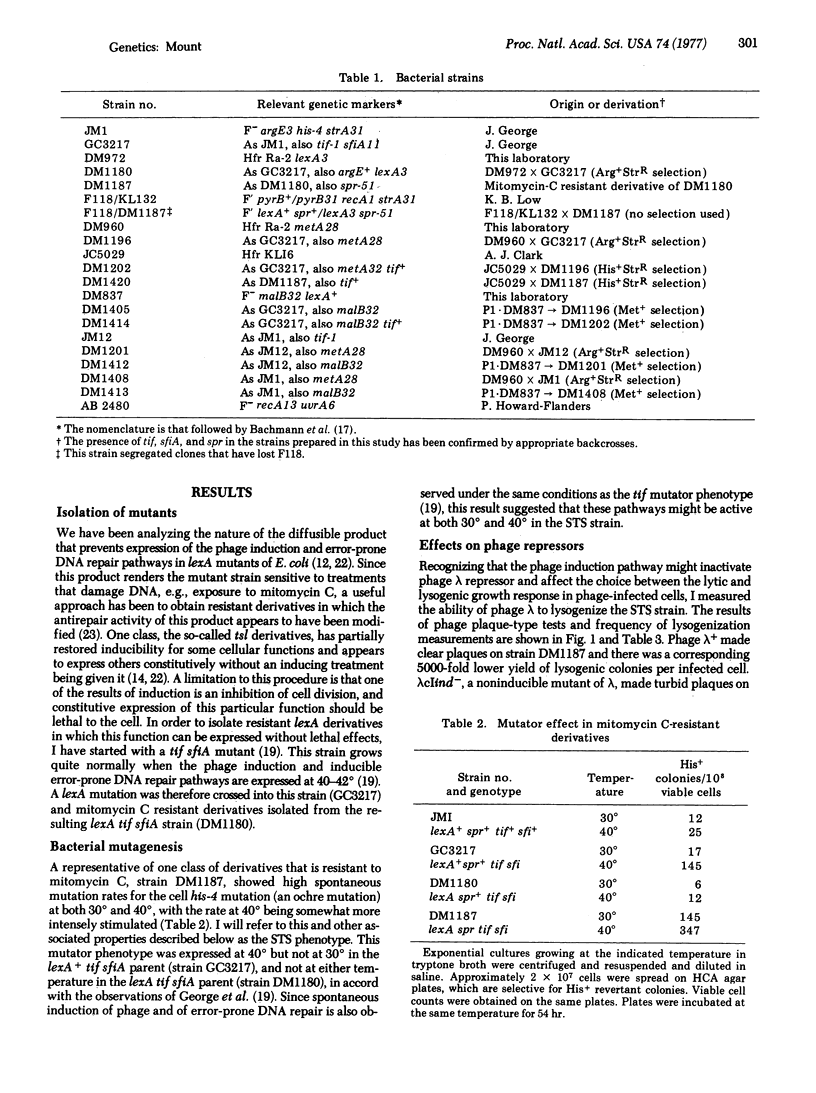
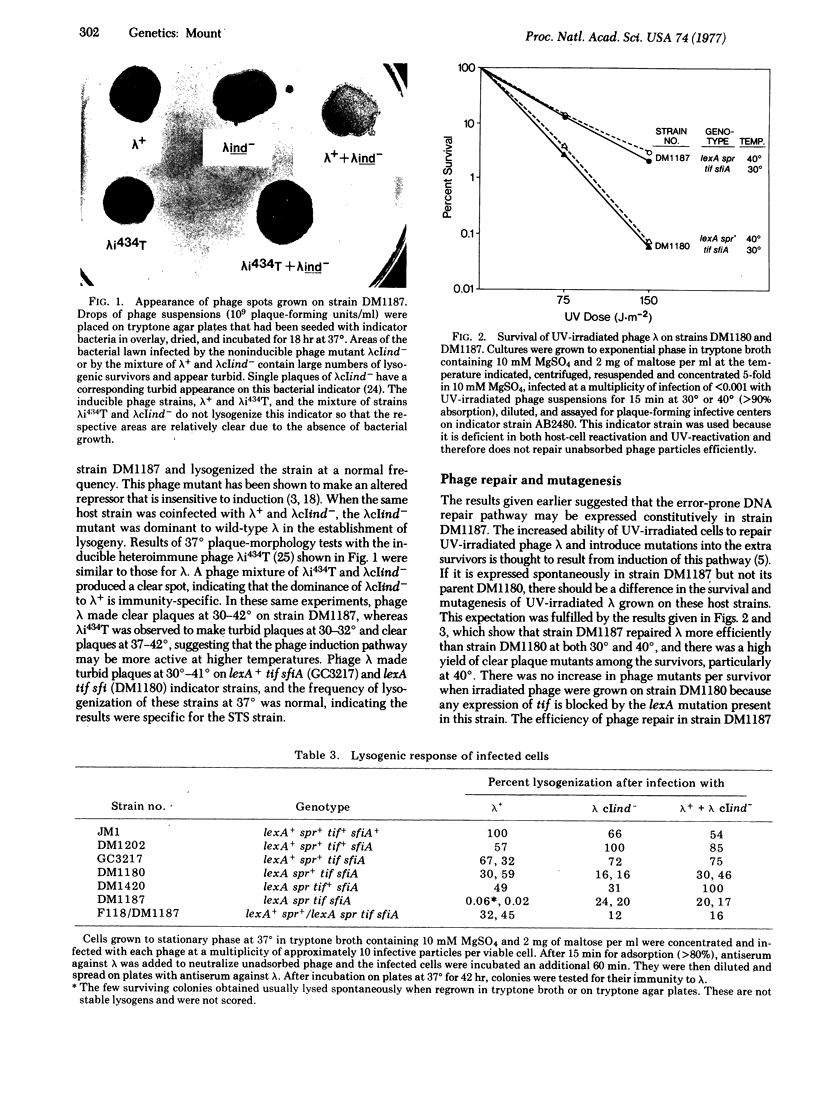
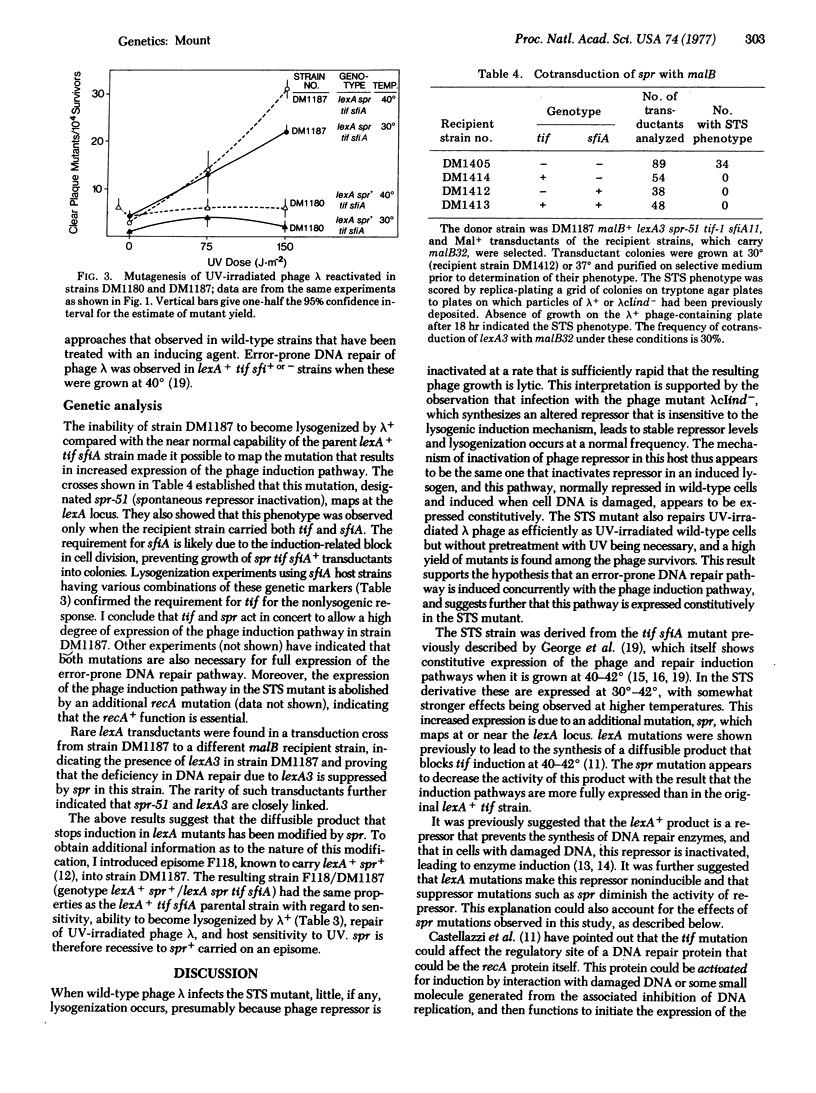
A mutant of E. coli (designated the STS mutant) has been isolated in which the phage induction and error-prone DNA repair pathways appear to be expressed constitutively without the cells having received an inducing signal. Phage lambda was not able to lysogenize this mutant, whereas a noninducible mutant of lambda, lambdacIind-, known to synthesize a repressor that is insensitive to the induction mechanism, lysogenized it normally. This result suggested that normal phage repressor was synthesized in the STS mutant but was then inactivated by the induction mechanism. The STS strain also had mutator characteristics, and showed spontaneous, error-prone repair of UV-damaged phage lambda. Derived from a lexA tif sfiA parent strain, the STS mutant carried an additional mutation spr at the lexA locus that resulted in a high level of expression of the induction pathways. The properties of this and related strains provide additional evidence that induction of phage and induction of error-prone DNA repair occur by a similar mechanism, and further suggest a model for the regulation of these pathways.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek E., Ryan A. Lysogenic induction. Prog Nucleic Acid Res Mol Biol. 1973;13:249–300. doi: 10.1016/s0079-6603(08)60105-1. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. [Prophage induction and cell division in E. coli. II. Linked (recA, zab) and unlinked (lex) suppressors of tif-1-mediated induction and filamentation]. Mol Gen Genet. 1972;119(2):153–174. doi: 10.1007/BF00269134. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Donch J. J., Green M. H., Greenberg J. Conditional induction of lambda-prophage in exrA mutants of Escherichia coli. Genet Res. 1971 Apr;17(2):161–163. doi: 10.1017/s0016672300012155. [DOI] [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- George J., Devoret R., Radman M. Indirect ultraviolet-reactivation of phage lambda. Proc Natl Acad Sci U S A. 1974 Jan;71(1):144–147. doi: 10.1073/pnas.71.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Model for regulation of Escherichia coli DNA repair functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2330–2334. doi: 10.1073/pnas.72.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., CAMPBELL A. Sur le système de répression assurant l'immunité chez les bactéries lysogenes. C R Hebd Seances Acad Sci. 1959 Jun 1;248(22):3219–3221. [PubMed] [Google Scholar]
- Kirby E. P., Jacob F., Goldthwait D. A. Prophage induction and filament formation in a mutant strain of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1903–1910. doi: 10.1073/pnas.58.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody E. E., Hayes W. Chromosome transfer by autonomous transmissible plasmids: the role of the bacterial recombination (rec) system. J Bacteriol. 1972 Jul;111(1):80–85. doi: 10.1128/jb.111.1.80-85.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mound D. W., Walker A. C., Kosel C. Suppression of lex mutations affecting deoxyribonucleic acid repair in Escherichia coli K-12 by closely linked thermosensitive mutations. J Bacteriol. 1973 Nov;116(2):950–956. doi: 10.1128/jb.116.2.950-956.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W. A method for the isolation of phage mutants altered in their response ot lysogenic induction. Mol Gen Genet. 1976 May 7;145(2):165–167. doi: 10.1007/BF00269589. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Kosel C. Ultraviolet light-induced mutation in UV-resistant, thermosensitive derivatives of lexA-strains of Escherichia coli K-12. Mol Gen Genet. 1975;136(2):95–106. doi: 10.1007/BF00272033. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Low K. B., Edmiston S. J. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet lght-induced mutations. J Bacteriol. 1972 Nov;112(2):886–893. doi: 10.1128/jb.112.2.886-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V., Ptashne M. Isolation of the 434 phage repressor. Nature. 1969 May 10;222(5193):541–544. doi: 10.1038/222541a0. [DOI] [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G. Inducible error-prone repair in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2753–2757. doi: 10.1073/pnas.72.7.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa H., Ito T. Inactivation of DNA-binding activity of repressor in extracts of lambda-lysogen treated with mitomycin C. Mol Gen Genet. 1973 Nov 2;126(2):103–110. doi: 10.1007/BF00330987. [DOI] [PubMed] [Google Scholar]
- West S. C., Powell K. A., Emmerson P. T. recA+-dependent inactivation of the lambda repressor in Escherichia coli lysogens by gamma-radiation and by tif expression. Mol Gen Genet. 1975 Nov 3;141(1):1–8. doi: 10.1007/BF00332374. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. The radiation sensitivity of Escherichia coli B: a hypothesis relating filament formation and prophage induction. Proc Natl Acad Sci U S A. 1967 May;57(5):1275–1279. doi: 10.1073/pnas.57.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]




