Summary
The treatment of advanced prostate cancer has been transformed by novel antiandrogen therapies such as enzalutamide. Here we identify induction of glucocorticoid receptor (GR) expression as a common feature of drug resistant tumors in a credentialed preclinical model, a finding also confirmed in patient samples. GR substituted for the androgen receptor (AR) to activate a similar but distinguishable set of target genes and was necessary for maintenance of the resistant phenotype. The GR agonist dexamethasone was sufficient to confer enzalutamide resistance whereas a GR antagonist restored sensitivity. Acute AR inhibition resulted in GR upregulation in a subset of prostate cancer cells due to relief of AR-mediated feedback repression of GR expression. These findings establish a novel mechanism of escape from AR blockade through expansion of cells primed to drive AR target genes via an alternative nuclear receptor upon drug exposure.
Introduction
Recently approved drugs that target androgen receptor (AR) signaling such as abiraterone and enzalutamide have rapidly become standard therapies for advanced stage prostate cancer (Scher et al., 2012b) (de Bono et al., 2011). Despite their success, sustained response with these agents is limited by acquired resistance which typically develops within ~6-12 months. Clinical success of kinase inhibitors in other tumors such as melanoma, lung cancer, leukemia and sarcoma is similarly transient (Sawyers et al., 2002) (Chapman et al., 2011) (Demetri et al., 2002) (Maemondo et al., 2010), resulting in numerous efforts to define mechanisms of acquired resistance. One strategy that has proven particularly useful is prolonged treatment of drug-sensitive preclinical models to derive drug-resistant sublines, followed by genome-wide profiling studies to ascertain differences that may play a causal role in conferring drug resistance. A common mechanism that has emerged from these kinase inhibitor studies is reactivation of the signaling pathway targeted by the drug, directly by mutation of the kinase target or indirectly by bypassing pathway inhibitor blockade through amplification of an alternative kinase (Glickman and Sawyers, 2012). Both scenarios have been validated in clinical specimens and are guiding efforts to discover next generation inhibitors and to develop rational drug combinations.
Clinically relevant mechanisms of resistance to hormone therapy in prostate cancer have also been elucidated using preclinical models. Hormone therapy, through the use of drugs that lower serum testosterone or competitively block the binding of androgens to AR, has been the mainstay of treatment for metastatic prostate cancer for decades but is not curative. The late stage of disease, which is refractory to hormone therapy, is termed castration resistant prostate cancer (CRPC). We previously examined the molecular basis of progression to CRPC in mouse models and discovered that increased AR expression was the primary mechanism (Chen et al., 2004). We then used this observation to screen for novel anti-androgens that restore AR inhibition in the setting of increased AR levels. These efforts yielded three second-generation anti-androgens: enzalutamide, ARN-509, and RD162 (Tran et al., 2009) (Clegg et al., 2012). Enzalutamide and ARN-509 were further developed for clinical use, culminating in FDA approval of enzalutamide in 2012 based on increased survival (Scher et al., 2012b).
Now with widespread use, resistance to enzalutamide is a major clinical problem. We and others have recently identified an AR point mutation as one resistance mechanism by derivation of drug-resistant sublines following prolonged exposure to enzalutamide or ARN-509 (Balbas et al., 2013) (Joseph et al., 2013) (Korpal et al., 2013). This AR mutation has also been recovered from patients with resistance to ARN-509 but only in a minority of cases (Joseph et al., 2013). Here we define a novel and potentially more prevalent mechanism of resistance by which tumors bypass AR blockade through upregulation of the glucocorticoid receptor (GR).
Results
GR is expressed in antiandrogen-resistant tumors
We previously showed that LNCaP/AR xenograft tumors regress during the first 28 days of treatment with ARN-509 (Clegg et al., 2012), enzalutamide or RD162 (Tran et al., 2009). In a pilot study to explore mechanisms of acquired resistance to these drugs, we treated mice continually and harvested tumors after progression (mean 163 days, Supplemental Table 1A). Tissue from fifteen resistant tumors obtained from long term antiandrogen treated mice (n=6 ARN-509, n=9 RD162) and from three control tumors from vehicle treated mice were analyzed by expression array. Aggregated data from resistant and control tumors in this pilot cohort were compared to identify expression changes commonly associated with resistance (Figure 1A). Among the most up-regulated genes in the resistant tumors was the glucocorticoid receptor (GR, gene symbol NR3C1) which shares overlapping target specificity with AR (Mangelsdorf et al., 1995). Of note, several of the most differentially expressed genes were known androgen regulated genes (confirmed by transcriptome analysis of short term DHT treated LnCaP/AR cells, in vitro (Supplemental Table 1B)), but they were altered in directions that did not reflect restored AR signaling. On the one hand, SGK1 (Serum Glucocorticoid Induced Kinase 1), a known AR and GR-induced target gene, was among the most up-regulated genes, but several other androgen-induced genes (PMEPA1, SNAI2, KCNN2, LONRF1, SPOCK1) were among the most repressed. Conversely, several androgen-repressed genes (UGT2B15, PMP22, CAMK2N1, UGT2B17) were among the most up-regulated (Figure 1A). These findings indicated that resistance in this model system is unlikely to be mediated by simple restoration of AR activity and raised the possibility that GR may play a role.
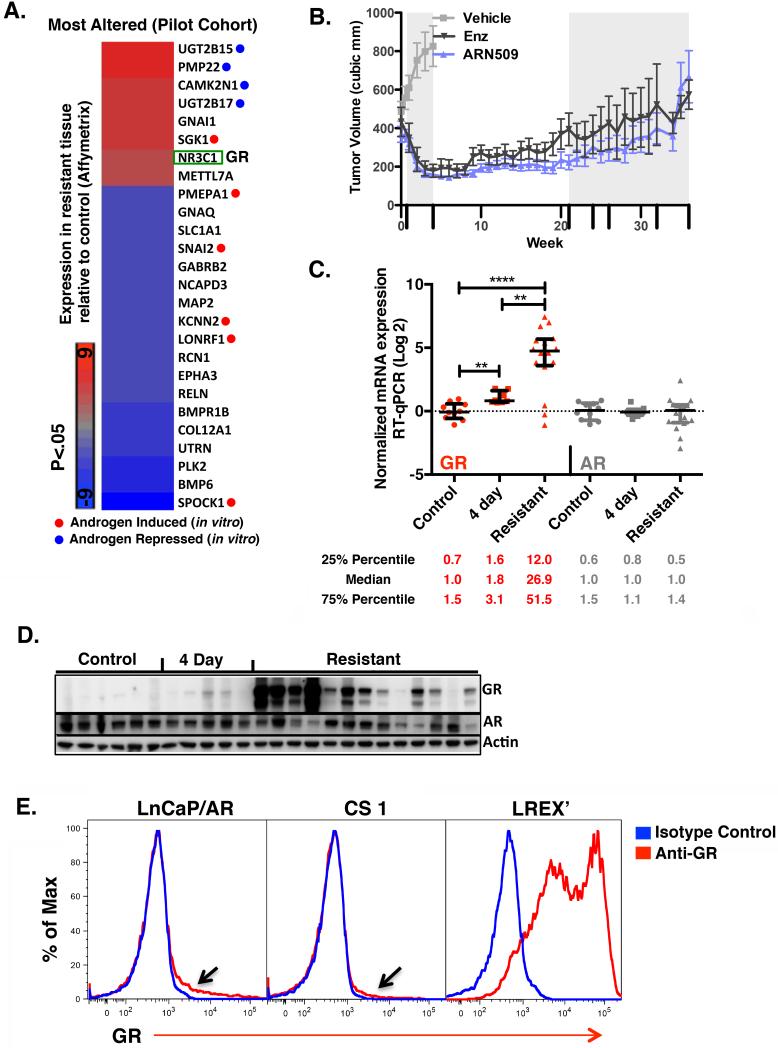
Figure 1. GR mRNA and protein is expressed in resistant tissues.
A. Most differentially expressed genes in a pilot cohort of LnCaP/AR xenograft tumors with acquired resistance to ARN-509 (n=6) or RD162 (n=9) compared to control (n=3) determined by microarray (Affymetrix Ex1.0). Mice with resistant tissues were continued on drug treatment through time of harvest. In vitro androgen-induced or -repressed genes are annotated (See also Supplementary Table 1B). B. Mean tumor volumes +/− s.e.m of LnCaP/AR xenografts in validation cohort. Days tumors were harvested are annotated on x-axis (long hash mark). C. RT-qPCR analysis of GR and AR mRNA expression in a validation cohort of LnCaP/AR xenograft tumors from mice treated with vehicle (control, n=10), 4 days of anti-androgen (n=8), or with acquired resistance to 10 mg/kg enzalutamide (n=8) or 10 mg/kg ARN-509 (n=8). See also Supplementary Table 1B. D. Western blot analysis of GR and AR protein expression in a subset of tissues also analyzed in B. Control (n=6), 4 day (n=5), Resistant (n=13). Resistant samples were loaded for protein analysis from highest to lowest GR levels based on corresponding mRNA analysis (See also Supplementary Table 1C.) E. Intracellular GR flow cytometric analysis of LnCaP/AR, CS1, and LREX’, cells passaged in vitro, under standard passage conditions (see methods). See also Figure S1.
To explore this question further, we generated an independent set of drug-resistant tumors (the validation cohort), focusing on the two second generation antiandrogens in clinical use, enzalutamide and ARN-509 (Figure 1B). GR mRNA levels in 10 control, 8 short term treated (4 day) and 16 resistant tumors were substantially higher in resistant tissues compared to control (median 26.9-fold increase) or 4 day treated tumors (Figure 1C). Of the tissues analyzed by RT-qPCR, most were also analyzed for GR expression by western blot, based on availability of protein lysates (control n=6, 4 day n=5, resistant n=13). No GR was detected in control samples, minimal expression was noted in 4 day treated samples, and substantial expression was found in most resistant tumors in a pattern that tended to correlate with GR mRNA levels (Figure 1D). There was no correlation between GR expression and the specific antiandrogen treatment used (Supplemental Table 1C). In contrast to GR, AR RNA or proteins levels were not consistently different across the treatment groups (Figure 1C,1D).
To explore AR and GR signaling in more detail, we established cells lines from control and drug-resistant tumors by adaptation to growth in vitro. LREX’ (LnCaP/AR Resistant to Enzalutamide Xenograft derived) was derived from an enzalutamide-resistant tumor with high GR expression, and CS1 was derived from a vehicle treated tumor. We also developed a flow cytometry-based assay to measure GR expression on a cell-by-cell basis. In both LNCaP/AR and CS1, most cells showed no evidence of GR expression, with the exception of a small subpopulation (black arrow, discussed later) (Figure 1E). In contrast, essentially all LREX’ cells expressed GR. Intracellular AR staining confirmed that AR levels in LREX’ did not notably differ from control cells (Figure S1A).
LREX’ tumors are dependent on GR for enzalutamide-resistant growth
Having established the LREX’ model as representative of high GR expression, we next confirmed that these cells maintain a resistant phenotype in vivo. LREX’ or control cells were injected into castrated mice that were then immediately initiated on antiandrogen treatment. LREX’ showed robust growth whereas LNCaP/AR or CS1 lines were unable to establish tumors in the presence of antiandrogen (Figure 2A,2B). Strong expression of GR was confirmed in multiple LREX’ xenograft tumors by western blot and by IHC (Figure S1B, 2C). As expected, untreated LNCaP/AR tumors were negative for GR expression with the exception of rare GR-positive cells (Figure 2C). Although many of these GR-positive cells had morphologic features of stromal or endothelial cells (blue arrows), some appeared epithelial (black arrow), consistent the with flow cytometry analysis (Figure 1E, black arrows).
Figure 2. GR is necessary for resistance in the LREX’ xenograft model.
A. Mean tumor volume +/− s.e.m. of LREX’ (n=20) or LnCaP/AR (n=14) cells in castrate mice treated with 10 mg/kg enzalutamide B. Mean tumor volumes +/− s.e.m. of CS1 in castrate mice treated with vehicle (n=10) or 10 mg/kg ARN-509 (n=10). C. GR IHC of enzalutamide (10 mg/kg)-treated LREX’ tumors and vehicle-treated LnCaP/AR xenograft tissues. Blue arrow = endothelial/stromal cells, Black arrow = epithelial cell. D. Mean tumor volumes +/− s.e.m of LREX’ xenografts in 10 mg/kg enzalutamide-treated castrate mice after infection with a non-targeting (n=14) or GR-targeting (n=12) hairpin. Comparison is by Mann-Whitney test. E. Tumor growth curve of CS1 in castrate mice after infection with the non-targeting (n=20) or GR-targeting (n=20) hairpin. F. Western blot analysis of GR expression in LREX’ cells prior to implantation and of available tissues from D at day 49. See also Figure S1.
To determine whether GR expression is required to maintain the drug-resistant phenotype, LREX’ cells were infected with a shRNA targeting GR (shGR) and stable knockdown of GR protein was confirmed (Figure 2F). Tumor growth of shGR infected LREX’ cells was significantly delayed relative to shNT (non targeted)-infected cells in castrated mice treated with enzalutamide (Figure 2D). In contrast, shGR had no impact on the growth of GR-negative CS1 xenografts, diminishing the possibility of an off-target effect (Figure 2E). Of note, shGR LREX’ xenografts harvested on day 49 showed decreased GR protein knockdown compared to the pre-implantation levels, indicative of selective pressure against GR silencing in the setting of enzalutamide treatment (Figure 2F). These findings provide direct evidence that GR drives enzalutamide resistance in vivo.
GR expression is associated with clinical resistance to enzalutamide
To determine whether GR expression is a feature of clinical antiandrogen resistance, we evaluated GR expression in bone metastases from patients receiving enzalutamide. Bone marrow samples were obtained prior to enzalutamide treatment (baseline) and again after 8 weeks of treatment, as previously reported in a cohort of abiraterone-treated patients (Efstathiou et al., 2012). Using a GR IHC assay optimized for use in bone marrow samples, we quantified the percentage of GR-positive tumor cells and dichotomized the data based on clinical response. Patients who continued to benefit from therapy for greater than 6 months were defined as good responders, while those in whom therapy was discontinued earlier than 6 months due to a lack of clinical benefit were classified as poor responders (Figure 3A). Consistent with the designation of good versus poor clinical response based on treatment status at 6 months, 11 of 13 good responders but only 1 of 14 poor responders had a maximal PSA decline greater than 50% (Figure 3C). Akin to the findings in the preclinical model, GR positivity at baseline was low: 3% of tumor cells in good responders and 8% in poor responders. Of note, 3 of 22 tumors had evidence of high GR expression at baseline (≥ 20% of tumor cells) and all three had a poor clinical response (Figure 3C,D). At 8 weeks, the mean percentage of GR positive cells was higher than baseline levels in both response groups but was more significantly elevated in poor responders (29% vs 8%, p=.009). In addition, the percentage of GR-positive cells at 8 weeks was significantly higher in poor compared to good responders (29% versus 10%, p=.02) (Figure 3C,D), and similar results were obtained when the analysis was limited to patients from whom matched baseline and 8 week samples were available for analysis (Figure 3E). Furthermore, when GR IHC data was dichotomized based on PSA decline instead of clinical response, GR induction was also associated with a limited PSA decline (Figure S2). These findings establish a correlation between GR expression and clinical response to enzalutamide and raise the possibility that AR inhibition may induce GR expression in some patients. The fact that PSA levels also correlate with GR expression raises the question of whether transcriptional regulation of a canonical AR target gene may be regulated by GR.
Figure 3. GR induction in disseminated tumor cells is associated with poor clinical response to enzalutamide and persistence of PSA.
A. Schematic of sample acquisition timeline and response groups. B. Number of good or poor responders who achieved PSA decline greater than 50%. C. Examples of GR IHC images from matched samples at baseline and 8 weeks. D. Percent GR positive epithelial cells in all tissue available at 0 and 8 weeks or E. matched samples obtained from the same patient at 0 and 8 weeks +/− s.e.m. Comparisons are by Mann-Whitney test. See also Figure S2.
GR expressing drug-resistant tumors show uneven restoration of AR target genes
Having implicated GR as a potential mediator of antiandrogen resistance, we next asked if restored AR pathway activity also plays a role by comparing the mRNA transcript levels of 74 direct AR target genes in control, 4 day, and resistant tumors from the validation cohort (Figures S3) as well as eight LREX’ tumors (Figure 4A) (see experimental procedures and Supplementary Table 2 for details on gene selection). Consistent with the data generated in the pilot cohort (Figure 1A), some AR target genes in resistant tissues showed elevated levels relative to control (SGK1, STK39) while other genes (NDRG1, TIPARP, PMEPA1) showed no evidence of restored expression.
Figure 4. Variable expression of AR target genes in LREX’, in vivo, and after glucocorticoid treatment, in vitro.
A. Normalized expression array signal (Illumina HT-12) of a suite of 74 AR target genes in control (n=10), 4 day (n=8), and LREX’ (n=8, right) xenograft tumors. Genes are ranked by degree of restoration of expression in resistant tissue ((Res-4 day) / (Control-4 day)). All resistant tissues were continued on anti-androgen treatment through time of harvest. B. Fractional restoration values of each of the 74 AR targets in LREX’ xenografts (n=8) or resistant tissues from the validation cohort (n=12, see also Figure S3). C. GR mRNA in resistant tissues used in B. D. Expression of AR target genes in the LREX’ cell line in steroid depleted media after 8 hours of treatment with the indicated agonists, in vitro. Enzalutamide = 10 micromolar, V = Vehicle. +/− s.e.m. See also Figures S3, S4.
To examine restoration of AR signaling across the entire set of 74 target genes, we calculated a fractional restoration value using log 2 transformed expression values and the equation (Resistant – 4 day) / (Control – 4 day). With this approach, a gene whose expression in resistant tissue equals the expression in control tumors calculates as 1, while a gene whose expression in resistance equals its expression after 4 days of antiandrogen treatment equals 0. (Values greater than one indicate hyper-restoration in resistance relative to control and values below zero suggest further inhibition as compared to acute treatment.) These data confirmed that the pattern of restoration varied gene by gene, but this pattern was consistent in LREX’ xenografts and in the validation cohort tumors (Pearson r .64, p = 7.54 × 10−10, Figure 4B). This finding is most consistent with a model in which AR remains inhibited in drug-resistant tumors but expression of certain AR target genes is restored by an alternative transcription factor, possibly GR. The fact that AR restoration values were somewhat higher in the LREX’ analysis correlates with higher GR expression in these tumors (Figure 4C).
GR drives expression of AR target genes in resistant tissues
To determine if GR can drive expression of this subset of AR target genes, we compared, in vitro, DHT-induced (AR) and dexamethasone (Dex)-induced (GR) expression of 7 AR targets that represent the spectrum of restoration noted in the in vivo analysis, as well as PSA (Figure 4D). All 8 genes were regulated by DHT as expected, and this regulation was blocked by enzalutamide. Thus, AR signaling remains intact and can be inhibited by antiandrogens in these drug-resistant cells, making an AR-dependent mechanism of drug resistance less likely.
In contrast to DHT, the effect of Dex on these same target genes was variable but closely matched the pattern observed in drug resistant xenografts. For example, Dex strongly induced SGK1 and STK39 but did not induce TIPARP, NDRG1, and PMEPA1. Of note, KLK3 (PSA) was comparably induced by either DHT or Dex, providing evidence that persistent PSA expression in patients responding poorly to enzalutamide could be driven by GR. As expected, enzalutamide did not notably affect Dex activity. To confirm that this pattern of GR-dependent gene expression is not unique to LREX’ cells, we introduced a GR expressing retrovirus into parental LNCaP/AR cells and observed a similar pattern of DHT- versus Dex-induced gene expression (Figure S4A, S4B). To be sure that the effects of Dex in these models are mediated through GR, we co-treated cells with a previously described competitive GR antagonist that lacks AR binding called compound 15 (Wang et al., 2006). Compound 15 significantly decreased expression of Dex-induced genes, confirming that Dex activity in the LREX’ model is GR-dependent (Figure S4C). Lastly, siRNA experiments targeting AR confirmed that AR is not necessary for Dex-mediated gene activation (Figure S4D). Collectively these experiments demonstrate that GR is able to drive expression of certain AR target genes independent of AR.
AR and GR have overlapping transcriptomes and cistromes
To explore AR and GR transcriptomes in an unbiased fashion, we performed expression profiling after short-term treatment of LREX’ cells with DHT or Dex in the presence or absence of enzalutamide. AR and GR signatures were respectively defined as all genes with absolute expression change greater than 1.6 fold (FDR<.05) after 1 nM DHT or 100 nM Dex treatment (Supplementary Table 3). Of the 105 AR signature genes and 121 GR signature genes, 52 were common to both lists (Figure 5A). An even larger proportion of AR or GR signature genes (>80%) showed evidence of regulation by the reciprocal receptor using different thresholds for expression differences (Supplementary Table 3). Heatmap analysis of these genes confirmed significant overlap in DHT- versus Dex-induced gene expression and showed that Dex-induced gene expression is not impacted by enzalutamide treatment (Figure 5B). These findings support the hypothesis that GR activity can bypass enzalutamide-mediated AR inhibition by regulating a distinct but significantly overlapping transcriptome.
Figure 5. Comparative AR and GR transcriptome and cistrome analysis in LREX’.
A. Venn diagram of AR and GR signature gene lists. AR or GR signatures were defined as all genes showing >1.6 (or <−1.6) fold change (FDR <. 05) after 8 hours of addition of DHT (1nM) or Dex (100nM) to charcoal stripped media, respectively. B. Heat map depiction of expression changes of AR signature genes (left) or GR signature genes (right) associated with the indicated treatment. Enzalutamide = 10 micromolar. C. Expression of AR- or GR-induced signature genes (as defined in A.) were compared in DHT (1nM) or Dex (100nM) treated samples. GR signature genes that also had higher expression in Dex samples (>1.1 fold, FDR <.05) were designated as GR-selective (n=67) and AR signature genes that showed higher expression in DHT samples (>1.1 fold, FDR <.05) were designated as AR-selective (n=39). D. Expression of AR- and GR-selective genes in LREX’ and control tumors in vivo compared by GSEA. E. AR cistrome defined by AR ChIP-seq after DHT (1nM) treatment of LREX’ in vitro in charcoal stripped media. Percent of AR defined peaks that overlap with GR peaks found by GR ChIP-seq after Dex (100nm) treatment of LREX’ in vitro are shown in pie graph. Top binding motifs in AR-unique and AR/GR overlap peaks are indicated below. F. GR cistrome defined by GR ChIP-seq after Dex treatment of LREX’ in vitro in charcoal stripped media. Percent of GR peaks that overlap with AR peaks found by AR ChIP-seq after DHT (1 nM) treatment of LREX’ in vitro are shown in pie graph. Top binding motifs in GR-unique and AR/GR overlap peaks are indicated below. See also Figure S5.
We next addressed the question of whether transcriptomes of enzalutamide-resistant tumors are more likely to be explained by AR- or GR-driven gene expression using gene set enrichment analysis (GSEA). To define gene sets that distinguish AR and GR activity, expression of AR and GR signature genes was first evaluated by GSEA in the DHT- and Dex-treated samples from which they were derived. As expected, GR signature genes were enriched in the Dex-treated samples and AR signature genes were enriched with DHT treatment (Figure 5C). Because several of the genes did not distinguish AR and GR status due to their overlapping transcriptional activities, we refined the lists into AR selective genes (defined as the AR induced signature genes that were also more highly expressed in DHT treated samples relative to Dex treated samples, n=39) and GR selective genes (defined as the converse, n=67) (Supplementary Table 3). GSEA analysis of these selective gene lists revealed that GR selective genes were strongly enriched in the enzalutamide-resistant LREX’ tumors whereas AR selective genes were strongly enriched in the control tumors (Figure 5D). These data provide compelling, unbiased evidence that drug resistance is associated with a transition from AR- to GR-driven transcriptional activity.
One prediction of this model is that GR should occupy a substantial portion of AR binding sites in drug resistant cells. To address this question, we conducted ChIP-seq experiments to define AR and GR DNA binding sites in LREX’ cells after DHT and Dex treatment respectively. Of note, 52% of the AR binding sites identified after DHT treatment were bound by GR after Dex treatment (Figure 5E). We examined the remaining 48% of AR peaks more closely to be sure that these peaks were not scored as GR negative simply because they fell just below the threshold set by our peak calling parameters. When we plotted the average AR and GR signal as a measure of the relative strength of AR and GR peaks, we found little evidence of GR binding at the AR unique sites (Figure S5A), confirming that these peaks were indeed unique to AR. Next we conducted motif analysis to explore potential differences between AR/GR overlap versus AR unique sites. The core ARE/GRE consensus sequence was present in both groups (66% and 68% of peaks) but AR/GR overlap peaks were relatively enriched for the FoxA1 motif (64% versus 45% of peaks, p=2.2×10-16) (Figure 5E). Similar analysis of the GR cistrome defined GR unique and AR/GR overlap peaks and revealed that a higher proportion of GR binding sites were unique to GR. Interestingly, GR unique peaks were highly enriched for the FoxA motif (Figure 5F), while the classic ARE/GRE was not reported by the motif discovery algorithm (MEME) and was found only 25% of the time.
Although these cistrome studies provide evidence of substantial overlap between AR and GR binding sites in enzaluamide-resistant cells, several lines of evidence indicate that the transcriptional differences in DHT- versus Dex-induced gene expression cannot be explained solely by DNA binding. For example, ChIP RT-qPCR experiments showed significant AR and GR DNA binding at genes induced by both receptors (SGK1, FKBP5, PSA) but also at genes such as NDRG1 that are transcriptionally activated by DHT but not Dex (Figure S5B). Integrative ChIP-seq and transcriptome analysis provided further evidence that DNA binding is not sufficient to determine transcriptional competence. Of the 56 AR signature genes found to have an AR binding peak, 49 showed at least some transcriptional regulation by GR (1.2 fold expression change, p<.05). 38 of these 49 GR regulated genes (78%) had an overlapping AR/GR binding peak, confirming substantial overlap at co-regulated genes. But GR peaks were also found in 3 of the 7 AR targets genes (43%) with no apparent GR transcriptional regulation (Figure S4C). Others have reported evidence of allosteric regulation of hormone receptor complexes by specific DNA sequences independent of binding affinity (Meijsing et al., 2009), a phenomenon that may also be relevant here.
Activation of GR by dexamethasone is sufficient to confer enzalutamide resistance
Whereas LNCaP/AR cells acquire GR expression after prolonged exposure to enzalutamide, some prostate cancer cell lines derived from CRPC patients (DU145, PC3, VCaP) express endogenous GR (Figure 6A). DU145 and PC3 cells are AR-negative and hence resistant to enzalutamide but VCaP cells are enzalutamide-sensitive in vitro (Tran et al., 2009). IHC analysis showed diffuse, primarily cytoplasmic GR expression under standard culture conditions that lack glucocorticoid supplementation (Figure S6A). To test if GR activation by addition of glucocorticoids impacts antiandrogen sensitivity, we treated VCaP cells with enzalutamide in the presence or absence of Dex. Enzalutamide inhibited growth as expected, but co-treatment with Dex reversed this growth inhibition (Figure 6B). Additional studies with the GR antagonist, compound 15, or with GR shRNA restored enzalutamide sensitivity, provided pharmacologic and genetic evidence that GR confers resistance (Figure 6C, 6D, 6E). Of note, GR knockdown (which inhibits GR more completely than compound 15, which has mixed agonist/antagonist properties(Wang et al., 2006)) augmented the activity of enzalutamide even in the absence of Dex (Figure 6D,F), suggesting that even the weak basal GR activity seen under our standard cultures conditions can confer relative resistance to enzalutamide. This result also suggests that a pure GR antagonist could enhance the activity of enzalutamide in prostate cancers co-expressing GR and AR.
Figure 6. GR activity is sufficient to confer enzalutamide resistance in VCaP.
FOR ALL PANELS: VCaP cells do not tolerate charcoal stripped media and were cultured in standard culture conditions (fetal bovine serum with endogenous hormones). Enz=10 micromolar, Dex = 100 nM, CMP 15 = 1 micromolar. A. Western blot analysis of prostate cancer cell lines. B, C and D. Cell viability assessed by CellTiter-Glo (Promega) assay and normalized to day 1 value after indicated treatments +/− s.e.m. E. Confirmation of GR knock-down by western blot after infection with GR targeting shRNA. F. Apoptosis as assessed by cPARP western blot after 3 days of indicated treatment. G. A suite of AR targets relevant to VCaP was defined (see methods) and normalized expression of each gene after 24 hours of indicated drug treatments is depicted by heat map and ranked by degree of induction with Dex. H. Expression of the top two genes from B. (KLK2 and FKBP5) after 24 hours of indicated treatments +/− s.e.m. See also Figure S6.
To determine if Dex activates a subset of AR target genes in VCaP (as we observed in the LREX’ model), we derived a list of AR target genes in VCaP cells exposed to DHT and asked whether Dex could modulate these same AR target genes in the presence of enzalutamide. Dex restored expression of some targets (KLK2, FKBP5, HOMER2, SLC45A3) but not others (DHCR24, SLC2A3, TRPM8, TMEM79), analogous to the uneven restoration we observed in the LNCaP/AR model (Figure 6G). Dex also induced expression of the clinical biomarker PSA in these cells, further supporting the hypothesis that GR can drive PSA progression in enzalutamide-resistant patients (Figures S6B, C). To confirm that Dex activated genes via the glucocorticoid receptor, we evaluated the effect of compound 15 on Dex induced transcriptional activity. As expected, compound 15 reduced Dex induction of the GR targets KLK2 and FKBP5 (Figure 6H). Similarly, GR knock-down prevented Dex-mediated induction of target genes (Figure S6C). As in the LREX’ system (Supplementary Table 3), the vast majority of genes robustly regulated by GR activation in VCaP cells were also regulated by AR activation with DHT (Supplementary Table 4). Others have recently shown substantial overlap in the AR and GR cistromes in VCAP as well(Sahu et al., 2013). These findings extend our hypothesis that GR promotes enzalutamide resistance largely by replacing AR activity at a subset of genes to a second model system.
A subset of prostate cancer are primed for GR induction in the setting of AR inhibition
In considering potential mechanisms for increased GR expression in drug-resistant tumors, we noted several observations that suggested two distinct models. First, flow cytometry analysis of LNCaP/AR and CS1 cells revealed GR expression in a rare subset of cells (Fig 1E), raising the possibility that these cells clonally expand under the selective pressure of antiandrogen therapy. Consistent with this model, we observed rare GR-positive cells in a tissue microarray analysis of 59 untreated primary prostate cancers (Supplementary Table 5). However, we also observed a modest (~2 fold) but significant increase in GR mRNA levels in LNCaP/AR xenografts after only 4 days of antiandrogen treatment, reminiscent of an older report of increased GR expression in normal ventral rat prostate after castration (Davies and Rushmere, 1990). These findings suggest a second model of adaptive resistance whereby AR inhibition causes an increase in GR levels due to loss of AR-mediated negative feedback.
To investigate the relationship between AR activity and GR expression, we first asked if the high level of GR expression in LREX’ tumors is maintained after discontinuation of enzalutamide. Remarkably, GR mRNA levels dropped by ~5 fold 8 days after treatment discontinuation (Figure 7A). Because enzalutamide has a prolonged half-life in mice (Tran et al., 2009), it is difficult to make definitive conclusions about negative feedback loops using in vivo models. Therefore, we conducted similar enzalutamide withdrawal experiments in LREX’ cells cultured in vitro. GR mRNA levels dropped as early as 1 day after discontinuation and continued to decline throughout the 23 days of the experiment (Figure 7B). Additional experiments with LREX’ cells using earlier timepoints in charcoal stripped media showed reduced GR mRNA levels after only 8 hours DHT exposure and this reduction was reversed by co-treatment with enzalutamide (Figure 7C). This reduction correlated precisely with the recruitment of an AR binding peak in an intronic enhancer of GR identified by ChIP, suggesting AR directly represses GR expression in these cells (Figure 7D).
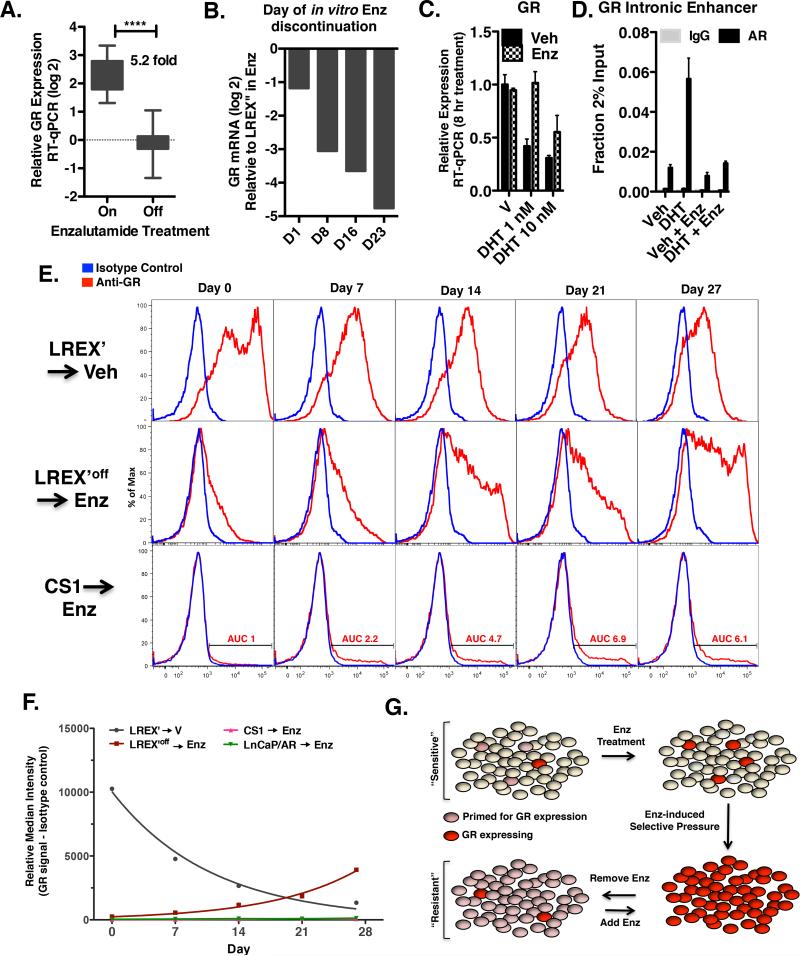
Figure 7. Resistant cells are primed for GR induction upon AR inhibition.
A. GR mRNA in LREX’ xenografts. Tumors were injected into castrated mice and immediately treated with 10 mg/kg enzalutamide (n=20) for 7 weeks . Half of the mice were then continued on 10 mg/kg enzalutamide (n=10) or discontinued for 8 days (n=10). B. LREX’ are maintained in vitro in the presence of enzalutamide 1 micromolar. GR mRNA was assessed in LREX’ cell line after passage for indicate number of days in standard fetal bovine serum containing media without enzalutamide. C. GR mRNA in LREX’ cultured in charcoal stripped media for 48 hours and then treated for 8 hours with vehicle or DHT with or without 10 micromolar enzalutamide. D. AR ChIP-qPCR with LREX’ cultured in charcoal stripped media and then treated for 1 hour with DHT (1nM) or Dex (100nM) at an intronic enhancer site +/− s.d. E. Intracellular GR flow cytometric analysis of indicated cells at indicated times points. AUC = area under curve. Enzalutamide = 1 micromolar F. Plotted median fluorescence (minus background) values from E and Figure S7C. For both LREX plots, R2 values for non-linear regression analysis is >.98. G. Model of GR induction in resistant tissues. See also Figure S7.
To determine if the loss of GR expression upon enzalutamide withdrawal occurs across the entire cell population or is restricted to a subset of cells, we conducted flow cytometry experiments, where a shift in median signal intensity can be used to identify expression changes in the bulk cell population. (Expression changes limited to a minority sub-population would not affect the median and would instead be identified as a tail population by histogram plot.) We observed an exponential decay in median GR protein signal (half-life 7.6 days) (Figure 7E,top row, 7F), confirming that the loss in GR expression occurs across the entire LREX’ cell population. Extension of this experiment to later time points (17 weeks) revealed a plateau in loss of GR expression by 7 weeks (Figure S7A).
Next we conducted the reciprocal experiment of re-exposure of LREX’ cells to enzalutamide following GR downregulation after prolonged enzalutamide withdrawal (LREX’off). GR expression was regained with induction kinetics essentially reciprocating the rate of decay previously seen with removal of drug (doubling time 6.8 days), establishing that the resistant line remained poised for GR induction in the setting of AR inhibition (Figure 7E,F). Consistent with the time scale, continued drug exposure for 7 weeks was associated with a clear shift in GR expression in essentially all cells (Figure S7A).
We next determined if AR inhibition is sufficient to induce GR expression in LNCaP/AR or CS1 cells that had not previously been exposed to enzalutamide. In contrast to LREX’, there was no change in median expression intensity in CS1 or LnCaP/AR over the 4 week experiment, indicating that most cells do not turn on GR expression simply as a consequence of AR inhibition (Figures 7E, 7F, S7C). However, the area under the GR staining population did increase. Given the weak antiproliferative effect of enzalutamide in vitro (Figure S7B), we conclude that this increase in GR expression is most likely explained by loss of AR-mediated negative feedback rather than by clonal expansion. Together, these findings support a model in which a subset of prostate cancer cells are “primed” for GR induction in the context of AR inhibition through an adaptive resistance mechanism (via AR-mediated negative feedback). We postulate that these cells then clonally expand under the selective pressure of AR blockade, eventually emerging as drug-resistant tumors whose expression profiles may resemble those of AR-driven tumors but are driven by GR (Figure 7G).
Discussion
Following the recent approvals of the next generation AR pathway inhibitors abiraterone and enzalutamide, the treatment of metastatic prostate cancer has evolved to a two-stage process. Initially patients receive conventional androgen deprivation therapy, typically with a gonadotropin-releasing hormone agonist that lowers testosterone (castration), often in conjunction with an anti-androgen such as bicalutamide. Preclinical and clinical studies have conclusively demonstrated that acquired resistance to conventional androgen deprivation therapy is caused by restoration of AR pathway activation, primarily due to increased AR expression. These discoveries provided the rationale for the development of next generation AR therapies.
Here we demonstrated that acquired resistance to at least one of these new next generation therapies, enzalutamide, can occur via a different mechanism – increased expression of GR. The evidence for GR-driven resistance emerged from two independent preclinical models (LNCaP/AR and VCaP) and was supported by correlative data showing increased GR expression in patients with enzalutamide resistance. Consistent with our mechanistic studies showing that GR can function independently of AR, increased GR expression was also associated with ARN-509 resistance, potentially forecasting a general mechanism of resistance to antiandrogens. Whether increased GR expression plays a role in abiraterone resistance remains to be determined. Unlike enzalutamide and ARN-509, abiraterone impairs AR signaling by lowering residual systemic and intratumoral androgen levels and preclinical evidence suggests that abiraterone resistance may be associated with increased AR expression (Mostaghel et al., 2011). We speculate that tumors can efficiently overcome the ligand deficiency conferred by traditional androgen-deprivation therapy or abiraterone by simply elevating AR levels, whereas the increased selection pressure conferred by second-generation antiandrogens requires an alternative strategy such as GR bypass or AR mutation (Balbas et al., 2013; Joseph et al., 2013; Korpal et al., 2013).
Comparative AR and GR transcriptome studies supported a model whereby GR bypasses enzalutamide-mediated AR blockade without the need for any restored AR function. This model is further supported by ChIP-seq analyses showing that GR can bind to just over half of all AR binding sites in enzalutamide resistant cells. Importantly, GR occupied a large number of sites that are not bound by AR, raising the possibility of a distinct GR transcriptional program that could contribute to resistance. However, our transcriptome analysis found that a large majority of genes robustly regulated by GR were also regulated by AR. Notably, several canonical AR targets genes including KLK3 and TMPRSS2 show regulation by GR (Supplementary Table 3). For these reasons, we believe that the antiandrogen resistance conferred by GR is most likely mediated by one or more of the unevenly restored AR target genes rather than a distinct set of “GR only” target genes. It will be of interest to explore whether just one or a small number of downstream targets are responsible for resistance and also why GR fails to activate transcription at the vast majority of the “GR unique” binding sites. We postulate that variables such as chromatin context, co-factors and other signaling events may be important.
The GR bypass model of AR pathway blockade reported here is reminiscent of recent reports that kinase inhibitor blockade in various cancers can be overcome by up-regulation of other kinases and/or their ligands (Engelman et al., 2007; Johannessen et al., 2010; Straussman et al., 2012; Wilson et al., 2012). To our knowledge, our observation is the first example of nuclear receptor bypass as a mechanism of acquired resistance to nuclear receptor blockade. In the case of kinase inhibitors, bypass is just one of many potential resistance mechanisms that also includes direct mutation of the kinase target and lineage switching to histologically distinct phenotypes that no longer require the drug target for survival (Katayama et al., 2012). We believe the same may be true here based on the fact that a subset of drug-resistant LNCaP/AR tumors had minimal GR expression, raising the possibility of other resistance drivers. For example, one of these low GR tumors contained the F876L AR mutation that converts both ARN-509 and enzalutamide to agonists and is associated with clinical resistance (Balbas et al., 2013; Joseph et al., 2013; Korpal et al., 2013). A second low GR tumor expressed high levels of N-Cadherin (Supplementary Table 1C), which can confer AR independence by morphological conversion to a tumor with mesenchymal features (Tanaka et al., 2010).
Expression of GR in antiandrogen-resistant prostate tumors appears to occur by a mechanism that includes features of adaptive resistance (via AR-mediated negative feedback of GR expression) as well as clonal selection. Our data showed that AR inhibition induced strong GR expression in drug-resistant prostate cancer cells as well as in a subset of drug-naïve cells that are somehow “primed” to respond. The molecular basis for this “primed” state remains to be defined but, based on the reversibility of GR expression in the presence or absence of AR inhibition, is likely to involve an epigenetic mechanism. Knowledge of baseline tumor GR expression in patients, as well as the “primed” state of these tumor cells, could have clinical relevance as a treatment response biomarker. We already have evidence that baseline GR expression may predict for a poor clinical outcome despite a limited clinical dataset and, based on the increase in GR expression in some patients after 8 weeks of treatment, that the “priming” phenomenon observed in our models may also be relevant in patients.
Whatever the precise mechanism regulating GR expression, one immediate implication is that corticosteroid therapy could be detrimental to prostate cancer patients in certain clinical contexts. Corticosteroids are currently administered routinely with both docetaxel and abiraterone to prevent side effects from each of these therapies. Our data suggest that corticosteroids might promote tumor progression in men whose tumors express GR. Indeed, reanalysis of the phase 3 clinical trial AFFIRM that demonstrated a survival benefit with enzalutamide treatment found that men receiving corticosteroids had a significantly worse survival that those who did not (Scher et al., 2012b) (Scher et al., 2012a). It is worth noting that corticosteroids can also confer clinical benefit in CRPC, an effect attributed to feedback suppression of pituitary ACTH production and resultant decrease in adrenal androgen production (Attard et al., 2009). This duality of potential glucocorticoid effects should prompt a reexamination of the appropriate clinical context for corticosteroid therapy.
Our findings also suggest that combined inhibition of both GR and AR could prolong the duration of response with next generation AR antagonists. Clinical studies of the GR antagonist mefipristone in patients with excess glucocorticoid production (Cushing syndrome) demonstrate that GR can be inhibited in humans with an acceptable risk-benefit profile (Fleseriu et al., 2012). Unfortunately both mefipristone and a related GR antagonist ORG34517 activate AR target gene expression, likely by direct AR agonism since mefipristone binds and activates AR (Klokk et al., 2007). The ability of compound 15 to overcome GR driven resistance should stimulate further efforts to optimize GR-specific antagonists that lack “off target” AR effects for use in preventing or overcoming enzalutamide resistance.
Experimental Procedures
Bone marrow evaluation
Patients were treated with enzalutamide 160 mg daily. Bone marrow biopsy and aspirate (~5 mL) were performed before treatment and at week 8. The bone marrow specimens were obtained by transiliac biopsy, and samples were processed according to standard MD Anderson Cancer Center decalcification and fixation procedures. After pathologic evaluation, samples were stored in the MD Anderson Cancer Center Prostate Cancer Tissue Bank. Imaging studies were performed at the time of suspected prostate cancer progression or at the treating physician's discretion, but generally not prior to 12 weeks post-treatment initiation. Therapy was discontinued at the treating physician's discretion in patients exhibiting progression. Retrospective analysis for GR was performed by IHC on 3.5-mm formalin-fixed, paraffin-embedded bone marrow biopsy sections with anti-GR at a dilution of 1:200 (BD Transduction Laboratories #611227). A Dako autostainer and standard 3,3-diaminobenzidine were used. GR expression was assessed in a blinded fashion by two pathologists scoring at least 100 tumor cells per specimen. Plotted are either data from all specimens or only from patients with usable material at baseline and 8 weeks.
AR target gene list derivation
The 74 AR target gene list utilized for evaluation of AR pathway status in the LnCaP/AR model includes all genes that showed at least a 1.6-fold change (FDR < .05) when comparing control and 4 day treated xenografts and that were also found to have an AR binding peak by ChIP-seq analysis of LNCaP/AR in vitro (Cai et al, in preparation). The VCaP AR target gene list includes all genes that that showed reciprocal expression change with 24 hour DHT (.1nM) or enzalutamide (10μM) of at least 1.4 fold (p<.05) (Illumina HT-12) and were also found to have an AR binding peak by ChIP-seq analysis of VCaP (Cai et al, in preparation).
AR/GR signature analysis and Gene Set Enrichment Analysis
AR and GR signature genes were defined as all genes showing >1.6 fold (FDR<.05) expression change with either 1 nM DHT or 100 nM Dex treatment, respectively, of LREX’ cells for 8 hours in charcoal stripped media. For GSEA, signature genes induced by either DHT or Dex treatment were used. GR selective genes showed at least 1.1 fold higher expression in Dex treated samples compared to DHT treated samples (FDR <.05). AR selective genes showed at least a 1.1 fold higher expression in DHT treated samples compared to Dex treated samples (FDR <.05).
Statistics
Microarray data analysis and comparisons were performed with Partek Software. All RT-qPCR comparisons are by two-sided t-test. Xenograft volumes and GR IHC of clinical specimens are compared by one-sided Mann-Whitney test. In vitro growth comparisons are by two-sided t-test. GSEA statistical analysis was carried out with publicly available software from the Broad Institute (Cambridge, MA: http://www.broadinstitute.org/gsea/index.jsp). In all figures, * = <.05, ** = <.01, *** = <.001, and ****=<.0001.
Supplementary Material
Research Highlights.
GR induction is a common feature of enzalutamide resistant prostate cancer
GR expression and activity promote resistance to enzalutamide
GR binds and regulates a subset of AR targets in a enzalutamide insensitive manner
AR inhibition induces high levels of GR in primed prostate cells
Acknowledgements
We thank Phil Iaquinta, Brett Carver, Yu Chen, Phil Watson, Haley Hieronymus, Bea Darimont and Howard Scher for helpful discussions. We thank Tom Scanlan (Portland, Oregon) for generously providing compound 15, Yu Chen for generously providing the pMItDT-EGFP vector, Agnes Viales and Liping Sun of the MSKCC Genomics Core Facility for ChIP-seq library prep and sequencing, Jeffrey Zhao for assistance with microarray data analysis, Ouathek Ouerfelli of the MSKCC organic synthesis core for synthesizing ORG34517, HuiYong Zhao and Philicia Moonsamy for technical assistance with xenograft experiments, Marina Asher of the MSKCC Pathology Core Facility for assistance with IHC, and Anuradha Gopalan for providing the tissue microarray of primary untreated prostate cancer. The work is funded by CDMRP Physician Research Training Award PC102106, NIH R01CA155169, NIH P50CA09262, U01 CA141502 and the Howard Hughes Medical Institute. The human tissue acquisition study at MDACC was given financial support from Medivation (San Francisco, CA). VKA is funded by a Young Investigator Award from the Prostate Cancer Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Attard G, Reid AH, A'Hern R, Parker C, Oommen NB, Folkerd E, Messiou C, Molife LR, Maier G, Thompson E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbas MD, Evans MJ, Hosfield DJ, Wongvipat J, Arora VK, Watson PA, Chen Y, Greene GL, Shen Y, Sawyers CL. Overcoming mutation-based resistance to antiandrogens with rational drug design. eLife. 2013;2:e00499. doi: 10.7554/eLife.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Clegg NJ, Wongvipat J, Joseph JD, Tran C, Ouk S, Dilhas A, Chen Y, Grillot K, Bischoff ED, Cai L, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer research. 2012;72:1494–1503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P, Rushmere NK. Association of glucocorticoid receptors with prostate nuclear sites for androgen receptors and with androgen response elements. Journal of molecular endocrinology. 1990;5:117–127. doi: 10.1677/jme.0.0050117. [DOI] [PubMed] [Google Scholar]
- de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr., Saad F, et al. Abiraterone and increased survival in metastatic prostate cancer. The New England journal of medicine. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. The New England journal of medicine. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- Efstathiou E, Titus M, Tsavachidou D, Tzelepi V, Wen S, Hoang A, Molina A, Chieffo N, Smith LA, Karlou M, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol. 2012;30:637–643. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- Fleseriu M, Biller BM, Findling JW, Molitch ME, Schteingart DE, Gross C. Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing's syndrome. The Journal of clinical endocrinology and metabolism. 2012;97:2039–2049. doi: 10.1210/jc.2011-3350. [DOI] [PubMed] [Google Scholar]
- Glickman MS, Sawyers CL. Converting cancer therapies into cures: lessons from infectious diseases. Cell. 2012;148:1089–1098. doi: 10.1016/j.cell.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, Moon M, Chow Maneval E, Chen I, Darimont B, et al. A clinically relevant androgen receptor mutation confers resistance to 2nd generation anti-androgens enzalutamide and ARN-509. Cancer discovery. 2013 doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, Jessop NA, Wain JC, Yeo AT, Benes C, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med. 2012;4:120ra117. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klokk TI, Kurys P, Elbi C, Nagaich AK, Hendarwanto A, Slagsvold T, Chang CY, Hager GL, Saatcioglu F. Ligand-specific dynamics of the androgen receptor at its response element in living cells. Mol Cell Biol. 2007;27:1823–1843. doi: 10.1128/MCB.01297-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, Yuan J, Kovats SG, Kim S, Cooke VG, et al. An F876L Mutation in Androgen Receptor Confers Genetic and Phenotypic Resistance to MDV3100 (Enzalutamide). Cancer discovery. 2013 doi: 10.1158/2159-8290.CD-13-0142. [DOI] [PubMed] [Google Scholar]
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. The New England journal of medicine. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijsing SH, Pufall MA, So AY, Bates DL, Chen L, Yamamoto KR. DNA binding site sequence directs glucocorticoid receptor structure and activity. Science. 2009;324:407–410. doi: 10.1126/science.1164265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, Nelson PS, Montgomery RB. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu B, Laakso M, Pihlajamaa P, Ovaska K, Sinielnikov I, Hautaniemi S, Janne OA. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer research. 2013;73:1570–1580. doi: 10.1158/0008-5472.CAN-12-2350. [DOI] [PubMed] [Google Scholar]
- Sawyers CL, Hochhaus A, Feldman E, Goldman JM, Miller CB, Ottmann OG, Schiffer CA, Talpaz M, Guilhot F, Deininger MW, et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood. 2002;99:3530–3539. doi: 10.1182/blood.v99.10.3530. [DOI] [PubMed] [Google Scholar]
- Scher HI, Fizazi K, Saad F, Chi K, Taplin M-E, Sternberg CN, Armstrong AJ, Hirmand M, Selby B, De Bono JS. ASSOCIATION OF BASELINE CORTICOSTEROID WITH OUTCOMES IN A MULTIVARIATE ANALYSIS OF THE PHASE 3 AFFIRM STUDY OF ENZALUTAMIDE (ENZA), AN ANDROGEN RECEPTOR SIGNALING INHIBITOR (ARSI), ESMO. 2012a [Google Scholar]
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. The New England journal of medicine. 2012b;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Kono E, Tran CP, Miyazaki H, Yamashiro J, Shimomura T, Fazli L, Wada R, Huang J, Vessella RL, et al. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat Med. 2010;16:1414–1420. doi: 10.1038/nm.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Shah N, Pantoja C, Meijsing SH, Ho JD, Scanlan TS, Yamamoto KR. Novel arylpyrazole compounds selectively modulate glucocorticoid receptor regulatory activity. Genes & development. 2006;20:689–699. doi: 10.1101/gad.1400506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–509. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.