Abstract
We correlated von Willebrand factor activity indices and brain natriuretic peptide (BNP) with measures of aortic stenosis (AS) severity, bleeding, symptoms, and freedom from death or aortic valve replacement. Patients with AS (n=66 [16 mild, 20 moderate, 30 severe]) and aortic valve replacement (n=21) were assessed with VWF antigen (VWF:Ag), VWF latex agglutination immunoturbidic activity (VWF:Ltx), platelet function analyzer collagen plus adenosine diphosphate (PFA-CADP), VWF multimer ratio (VWF:mult ratio), and BNP level after echocardiography. In AS patients, mean gradient correlated with BNP (Spearman r=0.29, P=.02), VWF:Ltx/VWF:Ag (r=–0.41, P<.001), PFA-CADP (r=0.49, P<.001), and VWF:mult ratio (r=–0.76, P<.001). The area under the curve (95% CI) for detection of severe AS was 0.62 (0.48-0.77) by elevated BNP, 0.81 (0.69-0.92) by PFA-CADP closure time, 0.69 (0.55-0.82) by VWF:Ltx/VWF:Ag ratio, and 0.86 (0.76-0.95) by VWF:mult ratio. For VWF:mult ratio, a threshold of 0.15 yielded sensitivity and specificity for severe AS of 77% and positive predictive value of 74%. Bleeding (in 14%) was associated with prolonged PFA-CADP and reduced VWF:Ltx/VWF:Ag. Symptoms were associated with elevated BNP and low Duke Activity Status Index score. In 66 AS patients, freedom from death (n=4) or aortic valve replacement (n=22) was associated with PFA-CADP (P=.003), VWF high molecular weight multimers (HMWM) (P=.009), and VWF:Ltx/VWF:Ag (P<.001), but not BNP (P=.32). In severe AS vs aortic valve replacement, PFA-CADP and VWF:mult ratio differed (P<.001), but BNP and VWF:Ltx/VWF:Ag did not. In conclusion, VWF activity indices are associated with AS severity and bleeding, and predictive of cardiovascular outcome.
Keywords: aortic stenosis, von Willebrand factor, brain natriuretic peptide, aortic valve replacement
Introduction
Decision making in aortic stenosis (AS) is confounded by overlapping moderate and severe designations (1-3) and uncertainty whether symptoms might be undetected or originate from other clinical conditions (4-6). Various echocardiographic severity criteria are inconsistent in assessments of prognosis (7,8). The case for the use of a biomarker that reflects disease severity, reliably detects progression, and assesses prognosis and response to therapy was made in a recent comprehensive review of natriuretic peptides by Steadman and colleagues (9). In light of the strong graded relationship between functional abnormalities of von Willebrand factor (VWF) and AS gradient in surgical cases (10-14) and in hypertrophic cardiomyopathy with obstruction (15) and the correction of laboratory VWF functional abnormalities and clinical bleeding tendency with aortic valve replacement (AVR) (10,16) or septal myectomy in hypertrophic cardiomyopathy (17), we sought to assess the performance of VWF activity indices as biomarkers of AS severity across a broad range of disease, in patients who had undergone aortic valve replacement, and in comparison with brain natriuretic peptide (BNP).
Methods
Patients referred for echocardiography with AS, peak transvalvular velocity greater than 2.5 m/s, or aortic valve replacement were eligible. Clinical data were collected and a bleeding questionnaire modified from the consensus conference on the assessment of von Willebrand disease (18) (Supplemental Table 1) and the Duke Activity Status Index (19) (supplemental material) were administered prospectively. Exclusion criteria were inadequate images, hemoglobin less than 8 g/dL, thienopyridine therapy, or severe left heart regurgitant lesions or mitral stenosis. Outcomes were determined by medical record review and/or contact by telephone to establish vital status and dates of surgery or death. Written informed consent was obtained, and the study complied with the Declaration of Helsinki and was approved by the Mayo Clinic Institutional Review Board.
The echocardiographic assessment of AS followed American Society of Echocardiography guidelines (20). Wall shear was calculated as 4 times blood viscosity estimated at 0.035 poise, times mean transvalvular blood velocity divided by the radius of the stenosis (10). Correlations between biomarkers and all usual measures of AS severity and wall shear stress were calculated, but for associations with bleeding risk, symptoms, and high-molecular-weight multimer (HMWM) loss, we used mean aortic gradient higher than 40 mm Hg as the definition of severe AS, 25 to 40 mm Hg as moderate AS, and less than 25 mm Hg as mild AS.
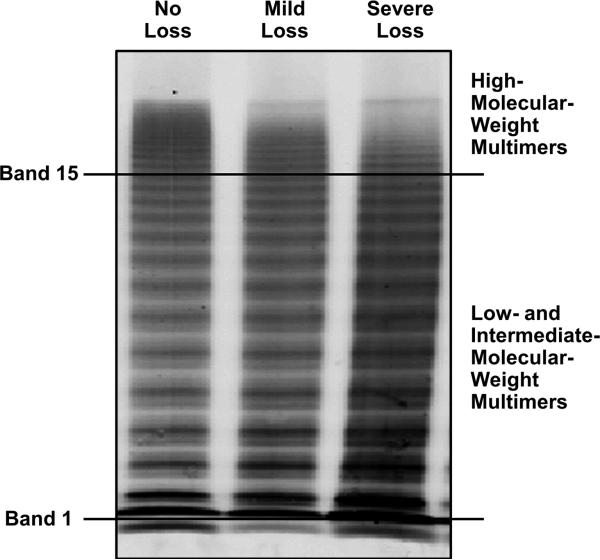
Citrated plasma was stored frozen until testing, usually after 1 to 3 months. VWF antigen (VWF:Ag) and VWF latex agglutination immunoturbidic activity (VWF:Ltx), were both expressed as a percentage of control as previously described (21,22). The primary clinical interpretation of VWF multimers at our institution is based on visual inspection of a patient's plasma and control pooled plasma (figure 1) recorded as subjective loss of highest molecular weight multimers (HMWM) or normal HMWM. This was recorded as a dichotomous variable. Quantification of VWF multimers, a research tool at our institution, involved scanning of raw fluorescent intensity of patients’ multimer electrophoresis using an Odyssey Infrared Imager version 2.0 (22), enclosing bands 2-15 containing low molecular weight multimers and then another adjacent one enclosing intermediate and high molecular weight mulimers, bands 16-20 (figure 1). The fluorescent intensity of bands16-20 divided by bands 2-15 was designated as VWF:mult ratio, and recorded as a continuous variable.. It was elected not to include the first band in our calculations due to significant artifact associated with this band, as can be seen in samples from type 3 von Willebrand disease. Platelet function analyzer collagen plus adenosine diphosphate (PFA-CADP) closure time (Siemens USA) was determined. The reference interval in the laboratory is 57 to 121 seconds.
Figure 1.
Gel electrophoresis of von Willebrand factor in aortic stenosis patients with no, moderate, or severe loss of high-molecular-weight multimer. The quantitative ratio is the in-gel infrared intensity of bands higher than 15 to bands 2 through 15.
The BNP immunoenzymatic assay (Biosite) was performed using patients’ fresh serum samples (Beckman Coulter DXI 800 immunoassay system). Normal values for this assay are age and sex adjusted (women have considerably higher normal values than men). In general, values of 200 to 400 ng/L suggested moderate heart failure, and values higher than 400 ng/L indicated moderate to severe heart failure.
Patients were defined as having nonclinically significant bleeding if they had all negative questionnaire responses or if they had incidental bruising, epistaxis, or blood in stool but no clinically relevant events in the history over the past 3 years; received remote surgery-related transfusion; or were postmenopausal women who reported remote menorrhagia. The Duke Activity Status Index is a 12-point activity index that yields a raw score and an estimate of maximum exercise oxygen consumption (19).
Associations of biomarkers with echocardiographic measures of AS severity were estimated by the Spearman rank correlation coefficient (r) and bootstrapped 95% CIs. Receiver operating characteristic curves were constructed to explore the diagnostic utility of these biomarkers for severe AS (defined as a mean gradient higher than 40 mm Hg); area under the curves and 95% confidence intervals were estimated. Comparison of the areas under the curve of each VWF biomarker to that of BNP was performed on the basis of methods proposed by DeLong et al (23). Sensitivity and specificity with exact binomial 95% confidence intervals were estimated for BNP above the individual's upper limit of normal value, PFA-CADP closure time longer than 121 seconds, VWF:Ltx/VWF:Ag ratio less than 0.8, and VWF:mult ratio higher than 0.15.
In secondary analysis, Kaplan-Meier curves estimating freedom from death and aortic valve replacement were constructed on the basis of predetermined cutoff values; age-adjusted hazard ratios from Cox regression models were estimated. Comparisons between patient groups were based on the Mann-Whitney test for numerical variables and the Fisher exact test for categorical variables. Because of the exploratory nature of the analyses, adjustment for multiple testing was not done. P values less than .05 were considered statistically significant. Analyses were performed using SAS (version 9.2; SAS Institute Inc) and R Statistical Software (version 2.14.0; R Foundation for Statistical Computing).
Results
A total of 87 patients had research biomarkers obtained, 32 women and 57 men, 66 with AS and 21 with aortic valve replacement (11 mechanical, 10 bioprosthetic). In 65 (75%), biomarkers were obtained within 48 hours of the echocardiogram. One AS patient's plasma was processed incorrectly for VWF measures, but PFA-CADP and BNP were performed. Baseline characteristics of the 66 AS patients are summarized in Table 1. Thirty-one patients (47%) had physician-identified AS-related symptoms. Over a median duration of 11.7 months, 4 AS patients died and 22 underwent aortic valve replacement.
Table 1.
Characteristics of Patients With Native Valve Aortic Stenosis Presenting for Clinically Indicated Echocardiography
| Variable | Aortic Stenosis (N=66) |
|---|---|
| Men | 43 (65%) |
| Age, (years) | 79 (70-83) |
| Hypertension | 46 (70%) |
| Glomerular filtration rate, (mL/min) | 60 (58-60) |
| Documented coronary disease | 15 (23%) |
| Stroke or emboli | 8 (12%) |
| Diabetes mellitus | 14 (21%) |
| Atrial fibrillation | 14 (21%) |
| Heart failure | 11 (17%) |
| Current/prior smoker | 43 (65%) |
| Aspirin use | 41 (62%) |
| Statin use | 32 (48%) |
| Symptoms of aortic stenosis | 31 (47%) |
| Left ventricular hypertrophy(ECG) | 22 (33%) |
Included as a control group were 21 patients who had prior aortic valve replacement. Their median age was 81 years (interquartile range, 76-86 years). Compared to the 30 severe AS patients, aortic valve replacement patients had significantly higher VWF:mult ratio (median, 0.173 vs 0.128; P<.001), had shorter PFA-CADP closure time (median, 92 vs 187 seconds; P<.001), and were less likely to have a loss of HMWM (10% vs 87%; P<.001). The BNP level and VWF:Ltx/VWF:Ag ratio were not significantly different between severe AS and patients with aortic valve replacement (Table 2). Indexed valve areas in valve replacement patients, not corrected for pressure recovery, suggested the presence of patient prosthesis mismatch (aortic valve area index ≤0.65 cm2/m2) in 5/21 patients, one of whom had VWF:mult ratio < 0.15, and none with loss of HMWM.
Table 2.
Comparison of Severe Aortic Stenosis (Mean Gradient>40 mm Hg) and Aortic Valve Replacement
| Variable | Severe Aortic Stenosis (n=30) | Aortic Valve Replacement (n=21) | P Value |
|---|---|---|---|
| Mean gradient (mm Hg) | 51 (45-62) | 15 (12-19) | <.001 |
| Peak aortic velocity (m/s) | 4.50 (4.30-4.93) | 2.46 (2.40-2.80) | <.001 |
| Brain natriuretic peptide (ng/L) | 177 (74-592) | 203 (115-369) | .84 |
| Elevated brain natriuretic peptide | 21 (70%) | 17 (81%) | .52 |
| Platelet function analyzer adenosine closure time (seconds) | 187 (128-223) | 92 (82-106) | <.001 |
| VWF latex activity / VWF antigen ratio | 0.87 (0.76-0.94) | 0.96 (0.86-1.00) | .09 |
| VWF:multimer ratio | 0.128 (0.110-0.149) | 0.173 (0.162-0.185) | <.001 |
| Loss of VWF high molecular weight multimers | 26 (87%) | 2 (10%) | <.001 |
Loss of HMWM was detected in 37 of the 65 patients with AS (Table 3), which was highly associated with severity of AS, as indicated by mean gradient (P<.001), peak aortic velocity (P<.001), and wall shear stress (P=.001). Those with versus those without HMWM loss had lower VWF:Ltx/VWF:Ag (median, 0.887 vs 0.956; P=.02) and VWF:mult ratios (median, 0.132 vs 0.171; P<.001). Among patients with VWF:mults interpreted as normal there were 10 / 47 in whom the VWF:multimer ratio was < 0.15, while among those with VWF:mults interpreted as abnormal, 10 / 39 had VWF:multimer ratio > 0.15. Loss of HMWM was associated with higher PFA-CADP closure times (median, 151 vs 107 seconds; P=.004) and Cornell ECG left ventricular hypertrophy scores (median, 2,720 vs 1,665; P=.001). Loss of HMWM was not associated with clinical symptoms, BNP level, or clinically significant bleeding.
Table 3.
Variables Associated With Loss of VWF high molecular weight multimers among 66 Aortic Stenosis Patients
| High Molecular | Weight Multimer | ||
|---|---|---|---|
| Variable | Normal (n=28) | Loss (n=37) | P Valueb |
| Mean gradient (mm Hg) | 26 (19-34) | 46 (38-56) | <.001 |
| Peak aortic velocity (m/s) | 3.3 (2.8-3.9) | 4.4 (3.9-4.8) | <.001 |
| Aortic valve area index (cm2/m2) | 0.68 (0.43-0.82) | 0.43 (0.37-0.60) | .005 |
| Wall shear stress (dynes/cm2) | 58 (39-72) | 90 (68-108) | <.001 |
| Significant bleeding | 2 (7%) | 7 (19%) | .28 |
| Symptoms | 12 (43%) | 19 (51%) | .62 |
| Duke Activity Status Index score | 36 (18-51) | 35 (19-50) | .57 |
| Cornell left ventricular hypertrophy score | 1,665 (1,077-2,568) | 2,720 (1,950-3,268) | .001 |
| Brain natriuretic peptide (ng/L) | 150 (67-240) | 122 (74-386) | .73 |
| Platelet function analyzer collagen ADP closure time (seconds) | 107 (83-134) | 151 (104-214) | .004 |
| VWF latex activity / VWF antigen ratio | 0.956 (0.881-1.052) | 0.887 (0.796-0.934) | .02 |
| VWF:multimer ratio | 0.171 (0.155-0.186) | 0.132 (0.110-0.157) | <.001 |
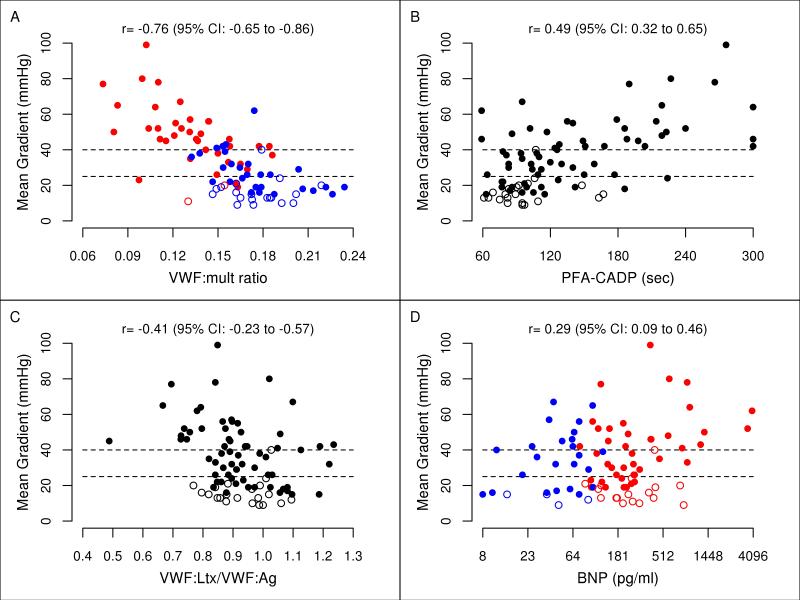
Spearman rank correlations of biomarkers with mean AS gradient are shown in Table 4. The VWF:mult ratio demonstrated the strongest relationship to mean gradient (r=–0.76; 95% CI, –0.65 to –0.86; P<.001; Figure 2A). Significant associations with the mean gradient were demonstrated for BNP (P=.02), PFA-CADP (P<.001), and VWF:Ltx/VWF:Ag (P<.001) (Figure 2B-D). Similar results were found when examining the correlations between the plasma biomarkers and other echocardiographic measures of AS.
Table 4.
Correlations of Echocardiographic Measurements of Stenosis Severity With Biomarkers Among Aortic Stenosis Patients (N=66)a
| Plasma Biomarkers |
||||||||
|---|---|---|---|---|---|---|---|---|
| Echocardiographic | Brain natriuretic peptide (ng/L) |
Platelet function analyzer collagen ADP closure time (seconds) |
VWF latex activity / VWF antigen ratio |
VWF:multimer ratio |
||||
| Measurements | r | P Value | r | P Value | r | P Value | r | P Value |
| Mean gradient (mm Hg) | 0.29 | .02 | 0.49 | <.001 | −0.41 | <.001 | −0.76 | <.001 |
| Peak aortic velocity (m/s) | 0.25 | .04 | 0.50 | <.001 | −0.41 | <.001 | −0.73 | <.001 |
| Aortic valve area (cm2) | −0.51 | <.001 | −0.51 | <.001 | 0.39 | .001 | 0.57 | <.001 |
| Aortic valve area index (cm2/m2) | −0.47 | <.001 | −0.51 | <.001 | 0.41 | <.001 | 0.57 | <.001 |
| Aortic valve time velocity integral (cm) | 0.34 | .005 | 0.57 | <.001 | −0.34 | .006 | −0.70 | <.001 |
| Time velocity integral ratio | −0.45 | <.001 | −0.51 | <.001 | 0.34 | .006 | 0.56 | <.001 |
| Wall shear stress(dyne/cm2) | 0.40 | <.001 | 0.55 | <.001 | −0.40 | .001 | −0.70 | <.001 |
Figure 2.
Aortic stenosis (AS) severity and biomarkers plotted in 66 AS patients (closed circles) and 21 aortic valve replacement patients (open circles). Cutoff gradient values are dashed lines for severe AS (>40 mm Hg) and moderate AS (25-40 mm Hg). Blue circles indicate patients with no loss of high-molecular-weight multimer (HMWM) and red circles indicate HMWM loss. A, von Willebrand factor multimer (VWF:mult) ratio. B, Platelet function analyzer collagen plus adenosine diphosphate (PFA-CADP) closure time. C, Ratio of von Willebrand factor latex agglutination immunoturbidic activity to von Willibrand factor antigen (VWF:Ltx/VWF:Ag). D, Brain nutriuretic peptide (BNP) level plotted on a base 2 log scale.
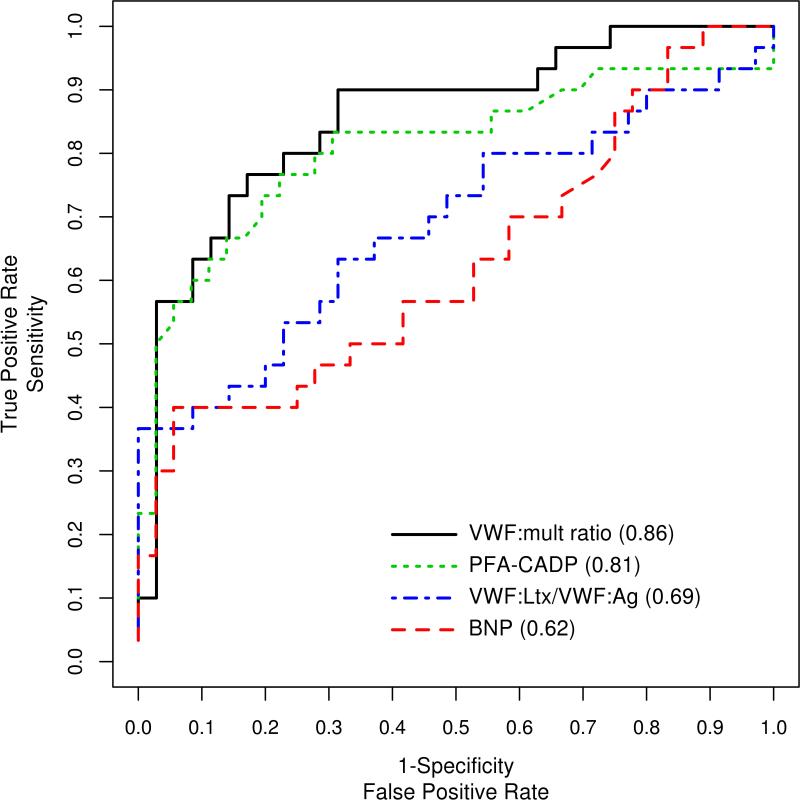
In the detection of severe AS based on a mean gradient > 40 mm Hg, estimates of the area under curves (Figure 3; Supplemental Table 2) showed that the PFA-CADP and VWF:mult ratio were best at detecting severe AS, with areas under curve of 0.81 and 0.86, respectively, while the areas under curve for VWF:Ltx/VWF:Ag and BNP were 0.69 and 0.62, respectively. Compared to BNP, the VWF:mult ratio had a significantly higher area under the curve in the detection of AS (P=.004), while neither PFA-CADP nor VWF:Ltx/VWF:Ag had a significantly higher area under the curve than BNP in the detection of AS (P=.06 and P=.45, respectively). In an exploratory analysis of possible cutoff points of VWF:mult ratio (Supplemental Table 3), a threshold of 0.15 demonstrated 77% sensitivity, 77% specificity, and 74% positive predictive value for the detection of a mean gradient higher than 40 mm Hg.
Figure 3.
Receiver operating characteristic curves and areas under the curves for brain natriuretic peptide (BNP), the ratio of von Willebrand factor latex agglutination immunoturbidic activity to von Willibrand factor antigen (VWF:Ltx/VWF:Ag), platelet function analyzer collagen plus adenosine diphosphate (PFA-CADP) closure time, and von Willebrand factor multimer quantitative ratio (VWF:mult ratio).
Clinically significant bleeding, noted in 9 of the 66 patients (14%, 7 gastrointestinal bleeding, 1 transfusion dependent anemia without underlying hematologic disease and bleeding from trivial wounds, 1 recurrent hemoptysis), was associated with significantly longer PFA-CADP closure time (median, 219 vs 115 seconds; P=.003) and lower VWF:Ltx/VWF:Ag ratio (median, 0.76 vs 0.91; P=.02), and multiple echocardiographic AS severity parameters (P≤.04) and symptoms (P=.01) (Supplemental Table 4).
Compared to patients without symptoms, those with AS-related symptoms had a higher BNP (median, 208 vs 111 ng/L; P=.03), lower Duke Activity Status Index score (P=.001), and echocardiographic and Doppler indices of AS severity (P≤.005). None of the VWF activity indices were associated with symptom status (Supplemental Table 5).
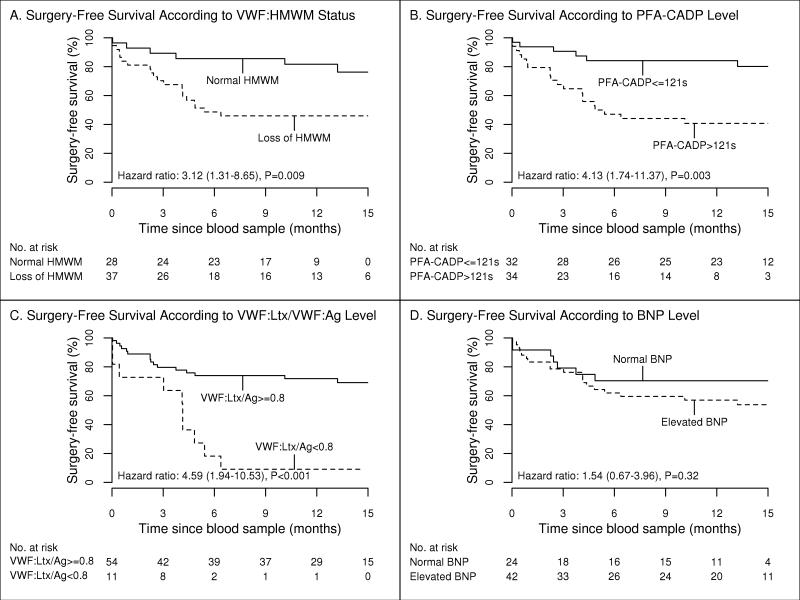
Overall survival curves (Figure 4) showed an increased risk of death or aortic valve replacement with a loss of HMWM (hazard ratio, 3.12; 95% confidence intervals, 1.31-8.65; P=.009); PFA-CADP closure time longer than 121 seconds (hazard ratio, 4.13; 95% confidence interval, 1.74-11.37; P=.003); and VWF:Ltx/VWF:Ag ratio less than 0.8 (hazard ratio, 4.59; 95% confidence interval, 1.94-10.53; P<.001). Elevated BNP was not significantly associated with aortic valve replacement or death (hazard ratio, 1.54; 95% confidence interval, 0.67-3.96; P=.32).
Figure 4.
Kaplan-Meier estimates of surgery-free survival. A, von Willebrand factor high-molecular-weight multimer (HMWM) loss vs normal. B, Platelet function analyzer collagen plus adenosine diphosphate (PFA-CADP) closure time >121 seconds vs ≤121 seconds. C, Ratio of von Willebrand factor latex agglutination immunoturbidic activity to von Willibrand factor antigen (VWF:Ltx/VWF:Ag) <0.8 vs ≥0.8. D, Brain natriuretic peptide (BNP) level, normal vs elevated. HR indicates hazard ratio.
Discussion
Defining symptoms and severity in AS is problematic, given the relatively high risk of surgical intervention in the elderly (4,5), and resolution of AS severity may require hemodynamic catheterization, dobutamine infusion, exercise testing, or biomarker assessment (24). AS severity designation varied between moderate and severe in 40% to 76% of cases in the study by Minners et al (2), depending on whether mean gradient or indexed aortic valve area was used. Bahlmann et al (3) reclassified 47.5% of severe AS patients to nonsevere AS after adjustment for pressure recovery. In a recent study, prognosis for patients with severe AS by valve area but with peak aortic velocity of 4 m/s or less and mean gradient of ≤40 mm Hg was not different than moderate AS (7), while elsewhere it was adverse for patients with low stroke volume (25), creating a quandary for writers of guideline publications (26). Since HMWM degradation is directly associated with high shear stress in AS, we tested the performance of VWF indices as AS severity biomarkers.
VWF:mult ratio, VWF:Ltx/VWF:Ag ratio, and PFA-CADP closure time were significantly associated with AS severity, as defined by mean gradient and other echocardiographic indices, more so than was BNP. There were significant differences between severe AS and prior aortic valve replacement patients in VWF:mult ratio, qualitative VWF HMWM loss and PFA-CADP, but not with BNP. Qualitative loss of HMWM by visual inspection had slightly better sensitivity (87%) for detection of severe AS than did VWF:mult ratio (77%), but the latter method was more precise. The 77% sensitivity figure for VWF:mult ratio is similar to prior reported surgical series of AS, in which the rates of abnormal quantitative VWF multimers in severe AS varied from 71% to 80% (10-14). These estimates contrast with other high shear entities: in hypertrophic cardiomyopathy with obstruction, VWF:mult ratios were abnormal in 100% of patients (12), and VWF multimer abnormalities are also universal in patients with nonpulsatile ventricular assist devices (27,28). Platelet function analyzer test results were abnormal in similar percentages, 80% of our severe AS patients, compared to 95% of hypertrophic cardiomyopathy patients with obstruction (15). These differences in shear-related laboratory abnormalities between AS patients and those with ventricular assist devices and hypertrophic cardiomyopathy patients could be consistent, with overestimation of AS by echocardiographic techniques in 20% to 30% of patients.
Although it is considered the gold standard for defining and subtyping type II von Willebrand disease, determination of VWF HMWM loss by qualitative visual inspection is not approved by the United States Food and Drug Administration and has a 15% error rate (29). Quantitative VWF multimer analysis is not standardized but, as previously noted, has been widely used in a variety of cardiovascular conditions, and pressure gradients have demonstrated a good correlation with a noncommercial VWF collagen binding activity assay using 95% equine tendon type I and 5% type III collagen (10,15). In commencing the Biomarkers of Aortic Stenosis Severity study, we contracted with a commercial laboratory, which performed an in-house VWF collagen binding assay using 100% human placental type III collagen. In evaluation of the first 19 patients enrolled, VWF HMWM abnormalities were seen in 5 patients, but none of these were detected by VWF collagen binding to VWF antigen ratios less than 0.7 or 0.8. This test was not further pursued.
Food and Drug Administration-approved and research-only methods widely used in special coagulation laboratories to give normal versus abnormal results in assessment of possible congenital von Willebrand disease have not been optimized for the detection of cardiac lesion–mediated shear-related VWF abnormalities. Furthermore, VWF ristocetin cofactor activity is insensitive to loss of HMWM (21), and it and the noncommercial VWF collagen binding assays have poor accuracy and precision (29). The VWF latex activity assay we employed has been shown to have good sensitivity in detecting HMWM loss in left ventricular assist device recipients (21). As in the ventricular assist device recipients (27,28), in native AS patients, VWF:Ltx/VWF:Ag was strongly associated with severe AS and AS-related clinically significant bleeding. However, VWF:Ltx/VWF:Ag, while very specific (see Table 2), was far less sensitive than HMWM loss for detecting AS-associated acquired von Willebrand syndrome in all grades of severity, detecting only 9 of 37 subjects with qualitative HMWM loss.
VWF multimer visual interpretation is associated with severe AS, but it can also be seen in mild and moderate AS, has significant operator to operator variation (29), and is not adequate for quantifying HMWM loss from year to year, as would be desirable in longitudinal studies. Multimer quantitation, as performed in our study and others (10-15), appears to overcome this. However, VWF multimer analysis may be impractical on a large scale because of its significant technical complexity, slow turnaround time, and high cost.
PFA-CADP closure time was strongly associated with all clinical end points except for AS-related symptoms. PFA-CADP tests whole blood under high shear and is sensitive to both platelet and VWF disorders. However, PFA-CADP testing must be performed on fresh whole blood, not stored plasma, and is invalid in the presence of hemolysis, anemia with hemoglobin less than 10 mg/dL, thrombocytopenia, or antiplatelet medications, such as clopidogrel. In one study, these limitations invalidated PFA-CADP determinations in 29% of patients (30).
A major finding of our study was that three independent VWF-related indices predicted death or aortic valve replacement, with hazard ratios from 3.12-4.59, while BNP had a non-significant hazard ratio of 1.54. Since the VWF indices were less predictive of symptoms than the DASI score or BNP, their association with lesion severity and not overall clinical status is the likely explanation for the prognostic value. Elsewhere BNP was found to be elevated versus controls, was modestly associated with severity of stenosis, and was more strongly associated with left ventricular mass, symptoms, and outcome (9). The ultimate clinical utility of these and other biomarkers in serial evaluation of patients and in attempt to slow disease progression remains to be defined.
Our study has several limitations. We did not record serial observations of VWF activity indices over time, but the stability over a 24-hour period and directionally appropriate gradient reductions have been seen with treatment of hypertrophic cardiomyopathy (15,17) and with aortic valve replacement for AS (10,11). Small sample size prohibited a multivariate analysis of predictors of bleeding, but the 14% bleeding rate is similar to previously published estimates of 20% (10) and 17% (13). These are the only 3 published estimates of bleeding in AS available. Aortic valve replacement patients, while a clinically relevant comparison group, are not a true control population. Indeed, 2 / 21 AVR patients had abnormal multimers, and 3 / 21 had VWF:mult ratio < 0.15, similar to the findings of Vincentelli, et al (10).
Supplementary Material
Acknowledgments
Grant Support: Mayo Foundation for Medical Research, and Center for Translational Science Activities grant support (UL1 RR024150)
References
- 1.Baumgartner H, Stefenelli T, Niederberger J, Schima H, Maurer G. “Overestimation” of catheter gradients by Doppler ultrasound in patients with aortic stenosis: a predictable manifestation of pressure recovery. J Am Coll Cardiol. 1999;33:1655–1661. doi: 10.1016/s0735-1097(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 2.Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J. 2008;29:1043–1048. doi: 10.1093/eurheartj/ehm543. [DOI] [PubMed] [Google Scholar]
- 3.Bahlmann E, Cramariuc D, Gerdts E, Gohlke-Baerwolf C, Nienaber CA, Eriksen E, Wachtell K, Chambers J, Kuck KH, Ray S. Impact of pressure recovery on echocardiographic assessment of asymptomatic aortic stenosis: a SEAS substudy. JACC Cardiovasc Imaging. 2010;3:555–562. doi: 10.1016/j.jcmg.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz CE, Otto CM. Aortic stenosis: clinical aspects of diagnosis and management, with 10 illustrative case reports from a 25-year experience. Medicine (Baltimore) 2010;89:349–379. doi: 10.1097/MD.0b013e3181fe5648. [DOI] [PubMed] [Google Scholar]
- 5.Vahanian A, Otto CM. Risk stratification of patients with aortic stenosis. Eur Heart J. 2010;31:416–423. doi: 10.1093/eurheartj/ehp575. [DOI] [PubMed] [Google Scholar]
- 6.Aikawa E, Otto CM. Look more closely at the valve: imaging calcific aortic valve disease. Circulation. 2012;125:9–11. doi: 10.1161/CIRCULATIONAHA.111.073452. [DOI] [PubMed] [Google Scholar]
- 7.Jander N, Minners J, Holme I, Gerdts E, Boman K, Brudi P, Chambers JB, Egstrup K, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Rossebo A, Pedersen TR, Skjaerpe T, Willenheimer R, Wachtell K, Neumann FJ, Gohlke-Barwolf C. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation. 2011;123:887–895. doi: 10.1161/CIRCULATIONAHA.110.983510. [DOI] [PubMed] [Google Scholar]
- 8.Lancellotti P, Magne J, Donal E, Davin L, O'Connor K, Rosca M, Szymanski C, Cosyns B, Pierard LA. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol. 2012;59:235–243. doi: 10.1016/j.jacc.2011.08.072. [DOI] [PubMed] [Google Scholar]
- 9.Steadman CD, Ray S, Ng LL, McCann GP. Natriuretic peptides in common valvular heart disease. J Am Coll Cardiol. 2010;55:2034–2048. doi: 10.1016/j.jacc.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F, Bauters A, Decoene C, Goudemand J, Prat A, Jude B. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349:343–349. doi: 10.1056/NEJMoa022831. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida K, Tobe S, Kawata M, Yamaguchi M. Acquired and reversible von Willebrand disease with high shear stress aortic valve stenosis. Ann Thorac Surg. 2006;81:490–494. doi: 10.1016/j.athoracsur.2005.07.074. [DOI] [PubMed] [Google Scholar]
- 12.Sucker C, Feindt P, Zotz RB, Stockschlaeder M, Scharf RE. Functional von Willebrand Factor assays are not predictive for the absence of highest-molecular weight von Willebrand Factor multimers in patients with aortic-valve stenosis. Thromb Haemost. 2005;94:465–466. [PubMed] [Google Scholar]
- 13.Casonato A, Sponga S, Pontara E, Cattini MG, Basso C, Thiene G, Cella G, Daidone V, Gerosa G, Pagnan A. Von Willebrand factor abnormalities in aortic valve stenosis: pathophysiology and impact on bleeding. Thromb Haemost. 2011;106:58–66. doi: 10.1160/TH10-10-0634. [DOI] [PubMed] [Google Scholar]
- 14.Solomon C, Budde U, Schneppenheim S, Czaja E, Hagl C, Schoechl H, von Depka M, Rahe-Meyer N. Acquired type 2A von Willebrand syndrome caused by aortic valve disease corrects during valve surgery. Br J Anaesth. 2011;106:494–500. doi: 10.1093/bja/aeq413. [DOI] [PubMed] [Google Scholar]
- 15.Le Tourneau T, Susen S, Caron C, Millaire A, Marechaux S, Polge AS, Vincentelli A, Mouquet F, Ennezat PV, Lamblin N, de Groote P, Van Belle E, Deklunder G, Goudemand J, Bauters C, Jude B. Functional impairment of von Willebrand factor in hypertrophic cardiomyopathy: relation to rest and exercise obstruction. Circulation. 2008;118:1550–1557. doi: 10.1161/CIRCULATIONAHA.108.786681. [DOI] [PubMed] [Google Scholar]
- 16.Warkentin TE, Moore JC, Anand SS, Lonn EM, Morgan DG. Gastrointestinal bleeding, angiodysplasia, cardiovascular disease, and acquired von Willebrand syndrome. Transfus Med Rev. 2003;17:272–286. doi: 10.1016/s0887-7963(03)00037-3. [DOI] [PubMed] [Google Scholar]
- 17.Blackshear JL, Schaff HV, Ommen SR, Chen D, Nichols WL. Hypertrophic obstructive cardiomyopathy, bleeding history, and acquired von Willebrand syndrome: response to septal myectomy. Mayo Clin Proc. 2011;86:219–224. doi: 10.4065/mcp.2010.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols WL, Hultin MB, James AH, Manco-Johnson MJ, Montgomery RR, Ortel TL, Rick ME, Sadler JE, Weinstein M, Yawn BP. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia. 2008;14:171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 19.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 20.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M, American Society of Echocardiography. European Association of Echocardiography Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 21.Chen D, Tange JI, Meyers BJ, Pruthi RK, Nichols WL, Heit JA. Validation of an automated latex particle-enhanced immunoturbidimetric von Willebrand factor activity assay. J Thromb Haemost. 2011;9:1993–2002. doi: 10.1111/j.1538-7836.2011.04460.x. [DOI] [PubMed] [Google Scholar]
- 22.Pruthi RK, Daniels TM, Heit JA, Chen D, Owen WG, Nichols WL. Plasma von Willebrand factor multimer quantitative analysis by in-gel immunostaining and infrared fluorescent imaging. Thromb Res. 2010;126:543–549. doi: 10.1016/j.thromres.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 23.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 24.American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Society of Cardiovascular Anesthesiologists. Society for Cardiovascular Angiography and Interventions. Society of Thoracic Surgeons. Bonow RO, Carabello BA, Kanu C, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 25.Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–2864. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 26.Zoghbi WA. Low-gradient “severe” aortic stenosis with normal systolic function: time to refine the guidelines? Circulation. 2011;123:838–840. doi: 10.1161/CIRCULATIONAHA.110.015826. [DOI] [PubMed] [Google Scholar]
- 27.Crow S, Chen D, Milano C, Thomas W, Joyce L, Piacentino V, 3rd, Sharma R, Wu J, Arepally G, Bowles D, Rogers J, Villamizar-Ortiz N. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg. 2010;90:1263–1269. doi: 10.1016/j.athoracsur.2010.04.099. [DOI] [PubMed] [Google Scholar]
- 28.Meyer AL, Malehsa D, Bara C, Budde U, Slaughter MS, Haverich A, Strueber M. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circ Heart Fail. 2010;3:675–681. doi: 10.1161/CIRCHEARTFAILURE.109.877597. [DOI] [PubMed] [Google Scholar]
- 29.Chandler WL, Peerschke EI, Castellone DD, Meijer P, NASCOLA Proficiency Testing Committee Von Willebrand factor assay proficiency testing: the North American Specialized Coagulation Laboratory Association experience. Am J Clin Pathol. 2011;135:862–869. doi: 10.1309/AJCPH5JK4ONENPAE. [DOI] [PubMed] [Google Scholar]
- 30.Tiede A, Priesack J, Werwitzke S, Bohlmann K, Oortwijn B, Lenting P, Eisert R, Ganser A, Budde U. Diagnostic workup of patients with acquired von Willebrand syndrome: a retrospective single-centre cohort study. J Thromb Haemost. 2008;6:569–576. doi: 10.1111/j.1538-7836.2008.02909.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.