Abstract
Background
The impact of parathyroidectomy on allograft function in kidney transplant patients is unclear.
Methods
We conducted a retrospective, observational study of all kidney transplant recipients from 1988 to 2008 who underwent parathyroidectomy for uncontrolled hyperparathyroidism (n = 32). Post-parathyroidectomy, changes in estimated glomerular filtration rate (eGFR) and graft loss were recorded. Cross-sectional associations at baseline between eGFR and serum calcium, phosphate, and parathyroid hormone (PTH), and associations between their changes within subjects during the first two months post-parathyroidectomy were assessed.
Results
Post-parathyroidectomy, the mean eGFR declined from 51.19 mL/min/1.73 m2 at parathyroidectomy to 44.78 mL/min/1.73 m2 at two months (p < 0.0001). Subsequently, graft function improved, and by 12 months, mean eGFR recovered to 49.76 mL/min/1.73 m2 (p = 0.035). Decrease in serum PTH was accompanied by a decrease in eGFR (p = 0.0127) in the first two months post-parathyroidectomy. Patients whose eGFR declined by ≥ 20% (group 1) in the first two months post-parathyroidectomy were distinguished from the patients whose eGFR declined by <20% (group 2). The two groups were similar except that group 1 had a higher baseline mean serum PTH compared with group 2, although not significant (1046.7 ± 1034.2 vs. 476.6 ± 444.9, p = 0.14). In group 1, eGFR declined at an average rate of 32% (p < 0.0001) during the first month post-parathyroidectomy compared with 7% (p = 0.1399) in group 2, and the difference between these two groups was significant (p = 0.0003). The graft function recovered in both groups by one yr. During median follow-up of 66.00 ± 49.45 months, 6 (18%) patients lost their graft with a mean time to graft loss from parathyroidectomy of 37.2 ± 21.6 months. The causes of graft loss were rejection (n = 2), pyelonephritis (n = 1) and chronic allograft nephropathy (n = 3). No graft loss occurred during the first-year post-surgery.
Conclusion
Parathyroidectomy may lead to transient kidney allograft dysfunction with eventual recovery of graft function by 12 months post-parathyroidectomy. Higher level of serum PTH pre-parathyoidectomy is associated with a more profound decrease in eGFR post-parathyroidectomy.
Keywords: graft dysfunction, hyperparathyroidism, kidney transplant, outcomes, parathyroidectomy
Secondary hyperparathyroidism is a serious complication of chronic kidney disease. With progression of kidney disease, abnormalities in calcium–phosphorus and vitamin D (1, 25 (OH)2 vitamin D) metabolism progress, directly affecting the expression of parathyroid hormone (PTH) and ultimately leading to asymmetric and nodular parathyroid gland hyperplasia (1). Successful kidney transplantation is thought to at least partially mitigate this process with amelioration of the endocrine and metabolic effects of secondary hyperparathyroidism. This effect usually occurs in the first few months after transplantation, but the effect is not universal. Despite relatively normal renal function, about 1–20% of patients may have persistent hyperparathyroidism post-transplant, often necessitating parathyroidectomy (2–5). Persistent hyperparathyroidism is known to be associated with tubulointerstitial calcifications and inferior graft outcomes in kidney allografts (6). In addition, high PTH levels and hypercalcemia promote vascular calcification, which is associated with increased post-transplant morbidity and mortality (7). Parathyroidectomy is indicated in renal transplant recipients with persistent hyperparathyroidism in association with hypercalcemia and/or progressive and otherwise unexplained renal insufficiency. Paradoxically, the procedure itself has been reported to adversely affect graft function. Several studies have reported an early decline in allograft function post-parathyroidectomy (8–10) with persistence of graft dysfunction in some patients (8, 9, 11). Conversely, recent data suggest no adverse impact on the long-term graft function (12–14).
We set out to identify the impact of parathyroidectomy on graft function post-parathyroidectomy at our institution.
Materials and methods
Subjects and study protocol
This was a retrospective study of kidney transplant recipients performed at The Ohio State University Medical Center from 1988 to 2008. Data were obtained from the OSUMC transplant database after getting an approval of institutional review board (IRB). Charts of the transplant recipients were reviewed, and patients who underwent parathyroidectomy post-transplant were identified. The following data were collected from the charts: age, sex, race, body mass index (BMI), diabetes, hypertension, panel reactive antibody, type of transplant, baseline immunosuppression, use of angiotensin-converting enzyme inhibitors, angiotensin receptor blocker, calcium, vitamin D, or cinacalcet, serum PTH, calcium, phosphate, estimated glomerular filtration rate (eGFR), time to parathyroidectomy, and subtotal vs. total parathyroidectomy.
Measurements and clinical outcomes
Serum intact PTH level was measured using an electrochemiluminescence immunoassay (Elecsys PTH; Roche Diagnostics, Mannhein, Germany). The eGFR (mL/min/1.73 m2) was calculated using four-variable MDRD calculation. Clinical variables including eGFR, serum calcium, PTH, and phosphate were recorded for 12 months prior and 12 months post-parathyroidectomy. Pre-parathyroidectomy eGFR was measured every 1–2 months with last data point one month prior to parathyroidectomy. Post-parathyroidectomy, eGFR was obtained at day 1, day 7, day 14, 1 month, 2 months, 3 months, 4 months, 6 months, 9 months, and 12 months. The degree of correlation was assessed between post-parathyroidectomy eGFR and PTH, calcium and phosphate using the first two months of repeated measures data. All episodes of acute kidney injury, rejection, graft failure, and patient deaths were recorded post-parathyroidectomy. Graft failure was defined as return to dialysis after transplantation.
Statistical analysis
The analysis was performed using SAS JMP statistical package (Version 9; SAS Inc., Cary, NC, USA). Demographic and procedural data are expressed as counts, percentages, or mean ± SD. Unpaired t tests, Fisher's exact test, or chi-squared analysis were used as appropriate to assess the differences between groups. Three PTH values that were recorded as below detection limits were imputed with randomly generated uniformly distributed values below the threshold. Longitudinal data on eGFR were analyzed on logarithmic scale using mixed model with subjects as random effects and time as a nominal effect. Interaction effects were used to differentiate between the patterns in the two groups based on the eGFR decline, and post-hoc t tests were used to test different contrasts. Similar mixed models were used to assess the association between the changes in eGFR and phosphate, log(PTH), and calcium. Pearson correlation (r) was used to measure the cross-sectional association between the baseline values of these variables. The GFR scatter plots were produced using mean eGFR data overtime. p-Value <0.05 was considered significant.
Results
From 1988 to 2008, kidney transplants were performed on 4052 patients at our center. Of these, 32 (0.8%) patients underwent parathyroidectomy for persistent hyperparathyroidism post-transplant. Thirty-one (97%) patients underwent subtotal parathyroidectomy. Serum PTH decreased in all patients afterward and none of them had recurrence of hyperparathyroidism post-parathyroidectomy. The demographics of study patients are listed in Table 1.
Table 1. Baseline demographics and clinical characteristics of the study patients (n = 32).
| Patient population Variables (n = 32) | Number (%) |
|---|---|
| Age (yr) | 48.9 ± 12.6 |
| Female sex (%) | 12 (37.5) |
| Caucasian race (%) | 26 (81.25) |
| BMI | 28.9 ± 7.1 |
| DM (%) | 9 (33.3) |
| HTN (%) | 32 (96.9) |
| PRA >10% (%) | 5 (15.2) |
| Deceased donor transplant (%) | 18 (57.25) |
| Non-primary transplant (%) | 7 (21.2) |
| Time to parathyroidectomy from KTx (months) | 45.3 ± 51.2 |
| Subtotal parathyroidectomy (%) | 31 (96.9%) |
| Serum PTH- baseline (ng/mL) | 623.7 ± 677.5 |
| Serum PTH (ng/mL) – 1 wk | 42.7 ± 53.5 |
| Serum calcium (mg/dL) – Baseline | 10.48 ± 0.83 |
| Serum calcium (mg/dL) – 1 wk | 8.21 ± 1.13 |
| eGFR mL/min/1.73 m2 – baseline | 51.2 ± 18.9 |
| Median time to follow up (months) (range) | 63.00 (0–168) |
BMI, body mass index; DM, diabetes mellitus; HTN, hypertension; PRA, panel reactive antibodies; KTx, kidney transplant; PTH, parathyroid hormone; eGFR, estimated glomerular filtration rate.
Plus-minus values are mean ± SD unless otherwise specified.
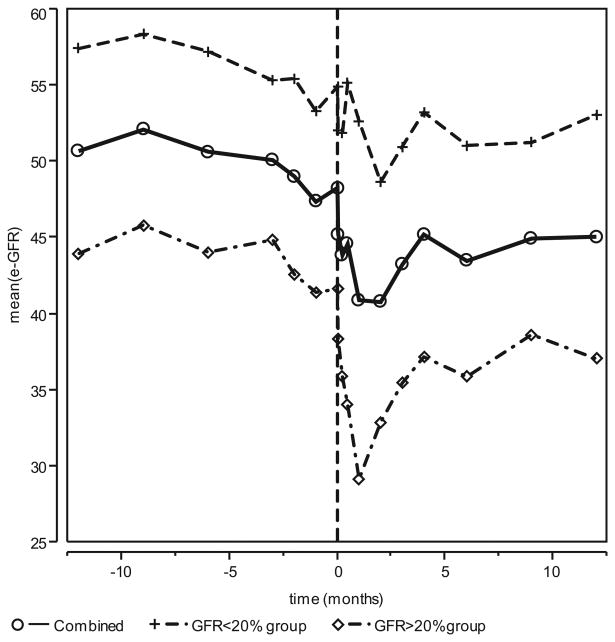
Pre-parathyroidectomy, eGFR was initially stable but began to decline approximately one month prior to surgery. Post-parathyroidectomy, graft function declined with mean eGFR declining from 51.19 mL/min/1.73 m2 at parathyroidectomy to 44.78 mL/min/1.73 m2 at two months. Subsequently, graft function improved, and by 12 months, mean eGFR recovered to 49.76 mL/min/1.73m2(Fig. 1).
Fig. 1.
Mean eGFR vs. time. Assessment of mean eGFR change from 12 months prior to parathyroidectomy to 12 months post-parathyroidectomy. Pre-parathyroidectomy eGFR was measured at −12, −9, −6, −3, −2, and −1 months prior to parathyroidectomy. Time 0 is eGFR immediately prior to parathyroidectomy. Post-parathyroidectomy, eGFR was obtained at day 1, day 7, day 14, 1 month, 2 months, 3 months, 4 months, 6 months, 9 months, and 12 months. For the entire group, eGFR began to decline immediately post-parathyroidectomy. eGFR declined from 51.19 mL/min/1.73 m2 at parathyroidectomy to 44.78 mL/min/1.73 m2 at 2 months. Subsequently, graft function improved and by 12 months mean eGFR recovered to 49.76 mL/min/1.73 m2. For Group 1 (≥20% decline), mean eGFR declined from 41.63 mL/min/1.73 m2 at the time of parathyroidectomy to 32.89 mL/min/1.73 m2 at month 2 and then recovered to 37.68 mL/min/1.73 m2 at 12 months. In group 2, eGFR declined from 54.93 mL/min/1.73 m2 at the time of parathyroidectomy to 49.64 mL/min/1.73 m2 at month 2 and then recovered to 54.35 mL/min/1.73 m2 by 12 months. Mean eGFR declined 32% in the first month post-parathyroidectomy for Group 1 compared with 7% for Group 2 (p = 0.0003). The profiles for the two groups are significantly different (p = 0.0010). eGFR, estimated glomerular filtration rate.
At parathyroidectomy, log(eGFR) was associated with log(PTH) (r = −0.4221; p = 0.0180), phosphate (r = −0.4209; p = 0.0206), but not with calcium (0.3460; p = 0.0611). Post-parathyroidectomy, the changes in log(eGFR) measurements within subjects were correlated with serum calcium, phosphate, and log(PTH) values at similar time points during the first two months. A significant positive correlation was found between PTH and eGFR (Fig. 2). A 15 times decrease in PTH, post-parathyroidectomy was associated with a 8.9% reduction in eGFR (p = 0.0127). There was also a statistically significant association between changes in calcium and eGFR (p = 0.0037). A 20% decline in serum calcium caused a 1% decline in eGFR. There was no significant correlation between changes in eGFR and serum phosphate (p = 0.0536).
Fig. 2.
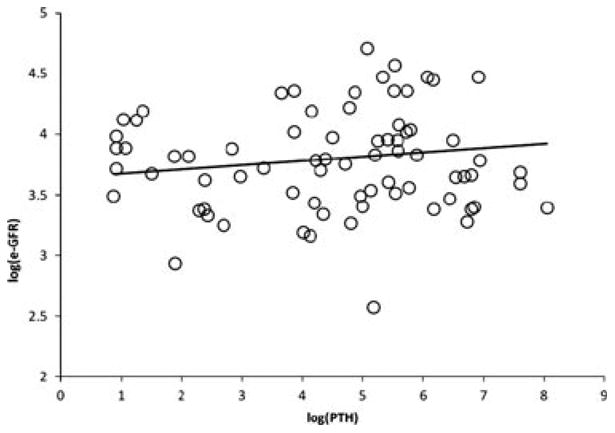
The scatter plot showing positive correlation between the mean logGFR and logPTH (p = 0.0127); A 15 times decrease in parathyroid hormone is associated with a 8.9% reduction in estimated glomerular filtration rate.
Patients whose eGFR declined by ≥20% (group 1, n = 9) in the first two months post-parathyroidectomy were distinguished from the patients whose eGFR declined by <20% (group 2, n = 23) (Table 2). A 20% decline in eGFR was chosen as cutoff to avoid analytical and biologic variability. The two groups were similar in terms of several demographic and clinical variables except that group 1 as compared with group 2 had a higher baseline mean serum PTH although not statistically significant (1046.7 ± 1034.2 vs. 476.6 ± 444.9, p = 0.14) and a longer median length of hospital stay (9 ± 8.9 vs. 3 ± 5.9 d, p = 0.03) (Table 2). In group 1, eGFR declined at a significant average rate of 32% (p < 0.0001) during the first month post-parathyroidectomy compared with a non-significant decline rate of 7% (p = 0.1399) in group 2. The difference between these two rates was significant (p = 0.0003). Post-parathyroidectomy, in group 1, the mean eGFR declined from 41.63 mL/min/1.73 m2 at the time of parathyroidectomy to 32.89 mL/min/1.73 m2 at month 2 and then recovered to 37.68 mL/min/1.73 m2 at 12 months with a continuous upward trend. In group 2, eGFR declined from 54.93 mL/min/1.73 m2 at the time of parathyroidectomy to 49.64 mL/min/1.73 m2 at month 2 and then recovered to 54.35 mL/min/1.73 m2 by 12 months.
Table 2. Baseline characteristics of those with ≥20% and <20% GFR decline in two months post-parathyroidectomy.
| Variables | GFR decline ≥20% N = 9 | GFR decline <20% N = 23 | p-Value |
|---|---|---|---|
| Age (yr) | 46.1 ± 17.3 | 50.0 ± 10.5 | 0.54 |
| Female sex (%) | 4 (44.4) | 8 (34.8) | 0.696a |
| Caucasian race (%) | 6(66.7) | 20 (87.0) | 0.3140a |
| Baseline eGFR (mL/min/1.73 m2) | 41.6 ± 9.0 | 54.9 ± 20.5 | 0.09b |
| eGFR decline at 1 month post-parathyroidectomy (%) | 32.4 ± 11.08 | 6.8 + 5.01 | 0.0003c |
| Baseline PTH (ng/mL) | 1046.7 ± 1034.2 | 476.6 ± 444.9 | 0.14 |
| Baseline serum phosphate (mg/dL) | 3.0 ± 0.75 | 2.6 ± 0.65 | 0.23 |
| Baseline serum calcium (mg/dL) | 10.0 ± 0.8 | 10.6 ± 0.8 | 0.08 |
| ACE inhibitor (%) | 3 (33.3) | 7 (30.4) | 1.00a |
| Cinacalcet (%) | 1 (11.1) | 2 (8.7) | 1.00a |
| Cyclosporine (%) | 8 (88.9) | 16 (69.6) | 0.39a |
| Time to parathyroidectomy post-transpant (months) | 33 ± 34.5 | 50 ± 55.2 | 0.39 |
| Total parathyroidectomy (%) | 1 (11.1) | 0 (0) | 0.64 |
| Cause of ESRD (%)d | |||
| Diabetes | 0 (0) | 9 (39.1) | |
| Hypertension | 2 (22.2) | 2 (8.7) | |
| Other | 7 (77.8) | 12 (52.2) | 0 |
| Mortality (%) | 3 (33.3) | 2 (8.7) | 0.12a |
| DDKtxp (%) | 5 (55.6) | 14 (60.9) | 1.00a |
| Re-transplant (%) | 3 (33.3) | 4 (17.4) | 0.37a |
| Initial length of hospital stay median (in d) (range) | 5 (1–30) | 3 (1–22) | 0.66b |
| Readmit for hypocalcemia (%) | 1 (11.1) | 3 (13.0) | 0.61 |
PTH, parathyroid hormone; eGFR, estimated glomerular filtration rate; ACE, angiotensin-converting enzyme; ESRD, end-stage renal disease; DDKtxp, deceased donor kidney transplant.
Plus-minus values are mean ± SD unless otherwise specified.
Fisher's exact test; use this test for comparing the two by two comparisons.
t Test for log-transformed data.
Estimates and p-value based on mixed model fit to log(eGFR) data.
Pearson chi-squared test (p = 0.0749).
Kidney allograft biopsy data were available in 16 patients pre-parathyroidectomy. Of the available allograft biopsies, four showed nephrocalcinosis with three also showing mild to moderate fibrosis, eight had acute rejection with four showing mild to moderate fibrosis.
Cyclosporine (CSA) is the calcineurin inhibitor used at our institution (Table 2). Pre-parathyroidectomy, 75% (n = 24) of patients were maintained on CSA, whereas 59% (n = 19) remained on CSA post-parathyroidectomy. A correlation between eGFR and CSA level post-parathyroidectomy was attempted but could not be made as the target range of CSA level differed from patient to patient depending on their time post-transplant.
Post-parathyroidectomy, four patients (12.5%) required readmission for hypocalcemia with mean time to readmission of 17 ± 18.9 d and length of hospital stay of 9.5 ± 5.3 d. During median follow-up of 66.00 ± 49.45 months post-parathyroidectomy, 6 (18.8%) patients lost their graft with a mean time to graft loss from parathyroidectomy of 37.2 ± 21.6 months. Patients who lost their graft (n = 5) had higher baseline mean serum PTH compared with those who did not lose their graft (n = 27) [1168.74 ± 777.51 ng/mL vs. 518.9 ± 619.1 ng/mL, p = 0.0245; t test applied to log(PTH)]. The two groups did not differ in terms of age [46.1 ± 17.3 vs. 50.0 ± 10.5 yr, p = 0.54], female gender [4 {44.4%} vs. 8 {34.8%}, p = 0.696], Caucasian race [6 {66.7} vs. 20 {87%}, p = 0.314], body mass index [28.6 ± 5.6 vs. 29.0 ± 7.68; p = 0.84], baseline eGFR [41.6 ± 9.0 vs. 54.9 ± 20.5, p = 0.09], baseline serum calcium [10.0 ± 0.8 vs. 10.6 ± 0.8, p = 0.08], deceased donor transplant [5 {44.4} vs. 14 {60.9}, p = 0.88], and median time to parathyroidectomy [33 ± 57 vs. 22 ± 50 months, p = 0.39] (Table 2).
Post-parathyroidectomy, there were two episodes of acute rejection within a year at the mean 3.0 ± 1.4 months. No graft loss occurred during the first year. The causes of graft loss were rejection in two patients, pyelonephritis in one patient, and chronic allograft nephropathy in the other three. Five patients died post-parathyroidectomy with two deaths within one yr of parathyroidectomy secondary to pneumonia and cardiac arrest. The other three deaths were attributed to cardiac arrest, sepsis, and unknown cause.
Discussion
In this single-center study of patients undergoing parathyroidectomy post-transplant for persistent hyperparathyroidism, we found an increased risk of early GFR decline post-parathyroidectomy. In our study population, GFR decline continued for the first two months post-parathyroidectomy but then began to recover approaching pre-parathyroidectomy levels by 12 months post-parathyroidectomy. These data suggest that post-parathyroidectomy GFR decline is transient and is likely to recover to pre-parathyroidectomy levels with time. Our data are in concordance with previous studies, which also concluded that parathyroidectomy may lead to stabilization of kidney function post-transplant (12–14).
The mechanism for early graft dysfunction post-parathyroidectomy is not clear. We postulate that this occurs due to hemodynamic alterations following surgery. The decline in graft function correlated inversely with changes in serum PTH levels. Pre-parathyroidectomy PTH was significantly higher in patients in Group 1. PTH has been shown to have significant vasodilator effects in the renal vascular bed in animal models, and it increases renal blood flow by stimulating adenylate cyclase activity in the renal vasculature (15–17). In addition, PTH increases nitric oxide production in endothelial cells leading to vasodilation (18). Overtime, the kidney develops a reliance on the increased blood flow. Therefore, abrupt reduction in PTH via parathyroidectomy could lead to decreased renal perfusion and transient graft dysfunction. Additionally, perioperative injury related to the procedure itself may also contribute to hemodynamic injury and early decline. Alternatively, continued graft decline after surgery may be a continuation of pre-parathyroidectomy GFR decline thought to be due to worsening nephrocalcinosis. As shown in Fig. 1, GFR began to decline at one month prior to surgery suggesting worsening graft function due to hyperparathyroidism. Presumably, this is a direct result of interstitial damage from worsening nephrocalcinosis and because this injury requires time to recover, it is also conceivable that the decline in GFR post-parathyroidectomy is at least in part the result of built-up nephrocalcinosis prior to surgery. Obtaining biomarkers for acute kidney injury post-parathyroidectomy may help elucidate the true mechanism for kidney injury post-parathyroidectomy.
Why the early rapid decline in graft function post-parathyroidectomy is slowed down subsequently is unknown. It could be due to recovery from hemodynamic injury, improvement in blood pressure post-parathyroidectomy (19), and/or due to probable reversal of graft nephrocalcinosis in the long term. Whether these factors were responsible for the improvement in graft function in our patients is not clear.
The calcimimetic cinacalcet was approved in 2004 and is being recommended as a promising alternative to parathyroidectomy in patients with persistent hyperparathyroidism after renal transplantation (20). Our data do not allow us a comparison of these two options, as we only had four patients who were treated with cinacalcet and three of them required parathyroidectomy. Analogous to the effect of parathyroidectomy on early allograft function cinacalcet may also lead to renal function decline (21), and its long-term effects on allograft function remains unknown. In addition, cinacalcet is expensive and may still not be enough to control PTH in those with severe parathyroid hyperplasia. In this circumstance, parathyroidectomy may still be necessary. Future studies need to confirm the risks vs. benefits and provide a cost analysis of cinacalcet therapy vs. parathyroidectomy.
There are few limitations to our study that must be mentioned. First, this was a single-center, retrospective cohort study. Second, due to a long duration of our study, the PTH assay at our institution changed over the study period. However, it is unlikely to affect our results as correlations of change in PTH with GFR decline were made in individual patients and only over a short-period post-parathyroidectomy. Finally, we could not study a correlation between CSA level and GFR change post-parathyroidectomy due to variability in therapeutic CSA range based on time post-transplant.
In conclusion, our observations indicate that the negative impact of parathyroidectomy on graft function is transient with recovery of graft function expected at 12-month post-parathyroidectomy. Higher level of serum PTH pre-parathyoidectomy is associated with a more profound decrease in eGFR post-parathyroidectomy.
Acknowledgments
Statistical work was supported in part by Award Number UL1RR025755 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Conflict of interest: None.
References
- 1.Drueke TB. Cell biology of parathyroid gland hyperplasia in chronic renal failure. J Am Soc Nephrol. 2000;11:1141. doi: 10.1681/ASN.V1161141. [DOI] [PubMed] [Google Scholar]
- 2.D'Alessandro AM, et al. Tertiary hyperparathyroidism after renal transplantation: operative indications. Surgery. 1989;106:1049. discussion 1055–1056. [PubMed] [Google Scholar]
- 3.Parfitt AM. Hypercalcemic hyperparathyroidism following renal transplantation: differential diagnosis, management, and implications for cell population control in the parathyroid gland. Miner Electrolyte Metab. 1982;8:92. [PubMed] [Google Scholar]
- 4.Schmid T, Muller P, Spelsberg F. Parathyroidectomy after renal transplantation: a retrospective analysis of long-term outcome. Nephrol Dial Transplant. 1997;12:2393. doi: 10.1093/ndt/12.11.2393. [DOI] [PubMed] [Google Scholar]
- 5.Evenepoel P, et al. Natural history of parathyroid function and calcium metabolism after kidney transplantation: a single-centre study. Nephrol Dial Transplant. 2004;19:1281. doi: 10.1093/ndt/gfh128. [DOI] [PubMed] [Google Scholar]
- 6.Gwinner W, et al. Early calcification of renal allografts detected by protocol biopsies: causes and clinical implications. Am J Transplant. 2005;5:1934. doi: 10.1111/j.1600-6143.2005.00938.x. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez D, et al. Clinical impact of preexisting vascular calcifications on mortality after renal transplantation. Kidney Int. 2005;67:2015. doi: 10.1111/j.1523-1755.2005.00303.x. [DOI] [PubMed] [Google Scholar]
- 8.Rostaing L, et al. Changes in blood pressure and renal function following subtotal parathyroidectomy in renal transplant patients presenting with persistent hypercalcemic hyperparathyroidism. Clin Nephrol. 1997;47:248. [PubMed] [Google Scholar]
- 9.Garcia A, et al. Effect of parathyroidectomy on renal graft function. Transplant Proc. 2005;37:1459. doi: 10.1016/j.transproceed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Evenepoel P, et al. Impact of parathyroidectomy on renal graft function, blood pressure and serum lipids in kidney transplant recipients: a single centre study. Nephrol Dial Transplant. 2005;20:1714. doi: 10.1093/ndt/gfh892. [DOI] [PubMed] [Google Scholar]
- 11.Lee PP, et al. Effects of parathyroidectomy on renal allograft survival. Kidney Blood Press Res. 2004;27:191. doi: 10.1159/000079810. [DOI] [PubMed] [Google Scholar]
- 12.Evenepoel P, et al. Parathyroidectomy after successful kidney transplantation: a single centre study. Nephrol Dial Transplant. 2007;22:1730. doi: 10.1093/ndt/gfm044. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira GF, et al. Parathyroidectomy after kidney transplantation: short-and long-term impact on renal function. Clinics (Sao Paulo) 2011;66:431. doi: 10.1590/S1807-59322011000300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kandil E, et al. Exploring the effect of parathyroidectomy for tertiary hyperparathyroidism after kidney transplantation. Am J Med Sci. 2010;339:420. doi: 10.1097/MAJ.0b013e3181d8b6ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musso MJ, et al. The vasodilator action of parathyroid hormone fragments on isolated perfused rat kidney. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:246. doi: 10.1007/BF00168976. [DOI] [PubMed] [Google Scholar]
- 16.Helwig JJ, et al. Parathyroid hormone stimulation of renal adenylate cyclase in various vertebrate species: evidence for an effect in the frog. Comp Biochem Physiol A Comp Physiol. 1987;88:349. doi: 10.1016/0300-9629(87)90496-8. [DOI] [PubMed] [Google Scholar]
- 17.Charbon GA. A rapid and selective vasodialtor effect of parathyroid hormone. Eur J Pharmacol. 1968;3:275. doi: 10.1016/0014-2999(68)90144-1. [DOI] [PubMed] [Google Scholar]
- 18.Kalinowski L, Dobrucki LW, Malinski T. Nitric oxide as a second messenger in parathyroid hormone-related protein signaling. J Endocrinol. 2001;170:433. doi: 10.1677/joe.0.1700433. [DOI] [PubMed] [Google Scholar]
- 19.Pizzarelli F, et al. Parathyroidectomy and blood pressure in hemodialysis patients. Nephron. 1993;63:384. doi: 10.1159/000187239. [DOI] [PubMed] [Google Scholar]
- 20.Kruse AE, et al. The calcimimetic cinacalcet normalizes serum calcium in renal transplant patients with persistent hyperparathyroidism. Nephrol Dial Transplant. 2005;20:1311. doi: 10.1093/ndt/gfh924. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz A, et al. The effect of cinacalcet on bone remodeling and renal function in transplant patients with persistent hyperparathyroidism. Transplantation. 2011;91:560. doi: 10.1097/TP.0b013e3182079431. [DOI] [PubMed] [Google Scholar]