Abstract
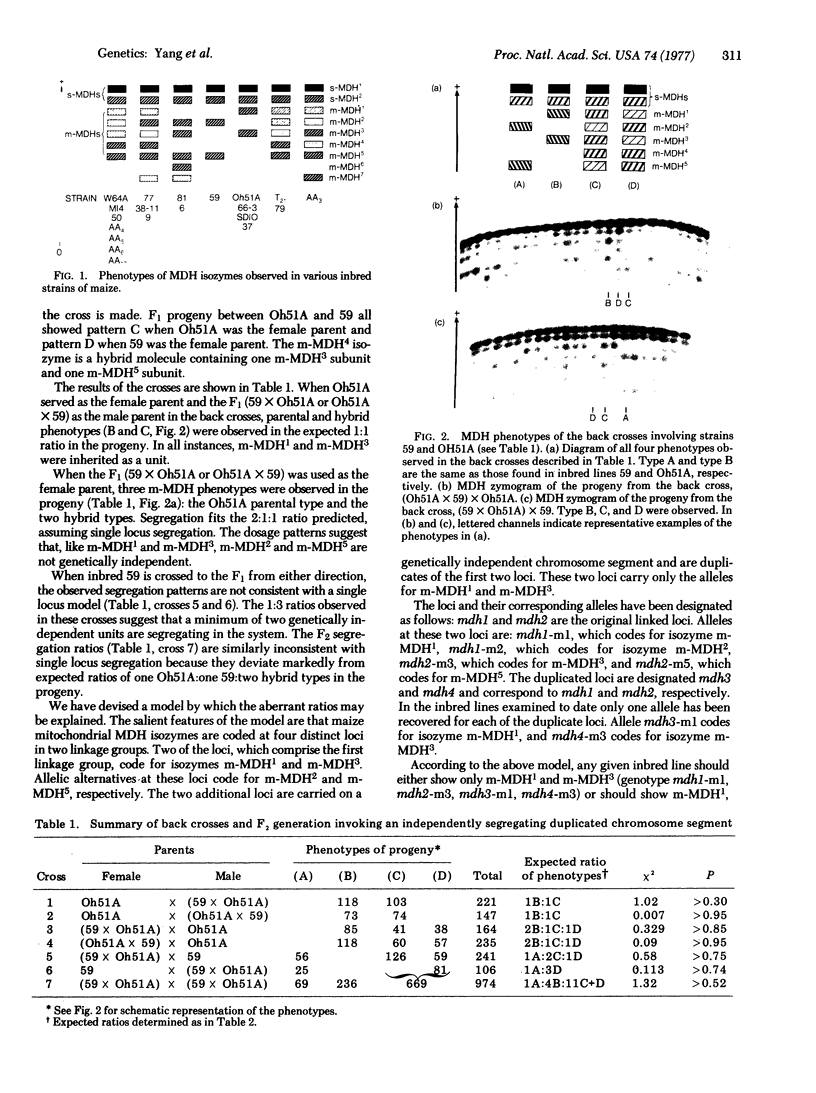
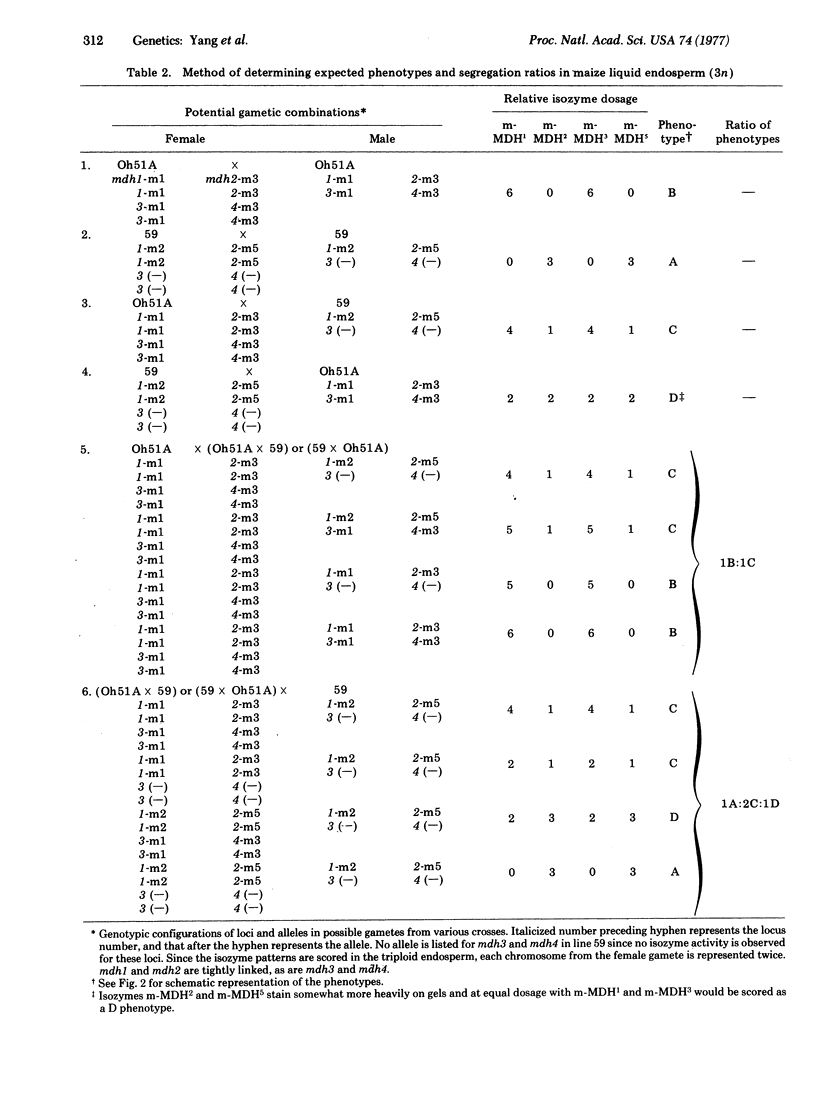
The genetic control of the major mitochondrial isoenzymes of malate dehydrogenase (L-malate:NAD+ oxidoreductase; EC 1.1.1.37) has been investigated in Zea mays. The mitochondrial isozymes are coded at four nuclear gene loci. Two of the loci (mdh1 and mdh2) are diallelic and tightly linked. The other two loci (mdh3 and mdh4) appear to have arisen by duplication of the chromosome segment carrying mdh1 and mdh2, but are not linked to them. The segregation of such a duplicate segment can explain anomalous backcross and F2 segregation ratios.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspinwall N. Genetic analysis of duplicate malate dehydrogenase loci in the pink salmon, Oncorhynchus gorbuscha. Genetics. 1974 Jan;76(1):64–72. [PMC free article] [PubMed] [Google Scholar]
- Bailey G. S., Wilson A. C., Halver J. E., Johnson C. L. Multiple forms of supernatant malate dehydrogenase in salmonid fishes. J Biol Chem. 1970 Nov 25;245(22):5927–5940. [PubMed] [Google Scholar]
- Barath Z., Küntzel H. Cooperation of mitochondrial and nuclear genes specifying the mitochondrial genetic apparatus in Neurospora crassa. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1371–1374. doi: 10.1073/pnas.69.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. G., Cortner J. A. Genetic variant of human erythrocyte malate dehydrogenase. Nature. 1967 Aug 12;215(5102):761–762. doi: 10.1038/215761a0. [DOI] [PubMed] [Google Scholar]
- Dévényi T., Rogers S. J., Wolfe R. G. Structural studies of pig heart malate dehydrogenase. Nature. 1966 Apr 30;210(5035):489–491. doi: 10.1038/210489a0. [DOI] [PubMed] [Google Scholar]
- Gross S. R., McCoy M. T., Gilmore E. B. Evidence for the involvement of a nuclear gene in the productin of the mitochondrial leucyl-tRNA synthetase of Neurospora. Proc Natl Acad Sci U S A. 1968 Sep;61(1):253–260. doi: 10.1073/pnas.61.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karig L. M., Wilson A. C. Genetic variation in supernatant malate dehydrogenase of birds and reptiles. Biochem Genet. 1971 Jun;5(3):211–221. doi: 10.1007/BF00485792. [DOI] [PubMed] [Google Scholar]
- Kitto G. B., Wilson A. C. Evolution of malate dehydrogenase in birds. Science. 1966 Sep 16;153(3742):1408–1410. doi: 10.1126/science.153.3742.1408. [DOI] [PubMed] [Google Scholar]
- Longo C. P., Longo G. P. The development of glyoxysomes in peanut cotyledons and maize scutella. Plant Physiol. 1970 Mar;45(3):249–254. doi: 10.1104/pp.45.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo G. P., Scandalios J. G. Nuclear gene control of mitochondrial malic dehydrogenase in maize. Proc Natl Acad Sci U S A. 1969 Jan;62(1):104–111. doi: 10.1073/pnas.62.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A., Allebach E. Gene transfer in Caulobacter crescentus: polarized inheritance of genetic markers. Genetics. 1975 May;80(1):1–11. doi: 10.1093/genetics/80.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich E., Luck D. J. Replication and inheritance of mitochondrial DNA. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1600–1608. doi: 10.1073/pnas.55.6.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D. Production of mitochondrial peptide-chain elongation factors in yeast deficient in mitochondrial deoxyribonucleic acid. Biochemistry. 1971 Nov 23;10(24):4422–4425. doi: 10.1021/bi00800a011. [DOI] [PubMed] [Google Scholar]
- SAGER R. NONCHROMOSOMAL HEREDITY. N Engl J Med. 1964 Aug 13;271:352–357. doi: 10.1056/NEJM196408132710706. [DOI] [PubMed] [Google Scholar]
- Shaw C. R. Electrophoretic variation in enzymes. Science. 1965 Aug 27;149(3687):936–943. doi: 10.1126/science.149.3687.936. [DOI] [PubMed] [Google Scholar]
- Shows T. B., Chapman V. M., Ruddle F. H. Mitochondrial malate dehydrogenase and malic enzyme: Mendelian inherited electrophoretic variants in the mouse. Biochem Genet. 1970 Dec;4(6):707–718. doi: 10.1007/BF00486384. [DOI] [PubMed] [Google Scholar]
- Weimberg R. An electrophoretic analysis of the isozymes of malate dehydrogenase in several different plants. Plant Physiol. 1968 Apr;43(4):622–628. doi: 10.1104/pp.43.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat T. E., Whitt G. S., Childers W. F. Linkage Relationships between the Homologous Malate Dehydrogenase Loci in Teleosts. Genetics. 1972 Feb;70(2):337–340. doi: 10.1093/genetics/70.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt G. S. Genetic variation of supernatant and mitochondrial malate dehydrogenase isozymes in the teleost Fundulus heteroclitus. Experientia. 1970;26(7):734–736. doi: 10.1007/BF02232514. [DOI] [PubMed] [Google Scholar]
- Yang Ning-Sun, Scandalios J. G. De novo synthesis and developmental control of the multiple gene-controlled malate dehydrogenase isozymes in maize scutella. Biochim Biophys Acta. 1975 Apr 19;384(2):293–306. doi: 10.1016/0005-2744(75)90031-5. [DOI] [PubMed] [Google Scholar]
- Yang N. S., Scandalios J. G. Purification and biochemical properties of genetically defined malate dehydrogenase in maize. Arch Biochem Biophys. 1974 Apr 2;161(2):335–353. doi: 10.1016/0003-9861(74)90314-2. [DOI] [PubMed] [Google Scholar]
- Yang N., Scandalios J. G. Cytoplasmic synthesis of soluble and mitochondrial malate dehydrogenase isozymes in maize. Arch Biochem Biophys. 1975 Dec;171(2):575–585. doi: 10.1016/0003-9861(75)90067-3. [DOI] [PubMed] [Google Scholar]
- Zee D. S., Isensee H., Zinkham W. H. Polymorphism of malate dehydrogenase in Ascaris suum. Biochem Genet. 1970 Apr;4(2):253–257. doi: 10.1007/BF00485776. [DOI] [PubMed] [Google Scholar]