Summary
Strategies to reduce arsenic in rice grain, below levels that represent a serious human health concern, require that the mechanisms of arsenic accumulation within grain be established. Therefore, re-translocation of arsenic species from flag leaves into filling rice grain was investigated.
Arsenic species were delivered through cut flag leaves during grain fill. Spatial unloading within grains was investigated using synchrotron X-ray fluorescence (SXRF) microtomography. Additionally, the effect of germanic acid (a silicic acid analogue) on grain arsenic accumulation in arsenite treated panicles was examined.
Dimethylarsinic acid (DMA) and monomethylarsonic acid (MMA) were extremely efficiently re-translocated from flag leaves to rice grain; arsenate was poorly re-translocated, and was rapidly reduced to arsenite within flag leaves; arsenite displayed no re-translocation. Within grains, DMA rapidly dispersed while MMA and inorganic arsenic remained close to the entry point. Germanic acid addition did not affect grain arsenic in arsenite treated panicles. 3D SXRF microtomography gave further information on arsenite localization in the ovular vascular trace (OVT) of rice grains.
These results demonstrate that inorganic arsenic is poorly re-mobilized, while organic species are readily re-mobilized, from leaves to grain. Stem translocation of inorganic arsenic may not rely solely on silicic acid transporters.
Keywords: arsenic, grain filling, phloem, rice, translocation
Introduction
Rice (Oryza sativa), the staple food for over half the world's population, represents a significant dietary source of inorganic arsenic (As), a nonthreshold, class 1 human carcinogen (Meharg et al., 2009). It is imperative that strategies to reduce grain As are developed and those mechanisms which enable As to reach, and accumulate within, the rice grain be determined (Zhao et al., 2009, 2010). The main species of As found in rice grain are inorganic arsenic (arsenate and arsenite), and DMA, with trace amounts of MMA and tetramethylarsonium sometimes present (Williams et al., 2005; Hansen et al., 2010). Arsenate, the dominate soil species under aerobic conditions with a high affinity for iron oxides, is an analogue of phosphate, and enters rice roots through phosphate transporters, while the markedly more mobile arsenite, the dominant redox form under anaerobic conditions, enters the rice plant through silicic acid transporters (Abedin et al., 2002a; Ma and Yamaji, 2008; Ma et al., 2008). Consequently, as paddy rice cultivation relies on anaerobic soils, rice is particularly effective at accumulating arsenic, compared with other cereals (Williams et al., 2007). Within the rice root, arsenate is reduced to arsenite, which then enters the stele via the Lsi2 silicic acid effluxer (Xu et al., 2008; Ma and Yamaji, 2008; Zhao et al., 2009). Su et al. (2010) reported that arsenite was the main species found in the xylem sap of rice plants fed either arsenite or arsenate. Arsenite may be complexed by phytochelatins, followed by sequestration into cell vacuoles (Bleeker et al., 2006; Raab et al., 2007; Zhao et al., 2009). Song et al (2010) recently identified phytochelatin transporters responsible for transporting chelated arsenite into cell vacuoles, in Arabidopsis thaliana. The organic species DMA and MMA are assimilated at a much slower rate by the root than inorganic As (Abedin et al., 2002b), with the protonated neutral forms also transported through silicic acid pathway (Li et al., 2009). Raab et al (2007) and Li et al (2009) found that methylated As species are more readily translocated within the plant than their inorganic counterparts. Once in the phloem/xylem bundle of rice DMA is translocated into grain with over an order of magnitude greater efficiency than inorganic As species (Carey et al., 2010). Phloem transport accounted for 90% of arsenite unloaded into grain and for 55% of DMA. A study of arsenic transport in castor bean plants reported that arsenite was not complexed with thiols in the phloem, as thiol complexes are not stable in the alkaline conditions of the phloem (Ye et al., 2010).
Carey et al (2010) used XANES (X-ray Absorption Near Edge Structure) spectroscopy to examine the speciation of As in the developing rice grain for excised panicles delivered a pulse of either arsenate, arsenite or DMA through the cut stem (i.e. xylem plus phloem). DMA was partially thiol-complexed within the grain and the redox status of inorganic arsenic seemed to be concentration dependant (Carey et al., 2010). When arsenite was delivered to excised rice panicles at 13.3μM, arsenite was oxidized to arsenate within the rice grain, while under exposure to 133μM, arsenite remained stable within the grain as free arsenite (Carey et al., 2010). Inorganic arsenic is localised in the bran layer of rice grain, while the endosperm contains high levels of DMA (Sun et al., 2008; Carey et al., 2010). The use of synchrotron X-ray fluorescence techniques to map the As distribution in rice grain with high levels of inorganic arsenic, has shown As to be concentrated in the region of the ovular vascular trace (OVT), the point of entry into the grain (Lombi et al., 2009; Meharg et al., 2008). Minerals are unloaded into the rice grain from the OVT, which contains both phloem and xylem cells, into the nucellar tissue and are then uploaded, via the apoplast, into the filial tissue (the aleurone and the endosperm) (Krishnan and Dayanandan, 2003). Lombi et al (2009) suggested that this transport of material from the maternal to the filial tissues may represent a physiological barrier which As species cross with differential efficiency. Synchrotron x-ray fluorescence (SXRF) microtomography of developing rice grains fed arsenite or DMA through excised stems found that arsenite was localised in the OVT region while DMA had rapidly spread throughout the outer layers and into the endosperm. These findings explain why inorganic As is concentrated in rice bran while the endosperm contains higher levels of DMA. Zheng et al (2010) examined temporal variation in As levels in rice plants at different stages of growth and reported that the concentration of DMA decreased during grain fill, suggesting that DMA accumulation in grain occurred via the re-translocation of DMA accumulated in the plant before flowering, while inorganic arsenic levels remained constant, suggesting it was taken up during the period of grain fill. However, whether As is actually re-translocated to the grain from flag leaves has yet to be established and the redox status of inorganic As within the flag leaves remains unknown.
The aim of this study was to investigate the re-translocation of As species from flag leaves into the filling rice grain, by feeding arsenic species directly into the flag leaves during grain fill, and to determine the spatial unloading of key As species within the developing rice grain and establish whether differences existed between leaf re-translocated and direct stem transported As. Additionally, to investigate the role of silicic acid and arsenite pathways, germanic acid, a silicic acid analogue (Ma et al., 2002; Nikolic et al., 2007) was used as a tracer in competitive inhibition in shoot and stem feeding studies. Furthermore, three-dimensional SXRF microtomography gave further insights into arsenite localization in the OVT after arsenite treatment.
Materials and Methods
Plant growth
For all experiments a quick flowering Oryza sativa L. cultivar Italica Carolina was utilized, and grown to maturity with healthy panicles labelled at anthesis as described in Carey et al 2010.
Leaf feeding of As species
To investigate the re-mobilization of arsenic species from flag leaves during grain development, rice panicles were exposed to 333 μM of arsenite, arsenate, DMA or MMA at 10 d post anthesis. Treatment stock solutions of 13.3 mM were prepared by dissolving the appropriate salt in Milli-Q deionised water, and 7 subsamples of each stock were frozen for subsequent removal and use on the relevant day. Treatment vials were prepared by making 0.25 ml of the defrosted treatment stock up to 1 ml with Milli-Q deionised water and then pipetting 0.1 ml of this diluted stock into a weighed Eppendorf vial, together with MES buffer at a final concentration of 5 mM, and rubidium (Rb) and strontium (Sr), as markers for phloem and xylem transport, respectively, at final concentrations of 1mM (Kuppelwieser and Feller, 1991; Carey et al., 2010). Solutions were pH adjusted to 6.4 with NaOH. This was then made up to 1 ml by weight by the addition of Milli-Q, yielding a treatment concentration of 333 μM arsenite, arsenate, DMA or MMA. For arsenite and DMA, additional treatments were included, 0, 33, and 133 μM, to generate a dose response and establish the best concentration to use in the primary experiments.
A sharp razor blade was used to remove the flag leaf tip and this cut end was inserted into the Eppendorf vial. A strip of aluminium foil was applied to limit evaporation, and dust entry, and the vials were held in place by strong tape. Treatments were delivered through the cut panicle flag leaf on intact plants for 7 d, with a fresh vial applied every 24 h. Each time a fresh vial was applied, the tip of the flag leaf was re-cut to ensure a fresh and open entry point. Treatment vials were frozen on removal from the flag leaves. For each treatment and control, 3 replicate panicles were used. Control ± Rb and Sr were also included.
Inorganic As speciation in flag leaf
For flag leaves fed arsenite or arsenate, As speciation in the fresh flag leaf was analyzed to evaluate inorganic As status. Immediately following removal of the last treatment vial, a section of flag leaf lamina was cut from the ligule/sheath end of the treated leaf, at least 3-4 cm from solution contact, and ground rapidly in liquid nitrogen. This was then suspended in 5 ml of chilled phosphate buffer (a solution of 6.66 mM ammonium nitrate and 6.66 mM ammonium hydrophosphate, adjusted to pH 6.2 using ammonia) and 1 ml of the resulting slurry was decanted into a 1.5 mL microcentrifuge tube and centrifuged at 12000xg for 2 min. The supernatant was diluted (0.5 ml made up to 5 ml) with chilled phosphate buffer solution and a 1 ml subsample was taken for As speciation analysis via anion exchange high performance liquid chromatography coupled with inductively coupled plasma mass spectrometry, HPLC-ICP-MS, as described in Sun et al (2009). Each sample was analysed on the HPLC-ICP-MS within 20-30 min of removal from the rice plant. The remaining contents of treatment vials were also speciated to confirm As species stability. All treatment vials were frozen on removal from the plant and defrosted on the day of speciation analysis. For the arsenite and arsenate treatments, 0.1 ml was subsampled from each of the 7 daily treatment vials for all 3 reps and diluted 1:1000 with chilled phosphate buffer and analysed for As speciation by HPLC-ICP-MS.
Total As analysis for leaf fed panicles
All treatments and controls were analysed for total As via ICP-MS. Flag leaf sections were cut from the ligule/sheath end of the blade, at least 3-4 cm from contact with tape or solution where they had been leaf fed. Grains were selected from the top and middle regions of the panicle and husks were separated manually, with a portion of the grain from each panicle being kept chilled for synchrotron analysis and the remainder oven dried for ICP-MS analysis. One fresh grain from one replicate of each treatment was then randomly selected for SXRF microtomography. Time limitations at the synchrotron prohibited the analysis of more than one replicate per treatment. Oven dried samples were microwave digested and analysed for total As, Rb and Sr concentrations, by ICP-MS (7500 Agilent Technologies), as described in Sun et al (2008). Quality control procedures were as described in Sun et al (2008).
SXRF microtomography
For leaf fed arsenic treated grain, SXRF microtomography was conducted on fresh rice grain, pulsed with 333 μM of either arsenite, arsenate, DMA or MMA, at GeoSoilEnviroCARS (Sector 13) of the Advanced Photon Source, Argonne National Laboratory. Details were as described in Carey et al (2010). The electron storage ring operated at 7 GeV with a top-up fill mode. Fresh rice grain was suspended from a rotation-translation stage in the path of a 3 μm X-ray beam and translated across in 3 μm steps with a dwell time of 0.05 s per step. The grain was then rotated 0.5° and the scan process repeated until a rotation of 0 to 180° was complete. Fluorescence data were collected using a multi-element fluorescence detector and the resulting 2D sine wave plots were reconstructed as described in McNear et al (2005).
Additionally, as it was established that grain arsenite is not re-translocated from flag leaves, further SXRF tomography was conducted for fresh developing rice grain pulsed with 133 μM arsenite via the excised panicle stem, yielding images that offer an improved resolution compared with Carey et al (2010). Fully rendered 3D microtomographic reconstructions of arsenic in the grain were collected at beamline X26A of the National Synchrotron Light Source (Brookhaven National Laboratory, Upton, NY) with the monochromator tuned to 12.5 keV to resolve the arsenic edge. Microtomography was carried out as described in Kim et al (2006). Installation of an additional silicon drift detector at 180° to the existing multi-element Ge array detector effectively removed self-absorption effects for the low Z elements, and allowed the use of on-the-fly data processing. This reduced spectral collection times to 50 milliseconds per point. Arsenite-pulsed grain was mounted on a Huber Goniometer (x,y,z,θ stage). A total of 20 tomographic sections were collected from the awl to the rachilla at intervals of 240 μm. The beam dimensions were 10 μm horizontal × 6 μm vertical, with 20 μm pixel sizes. Data was rendered using tomographic display software written for the Interactive Data Language (IDL version 8.0) program (©ITT Visual Information Solutions).
Phloem vs xylem transport of germanic acid in excised panicles
The comparative contributions of phloem and xylem in the shoot to grain translocation of germanic acid, a silicic acid analogue, in ± stem girdled plants was investigated, with panicles also exposed to Rb and Sr, as markers for phloem and xylem flow, respectively (Kuppelwieser and Feller, 1991; Carey et al., 2010). Panicle stems were girdled with a jet of steam 1 to 2cm below the panicle head, 24 h prior to excision from the plant, as described in Carey et al (2010). This destroys the phloem cells preventing further phloem transport in to the rice grain while xylem vessels remain functional (Martin 1982; Kuppelwieser and Feller; 1991; Chen et al., 2007; Carey et al., 2010).
At 10 d post anthesis, plants were placed in darkness for 2 h to reduce transpiration, limiting the formation of air bubbles in the xylem during excision. Panicles were then excised below the flag leaf node and placed in Pyrex tubes of autoclaved nutrient solution that had been spiked with the appropriate treatment, as described in Carey et al (2010). Panicles were placed directly into treatment solutions. Solutions contained Rb and Sr at a final concentration of 1 mM. MES buffer, at a final concentration of 5 mM, was added to autoclaved nutrient solutions through a sterile filter. Germanic acid treatment stocks were prepared by dissolving GeO2 in Milli-Q deionised water containing 0.2% 4 M NaOH to form germanic acid. Panicles were then transferred to a growth chamber with a 12 h photoperiod, a day: night temperature of 28: 23°C, relative humidity of 80 : 60 and light intensity of 1200 μmol m-2 s-1. For each treatment and control 3 replicate panicles were used. Controls ± Rb and Sr were included. Following the 48 h treatment period flag leaves and grains were oven dried and grains were selected from the top and middle regions of the panicle and manually de-husked.
Additionally, to assess whether shoot-to-grain translocation of arsenite utilizes silicic acid transporters, a competition experiment was conducted by treating excised rice panicles with a hydroponically administered 48 h pulse of 133 μM arsenite or arsenate (or Milli-Q for the control panicles), ± 1.33 mM Ge during grain development.
Samples were microwave digested and total Ge was measured by ICP-MS as described above.
Re-translocation of Ge from flag leaf
To investigate the remobilization of Ge from flag leaves during grain development, rice panicles were exposed to 333 μM of Ge, via the cut flag leaf on intact plants, at 10 d post anthesis. This was conducted alongside the leaf fed arsenic experiment and followed the same procedure.
Results
Leaf feeding of As species
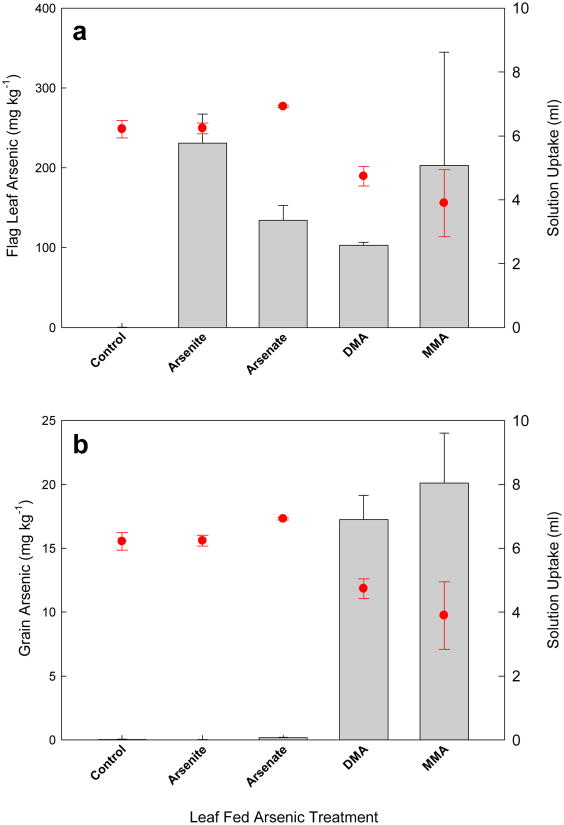
Total solution uptake from feeding vials was less for the DMA and MMA treatments than that for the control and inorganic treatments (Fig. 1). ANOVA demonstrated that As treatment had a significant effect on solution uptake from feeding vials (P=0.01) with inorganic As treatments and the As control having significantly greater total solution uptake than the MMA treatment, and the arsenate treatment having significantly greater uptake than DMA, with no difference between the two organic treatments.
Fig. 1.
Mean total arsenic (As) concentrations (bars) in (a) flag leaf and (b) grain, for rice panicles exposed to 333 μM of 1 of 5 arsenic treatments together with 1 mM rubidium and strontium. Treatments were delivered through the cut flag leaf on intact plants for 7 d, with a fresh vial applied every 24 h. Total solution uptake is also shown for each treatment (circles). Error bars represent ± SE of three replicates.
Mean total As concentrations in flag leaf and grain for leaf fed As species are reported in Fig 1. ANOVA revealed that As treatments had a highly significant effect on flag leaf As levels (P<0.001) with mean flag leaf As significantly lower for As controls than all other As treatments, with no significant differences between the 4 As species, although there was a large degree of variation in MMA treated leaves. In the grain, ANOVA determined that for all As species, with the exception of arsenite which was not significantly different to the control, mean grain As was significantly increased by flag leaf exposure (P<0.001). Fisher's pairwise comparisons showed that organic As treatments, between which there was no significant difference, led to significantly greater mean grain As than inorganic treatments, with levels 100-fold those for arsenate treated plants, and that leaf feeding of arsenate led to significantly higher grain As than feeding with arsenite.
Mean total Rb and Sr concentrations in flag leaf and grain for leaf fed As species are reported in the Supporting Information, Fig S1. The results demonstrate that there is significant phloem transport to the developing grain from the flag leaf, and that this is not affected by the addition of As. There was no measurable transport of Sr from the flag leaf to the grain suggesting that there was little xylem transport from the flag leaf to the grain although, in view of the large degree of variation between replicates (Fig. S1), it cannot be definitively concluded that there is no xylem transport from the flag leaf to the grain.
As speciation in flag leaf
HPLC-ICP-MS analysis of As speciation in freshly extracted flag leaves exposed to arsenite and arsenate for 7 d with feeding vials changed daily showed that arsenite remained stable as arsenite in the leaf, with no conversion to other species, while arsenate was completely converted to arsenite (Fig. S4). Speciation of the remaining feeding vial contents after 24 h attachment to the flag leaf found that arsenite and arsenate treatments had remained stable within the vial for the duration of the feeding period, with As in arsenate feeding solutions predominantly arsenate (99% arsenate and 1% arsenite) and arsenite feeding vials containing 100% arsenite (Fig. S5).
Imaging arsenic unloading
SXRF microtomography images collected for fresh developing rice grains fed with arsenic species via flag leaves are compared with those collected previously for developing rice grains fed via the excised stem (Fig. S6). It appears that arsenic species delivered to the rice grain via re-translocation from the flag leaf do not differ in their unloading and distribution from those delivered via direct stem transport.
MMA and arsenate, not previously analysed, appear to be retained in the ovular vascular trace (OVT) region of the fresh grain, whether fed via the cut flag leaf or excised panicle stem (Fig. S6).
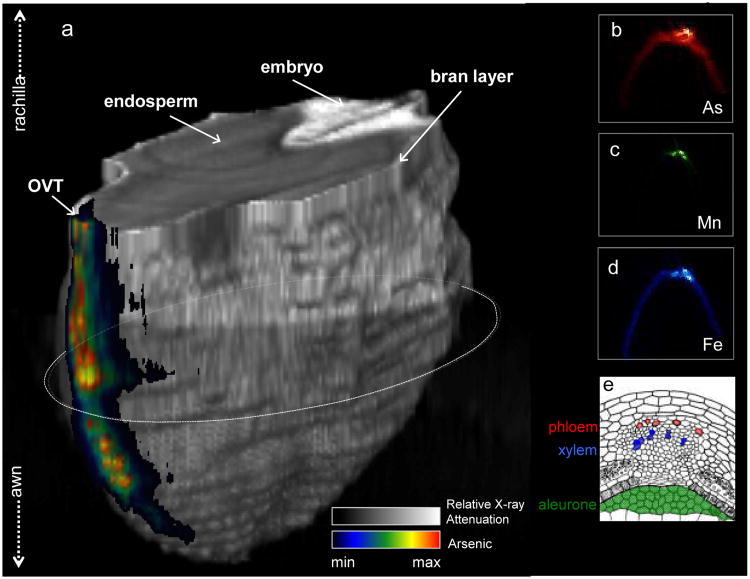
SXRF microtomography images in Fig. 2 show arsenic distribution, during unloading into the rice grain, for an excised panicle pulsed with 133 μM arsenite for 48 h, enhancing the information provided in Carey et al (2010). In the 3D image in Fig. 2a, arsenic can clearly be seen in the OVT, which runs down the ventral side of the grain (Krishnan and Dayanandan, 2003), apparently coincident with Fe and Mn, in agreement with previous studies (Meharg et al., 2008; Lombi et al., 2009; Carey et al., 2010). However, closer inspection (2b-d) reveals that Fe and Mn appear to be located on either side of the arsenic. The 2D tomograms in Fig. 2(b-d) demonstrate that arsenic, while remaining largely localised in the OVT, has begun to spread beyond it, indicating that arsenite is being unloaded into the nucellar epidermis that encircling the endosperm, but much less efficiently than DMA. With regard to Fe and Mn, Fe appears to be more efficiently unloaded into the nucellar epidermis than arsenite, while Mn is more strongly retained within the OVT.
Fig. 2.
(a) Three dimensional rendering of total x-ray absorption (greyscale colourbar) and arsenic (As) (rainbow colourbar) fluorescence of an immature rice grain pulsed with 133 μM arsenite. (b-d) Individual tomograms of As, manganese (Mn) and iron (Fe) in the ovular vascular trace. (e) Transverse section of ventral side of immature rice grain at rapid grain filling stage (c. 10 DAF) showing the vasculature of the ovular vascular trace, re-printed, with the kind permission of the authors, from Krishnan and Dayanandan (2003).
Ge phloem and xylem transport
Excised stem fed Ge Mean concentrations of the phloem marker, Rb, and the xylem marker, Sr, in the grain, husk and flag leaves for the stem-girdling experiment are reported in Fig. S7. These results demonstrate that the stem-girdling treatment was effective in interrupting phloem transport without damaging xylem transport and that germanic acid addition did not significantly affect phloem or xylem transport to the rice grain and husk.
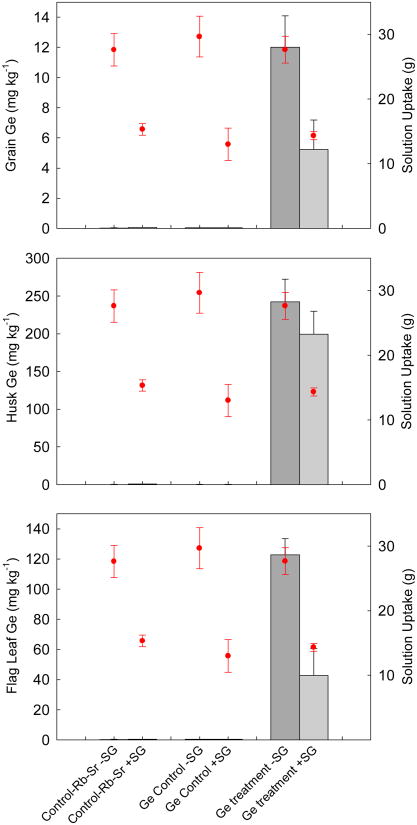
Silicic acid itself was originally utilized to examine silicic acid/arsenite competition in shoot to grain transport of inorganic arsenic, however, addition of silicic acid at 10-fold the molar concentration of As proved so phytotoxic to the excised panicles that the data could not be meaningfully interpreted. Therefore, germanic acid, a silicic acid analogue, was used. Mean concentrations of Ge in the flag leaf, grain, and husk for the ± stem-girdled panicles stem fed germanic acid are reported in Fig. 3. Since stem girdled panicles were generally smaller and took up less solution than nonstem girdled panicles, solution uptake has been included in Fig. 3. This demonstrates that any observed differences between stem girdled and nonstem girdled germanic acid treatments in grain, husk and flag leaf Ge concentration corresponds with differences in solution uptake. Two-way ANOVA showed that germanic acid treatment had a highly significant effect on mean flag leaf Ge concentrations (P<0.001), resulting in 100-fold more Ge than control panicles, and that there was a significant effect of stem girdling (P=0.037).
Fig. 3.
Mean total concentrations of germanium (Ge) in grain (a), husk (b) and flag leaf (c) for excised rice panicles subjected to a ± stem girdling treatment and hydroponically fed, over a 48 h period, nutrient solution amended with 133 μM germanic acid and 1 mM rubidium (Rb) and strontium (Sr); 1mM Rb and Sr only (germanic acid controls) or, for zero exposure controls, no amendment. Total solution uptake is also shown for each treatment (circles). Error bars represent the ± SE of three replicates.
Two-way ANOVA revealed that the germanic acid treatment had a highly significant effect on mean grain Ge concentrations (P<0.001), with germanic acid treated panicles being at least 100-fold greater than the controls. The stem girdling treatment did not result in significant differences in grain Ge, with no significant differences between stem girdled and nonstem girdled counterparts in all cases, although there was a significant interaction between the germanic acid and stem girdling treatments (P=0.011).
For husk samples, two-way ANOVA revealed that the germanic acid treatment had a highly significant effect on mean husk Ge, with germanic acid treatment yielding mean husk Ge levels 500-fold those of the control panicles, twice those of the germanic acid treated flag leaf and more than 15-fold those of the corresponding grain (P<0.001). However the stem girdling treatment did not have a significant affect on husk Ge and there was no significant interaction between the germanic acid and stem girdling treatments.
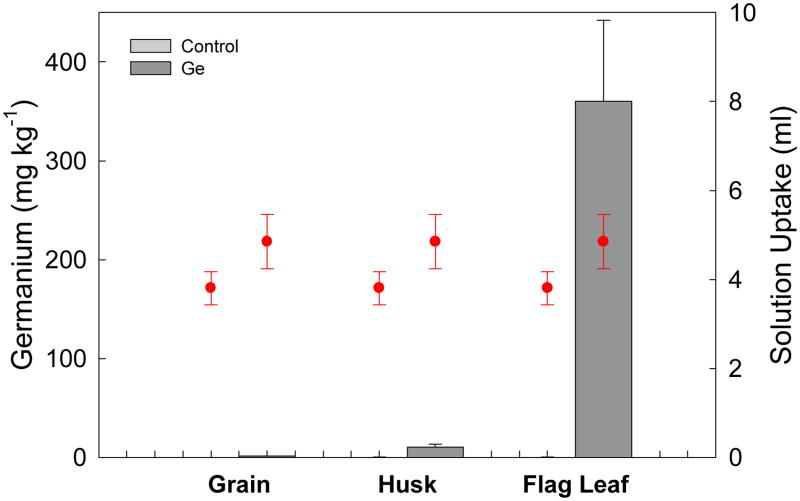
Leaf fed Ge For the leaf fed panicles, two-way ANOVA determined that exposure to 333 μM germanic acid via a cut flag leaf on the intact plant led to significantly higher levels of Ge in all examined plant parts compared with the germanic acid control (P<0.001) and that Ge levels differed significantly for plant parts, in the order flag leaf > husk> grain ((P<0.001) as shown in Fig. 4). For both the control and the germanic acid treated flag leaves, mean husk levels of Ge were 10-fold greater than those of the grain, although this is a markedly lower husk: grain ratio than demonstrated by excised stem fed panicles, where husk Ge levels were 20-fold those of the grain. This may be a result of Ge in leaf fed plants being largely restricted to phloem transport, which, as stem girdling demonstrates, is not the primary transport pathway for Ge into husk or grain. The retention of Ge in the flag leaf also indicates that Ge is not particularly phloem mobile.
Fig. 4.
Mean total germanium (Ge) concentrations in flag leaf, grain and husk, for rice panicles exposed to 333 μM germanic acid, and 1 mM rubidium (Rb) and strontium (Sr), through the cut flag leaf on intact plants for 7 d, with a fresh vial applied every 24 h. Total solution uptake is also shown for each treatment (circles). Error bars represent ± SE of three replicates. [Author, please note that the key will be deleted according to our journal style. As it is not possible to distinguish the colour of (supposedly?) pale grey bars, the key was not included in the legend. Please alter the legend if necessary.]
Germanic acid competition with inorganic As
Mean As concentrations in flag leaf, grain and husk for excised panicles treated with germanic acid and inorganic As are reported in Fig. 5. Flag leaf concentrations of Ge were below the limit of detection (LOD) for control treatments and were, therefore, excluded from statistical analysis. Two-way ANOVA demonstrated that pulsing excised panicles with arsenate led to significantly greater flag leaf As than treatment with arsenite (P=0.019), although at 1.5-fold, this difference was not as marked as that observed in grain and husk samples, while germanic acid addition had no significant effect. There was no interaction between As and germanic acid treatments.
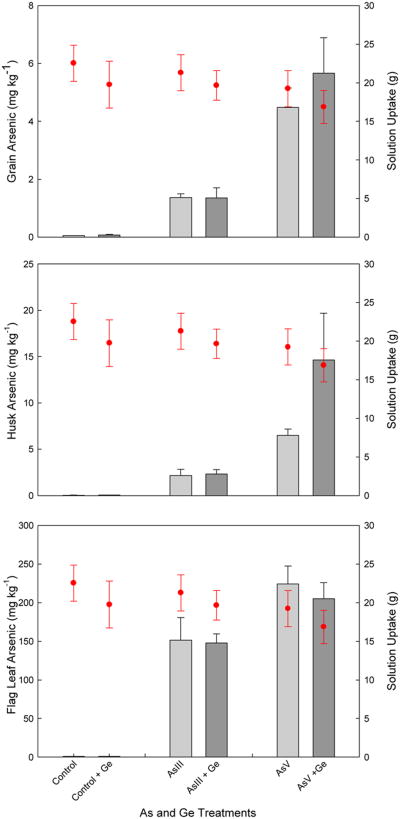
Fig. 5.
Mean total arsenic (As) concentrations rice grain (top), husk (middle) and flag leaf (bottom) for excised rice panicles hydroponically fed, over a 48 h period, nutrient solution amended with either 133 μM arsenite (AsIII) or arsenate (AsV) together with 1.33 mM germanic acid. Total solution uptake is shown for each treatment (circles). Error bars represent ± SE of three replicates.
Two-way ANOVA showed that As treatments had a significant effect on grain As levels (P<0.001), with arsenite and arsenate addition both yielding significantly higher grain As than the controls and arsenate leading to significantly higher (3-4 fold more) grain As than the arsenite treatment. Germanic acid, fed at 10-times the molar concentration of the As species, had no significant effect on grain As levels and there was no significant interaction between As and germanic acid treatments.
For the husk, two-way ANOVA showed that treatment with As had a highly significant effect on husk As levels (P<0.001) while germanic acid treatment did not lead to significant differences in husk As and there was no significant interaction between the As and germanic acid treatments. As with the grain, pairwise comparisons revealed that treatment with arsenate led to significantly greater (3 to 4-fold more) husk As than the arsenite treatment (P<0.001).
There were no significant differences in solution uptake (Fig. 5); two-way ANOVA confirmed that the As and germanic acid treatments did not lead to any significant differences in solution uptake and there was no significant interaction between treatments.
Discussion
Re-translocation of arsenic from shoot to grain
This study investigated the re-translocation of arsenic species from flag leaves to rice grain during grain filling, by feeding arsenic species directly into the panicle flag leaf of intact plants.
Re-translocation of the organic arsenic species, DMA and MMA, was extremely efficient, despite lower solution uptake compared with the inorganic treatments and controls. Flag leaves treated with DMA took up 25-30% less feeding solution than the inorganic As and control treatments, and MMA treated leaves took up 35-40% less feeding solution. This is consistent with their use as herbicides and defoliants (desiccants) (Marcus-Wyner and Rains, 1982; Mastradone and Woolson, 1983; Marin et al., 1993; Valette-Silver et al., 1999; Rosen and Liu, 2009). The dose response conducted for DMA and arsenite (Fig. S2) confirmed that DMA was efficiently transported to the grain, regardless of leaf dose, in contrast to arsenite which displayed no measureable re-translocation from flag leaf to grain. The relatively high mobility of DMA and MMA compared with inorganic arsenic agrees with previous studies (Raab et al., 2007; Li et al., 2009; Carey et al., 2010). Raab et al (2007) suggested this might be due to poorer thiol complexation limiting retardation during translocation. A recent study showed that DMA and MMA both remained stable and uncomplexed in the phloem and xylem (Ye et al., 2010).
Zheng et al (2010) reported that the DMA concentration in rice grain decreased during grain filling. The results presented here appear to contradict that study, as DMA was actively translocated into filling grain to reach relatively high concentrations. As DMA is so rapidly re-translocated from flag leaves to rice grain, DMA that has accumulated within the plant prior to panicle formation will swiftly partition into the panicle and ovary, during development, leading to a high proportion of DMA in the initial stages of panicle development and grain fill. As DMA is slowly taken up by rice roots (Abedin et al., 2002b), in contrast to inorganic arsenic, the amount of DMA taken up by the plant throughout the grain fill period will be comparatively low, and further diluted by the carbohydrate filling the grain. Oparka and Gates (1981) suggested that water entering the endosperm of developing grains, with the mass flow of assimilates, leaves the grain via the xylem of the pericarp bundle. It is possible that some DMA, being xylem mobile, does so too, although this would necessitate a corresponding increase in plant tissues elsewhere and Zheng et al (2010) did not detect this.
While MMA is similar to DMA in its rapid re-translocation from flag leaf to rice grain, SXRF microtomography demonstrates that MMA and DMA have very different degrees of mobility within the developing grain. DMA rapidly spreads throughout the outer layers and into the endosperm, whether arriving via direct stem transport or via re-mobilisation from flag leaves. MMA, conversely, is retained within the OVT region in a similar pattern to inorganic As species (Fig. S6).
With regard to inorganic As, arsenate was relatively poorly re-translocated to the rice grain, but proved more mobile than arsenite, which displayed no measureable re-mobilization from flag leaf to grain (Fig. 1). Arsenate was also 3-4 times more efficiently translocated into the rice grain than arsenite when fed through excised panicles at concentrations of 133μM (Fig. 5). Despite exhibiting a similar lack of mobility within the rice grain (Fig. S6), arsenate is clearly more mobile within the shoot than arsenite, probably due to arsenate's low affinity for thiol groups (Raab et al., 2007). However, arsenate is rapidly reduced to arsenite within the flag leaf and, in a hydroponic study, arsenite was the predominant species found in the xylem of rice plants fed arsenate (Su et al., 2010). Carey et al (2010) did not find any significant differences in shoot-to-grain transport of arsenite and arsenate when fed through excised panicles at concentrations of 13.3 μM. In that study, XANES speciation of developing rice grains demonstrated that, at 13.3 μM exposure, arsenite in the grain was oxidized to arsenate, while at 133 μM exposure, arsenite remained stable (Carey et al., 2010). The interpretation that arsenate is re-translocated from the flag leaf to the grain, albeit in small quantities, while arsenite is not, suggests that some of the initial arsenate is exported to grain, potentially through the phosphate pathways, before reduction takes place. Within the flag leaf, arsenite is likely detoxified through complexation with thiol-rich peptides, including phytochelatins and glutathione, and sequestered into leaf cell vacuoles (Bleeker et al., 2006; Raab et al., 2007; Zhao et al., 2009). Song et al (2010) recently identified phytochelatin transporters responsible for facilitating the chelation of arsenite prior to sequestration in cell vacuoles, in Arabidopsis thaliana. In this study, As speciation would not have distinguished between free arsenite and thiol-complexed arsenite. A number of studies have shown that translocation of As from shoot to rice grain becomes less efficient at higher shoot arsenic levels, indicating some threshold (Williams et al., 2007; Adomako et al., 2009; Lu et al., 2009; Norton et al., 2009). Norton et al (2009) reported that, for a range of cultivars, the percentage of DMA in rice grain increased with increasing total As while the percentage of arsenite decreased (Norton et al., 2009). This may be, in part, due to a greater proportion of arsenite being retained within leaf cell vacuoles. Zheng et al (2010) reported that the nodes of rice plants distribute 40% of any arsenic they receive to the closest leaf. The results presented here suggest that any arsenite distributed to the flag leaf will remain there, while a significant amount of any DMA transported to the flag leaf will be re-translocated to the grain. The rapid reduction of arsenate to arsenite within the flag leaf means that any arsenate accumulated within the rice plant during growth will have been reduced to arsenite and effectively immobilised. Therefore, arsenite and arsenate accumulating in the developing grain must arrive via direct transport through the stem during grain fill, while DMA (and MMA) will be delivered to the rice grain both from flag leaf stores and via the direct transport through the stem of any taken up during grain fill.
Spatial unloading of arsenic
The 3D and 2D images of grains from the leaf fed and excised stem agree with previous images of arsenic-fed rice grains (Carey et al., 2010). In addition, in Fig. 2, arsenite can clearly be seen in the ovular vascular trace, closely localized, with Fe and Mn apparently on each side of arsenite. Since phloem cells are situated on the outside of the xylem cells in the OVT (Krishnan and Dayanandan, 2003), it is likely that arsenite is localised in the phloem while Fe and Mn are present in both phloem and xylem, although the resolution is not high enough to definitively conclude this. Also, Fig. 2 demonstrates that a portion of the arsenic is being unloaded into the nucellar epidermis albeit much less efficiently than DMA. The concentration of inorganic As, particularly arsenite, within the bran layer of mature rice grain would suggest that arsenite does not move beyond the nucellar epidermis into the endosperm, as demonstrated for DMA (Meharg et al., 2008; Sun et al., 2008). Moore et al. (2010) showed that arsenic was concentrated both in hotspots in the OVT region and in the sub-aleurone layer that surrounds the endosperm for a rice grain containing c. 30% inorganic arsenic and 70% organic arsenic.
Ge phloem/xylem mobility
The highly efficient translocation of germanic acid to the Si accumulating tissues, particularly the husk, observed for excised panicles, agrees with the role of germanic acid as a silicic acid analogue. Stem girdling demonstrated that phloem transport is not important in germanic acid, and analogously, silicic acid translocation to the rice panicle. It may be that, as germanic acid is extremely mobile in xylem and that interruption of the percentage distributed to tissues via phloem is masked by the substantial xylem flow. In contrast to ‘leaf-locked’ arsenite and phloem-immobile Sr, there was some translocation of germanic acid from treated flag leaves to rice grain. This was limited and the markedly lower husk:grain ratio than that demonstrated by excised stem fed panicles indicated that this is not the primary pathway for germanic acid, and thus silicic acid, to the grain. As background Ge in tissues is low, even trace level translocation of germanic acid to grain can be detected, but it should be stressed that grain concentrations are 277-fold lower than leaf. It may be that silicic and germanic acids are phloem-mobile, but at the flag leaf node a high proportion, if not all, is transferred to the xylem, and, therefore, stem girdling at the base of the panicle (above the node) has no effect. Nodal vascular anastomoses in rice panicle nodes connect the enlarged vascular bundles to the surrounding diffuse vascular bundles and this intervascular connection is necessary for translocation to panicle tissues (Yamaji and Ma, 2009). Yamaji and Ma (2009) noted that intervascular transport via nodal vascular anastomoses does not sufficiently account for the rapid accumulation of Si in rice husks and demonstrated that Lsi6 transporters, in xylem transfer cells, mediate Si transport from enlarged vascular bundles in the panicle node to the diffuse vascular bundles that transport material to the panicle.
With regard to differential mobility of As species, organic As species are perhaps rapidly transferred via xylem transfer cells from enlarged vascular bundles to diffuse vascular bundles while arsenite, which is far less mobile in the xylem, is not.
Arsenite-germanic acid competition
Germanic acid has been shown to be an effective analogue of silicic acid in rice (Ma et al., 2002; Nikolic et al., 2007) and silicic acid transporters have been implicated in the transport of arsenite (and methylated arsenic species) into rice roots (Ma and Yamaji, 2008; Ma et al., 2008, Li et al., 2009). Germanic acid does not significantly reduce shoot to grain transport of arsenite, indicating that arsenite may not rely solely on silicic acid transporters for shoot-to-grain transport, or that competition is poor for these transporters, as may be the case for channels. In a study of field grown rice cultivars, Norton et al (2010) found a positive correlation between shoot As and shoot silicon but no correlation between shoot silicon and grain As levels. Ma et al (2008) showed that Lsi6 transporters are not involved in arsenite uptake into the roots of rice plants, although this may simply be due to low expression of Lsi6 in rice roots. Arsenite-silicic acid competition may be significant in rice roots, where there is strong control on uptake into the plant, but is perhaps less important in the rice shoot where transport is largely driven by mass flow. When Li et al (2009) found that silicic acid did not reduce root uptake of MMA and DMA, they suggested that aquaporins such as Lsi1 may allow rapid fluxes of solutes so competition will be less obvious (Li et al., 2009; Maure et al., 2008), this may also be the case for Lsi6 but further investigation is required.
In conclusion, this study demonstrates that DMA and MMA are extremely efficiently re-translocated, via the phloem, from the panicle flag leaf to the filling rice grain. In contrast to inorganic arsenic and MMA, DMA is extremely mobile within the grain, whether arriving via direct transport though the stem or re-translocation from flag leaves. Arsenate was relatively poorly re-translocated to the rice grain and was rapidly reduced to arsenite within the flag leaf. Leaf fed arsenite was not translocated to the rice grain, and was potentially sequestered into leaf cell vacuoles. Consequently, arsenite and arsenate accumulation in the developing grain must occur solely via direct transport through the stem during grain fill, while DMA will also be delivered to the rice grain from flag leaf stores. The results of this study also show that arsenite does not compete with germanic acid for direct transport to the rice grain through the stem, suggesting that arsenite does not rely solely on silicic acid transporters for shoot to grain translocation and, therefore, that shoot silicon status may not be important in rice grain As accumulation. Alternatively, arsenite-silicic acid competition is perhaps less obvious in the rice shoot where transport is largely driven by mass flow and/or that the rate of transport through channels is too rapid to allow competition to take place.
Supplementary Material
Fig. S1 Mean total concentrations of rubidium (Rb) and strontium (Sr) in flag leaf and grain, for rice panicles exposed to 333 μM of 1 of 5 arsenic treatments together with 1 mM Rb and Sr, or, in the case of the zero exposure controls, ultrapure, MilliQ water only.
Fig. S2 Dose response curves showing mean arsenic concentrations in grain and flag leaf for DMA treated panicles and for arsenite treated panicles under increasing levels of exposure.
Fig. S3 Mean total solution uptake for panicles treated with 0, 33, 133 and 333 μM arsenite or DMA. [Author, please note that there is no reference to this figure in the text. Please insert it.]
Fig. S4 Chromatograms showing arsenic speciation in fresh leaf extracts of arsenite and arsenate fed flag leaves together with a standard mix.
Fig. S5 Chromatograms showing arsenic speciation in leaf feeding vials of arsenite and arsenate.
Fig. S6 Fluorescence microtomography images showing distributions of arsenic for virtual cross sections of rice grain.
Fig. S7 Mean total concentrations of Rb and Sr in rice grain, husk and flag leaf for excised rice panicles subjected to a stem girdling treatment and hydroponically fed, over a 48 h period, nutrient solution.
Methods S1 Phloem transport of arsenic species from flag leaf to grain during grain filling. [Author, please insert a reference to Methods S1 in the text]
Acknowledgments
This work was supported by a Biotechnology and Biological Sciences Research Council Doctoral Training Grant and by grants from the National Institute of Environmental Health Sciences, Superfund Research Program (grant no. P42 ES007373-14) to TP and MLG. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH. Portions of this work were performed at GeoSoilEnviroCARS (Sector 13), Advanced Photon Source (APS), Argonne National Laboratory. GeoSoilEnviroCARS is supported by the National Science Foundation - Earth Sciences (EAR-0622171) and Department of Energy - Geosciences (DE-FG02-94ER14466). Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. A portion of this work was performed at Beamline X26A, National Synchrotron Light Source (NSLS), Brookhaven National Laboratory. X26A is supported by the Department of Energy (DOE) - Geosciences (DE-FG02-92ER14244 to The University of Chicago - CARS) and DOE - Office of Biological and Environmental Research, Environmental Remediation Sciences Div. (DE-FC09-96-SR18546 to the University of Kentucky). Use of the NSLS was supported by DOE under Contract No. DE-AC02-98CH10886. The U.S. Environmental Protection Agency, through its Office of Research and Development, funded and managed a portion of the research; it has not been subject to Agency review and, therefore, does not necessarily reflect the views of the Agency, no official product endorsement should be inferred. We would like to express our sincere gratitude to Norman Little and Dave Hadwen of the University of Aberdeen and to all the staff of Sector 13 at the APS for their support.
Footnotes
Supporting Information: Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office. [Author, please check if the short descriptions of Supporting Information files are ok]
References
- Abedin J, Cresser MS, Meharg AA, Feldmann J, Cotter-Howells J. Arsenic accumulation and metabolism in rice (Oryza sativa L.) Environmental Science and Technology. 2002a;36:962–968. doi: 10.1021/es0101678. [DOI] [PubMed] [Google Scholar]
- Abedin MJ, Feldmann J, Meharg AA. Uptake kinetics of arsenic species in rice plants. Plant Physiology. 2002b;128:1120–1128. doi: 10.1104/pp.010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adomako EE, Solaiman ARM, Williams PN, Deacon C, Rahman GKMM, Meharg AA. Enhanced transfer of arsenic to grain for Bangladesh grown rice compared to US and EU. Environment international. 2009;35:476–479. doi: 10.1016/j.envint.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Bleeker PM, Hakvoort HWJ, Bliek M, Souer E, Schat H. Enhanced arsenate reduction by a CDC25-like tyrosine phosphatase explains increased phytochelatin accumulation in arsenate-tolerant Holcus lanatus. Plant Journal. 2006;45:917–929. doi: 10.1111/j.1365-313X.2005.02651.x. [DOI] [PubMed] [Google Scholar]
- Carey AM, Scheckel KG, Lombi E, Newville M, Choi Y, Norton GJ, Charnock JM, Feldmann J, Price AH, Meharg AA. Grain unloading of arsenic species in rice (Oryza sativa L.) Plant Physiology. 2010;152:309–319. doi: 10.1104/pp.109.146126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Wu F, Dong J, Vincze E, Zhang G, Wang F, Huang Y, Wei K. Cadmium translocation and accumulation in developing barley grains. Planta. 2007;227:223–232. doi: 10.1007/s00425-007-0610-3. [DOI] [PubMed] [Google Scholar]
- Hansen HR, Raab A, Price AH, Duan G, Zhu Y, Norton GJ, Feldmann J, Meharg AA. Identification of tetramethylarsonium in rice grains with elevated arsenic content. Journal of Environmental Monitoring. 2011;13:32–34. doi: 10.1039/c0em00460j. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Dayanandan P. Structural and histochemical studies on grain-filling in the caryopsis of rice (Oryza sativa L.) Journal of Biosciences. 2003;28:455–469. doi: 10.1007/BF02705120. [DOI] [PubMed] [Google Scholar]
- Kuppelwieser H, Feller U. Transport of Rb and Sr to the ear in mature, excised shoots of wheat: Effects of temperature and stem length on Rb removal from the xylem. Plant and Soil. 1991;132:281–288. [Google Scholar]
- Li RY, Ago Y, Liu WJ, Mitani N, Feldmann J, McGrath SP, Ma JF, Zhao FJ. The rice aquaporin lsi1 mediates uptake of methylated arsenic species. Plant Physiology. 2009;150:2071–2080. doi: 10.1104/pp.109.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombi E, Scheckel KG, Pallon J, Carey AM, Zhu YG, Meharg AA. Speciation and distribution of arsenic and localization of nutrients in rice grains. New Phytologist. 2009;184:193–201. doi: 10.1111/j.1469-8137.2009.02912.x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Adomako EE, Solaiman ARM, Islam MR, Deacon C, Williams PN, Rahman GKMM, Meharg AA. Baseline soil variation is a major factor in arsenic accumulation in Bengal delta paddy rice. Environmental Science and Technology. 2009;43:1724–1729. doi: 10.1021/es802794w. [DOI] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Ichii M, Wu GF. A rice mutant defective in Si uptake. Plant Physiology. 2002;130:2111–2117. doi: 10.1104/pp.010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Yamaji N. Functions and transport of silicon in plants. Cellular and Molecular Life Sciences. 2008;65:3049–3057. doi: 10.1007/s00018-008-7580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proceedings of the National Academy of Science; USA. 2008. pp. 9931–9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Wyner L, Rains DW. Uptake, accumulation, and translocation of arsenical compounds by cotton. Journal of environmental quality. 1982;11:715–719. [Google Scholar]
- Marin AR, Masscheleyn PH, Patrick WH., Jr Soil redox-pH stability of arsenic species and its influence on arsenic uptake by rice. Plant and Soil. 1993;152:245–253. [Google Scholar]
- Marschner Horst. Mineral nutrition of higher plants. London; San Diego: Academic Press; 1995. [Google Scholar]
- Martin P. Stem xylem as a possible pathway for mineral retranslocation from senescing leaves to the ear in wheat. Australian Journal of Plant Physiology. 1982;9:197–207. [Google Scholar]
- Mastradone PJ, Woolson EA. Levels of arsenical species in cotton after field application of a cacodylic acid defoliant. Bulletin of environmental contamination and toxicology. 1983;31:216–221. doi: 10.1007/BF01607896. [DOI] [PubMed] [Google Scholar]
- Maure C, Verdoucq L, Luu DT, Santoni V. Plant aquaporins: Membrane channels with multiple integrated functions. Annual Review of Plant Biology. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- McNear DH, Jr, Peltier E, Everhart J, Chaney RL, Sutton S, Newville M, Rivers M, Sparks DL. Application of quantitative fluorescence and absorption-edge computed microtomography to image metal compartmentalization in Alyssum murale. Environmental Science and Technology. 2005;39:2210–2218. doi: 10.1021/es0492034. [DOI] [PubMed] [Google Scholar]
- Meharg AA, Lombi E, Williams PN, Scheckel KG, Feldmann J, Raab A, Zhu YG, Islam R. Speciation and localization of arsenic in white and brown rice grains. Environmental Science and Technology. 2008;42:1051–1057. doi: 10.1021/es702212p. [DOI] [PubMed] [Google Scholar]
- Meharg AA, Williams PN, Adomako E, Lawgali YY, Deacon C, Villada A, Cambell RCJ, Sun GX, Zhu YG, Feldmann J, Raab A, Zhao FJ, Islam R, Hossain S, Yanai J. Geographical variation in total and inorganic arsenic content of polished (white) rice. Environmental Science and Technology. 43:1612–1617. doi: 10.1021/es802612a. [DOI] [PubMed] [Google Scholar]
- Moore KL, Schroder M, Lombi E, Zhao FJ, McGrath SP, Hawkesford MJ, Shewry PR, Grovenor CRM. NanoSIMS analysis of arsenic and selenium in cereal grain. New Phytologist. 2010;185:434–445. doi: 10.1111/j.1469-8137.2009.03071.x. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Nikolic N, Liang Y, Kirkby EA, Römheld V. Germanium-68 as an adequate tracer for silicon transport in plants. Characterization of silicon uptake in different crop species. Plant Physiology. 2007;143:495–503. doi: 10.1104/pp.106.090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton GJ, Islam MR, Deacon C, Zhao FJ, Stroud JL, Mcgrath SP, Islam S, Jahiruddin M, Feldmann J, Price AH, Meharg AA. Identification of low inorganic and total grain arsenic rice cultivars from Bangladesh. Environmental Science and Technology. 2009;43:6070–6075. doi: 10.1021/es901121j. [DOI] [PubMed] [Google Scholar]
- Norton GJ, Islam MR, Duan G, Lei M, Zhu YG, Deacon CM, Moran AC, Islam S, Zhao FJ, Stroud JL, McGrath SP, Feldmann J, Price AH, Meharg AA. Arsenic shoot-grain relationships in field grown rice cultivars. Environmental Science and Technology. 2010;44:1471–1477. doi: 10.1021/es902992d. [DOI] [PubMed] [Google Scholar]
- Raab A, Williams PN, Meharg AA, Feldmann J. Uptake and translocation of inorganic and methylated arsenic species by plants. Environmental Chemistry. 2007;4:197–203. [Google Scholar]
- Rosen BP, Liu Z. Transport pathways for arsenic and selenium: A minireview. Environment international. 2009;35:512–515. doi: 10.1016/j.envint.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WY, Park J, Mendoza-Cózatl DG, Suter-Grotemeyer M, Shima D, Hörtensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, Schroeder JI, Lee Y, Martinoia E. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21187–21192. doi: 10.1073/pnas.1013964107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YH, McGrath SP, Zhao FJ. Rice is more efficient in arsenite uptake and translocation than wheat and barley. Plant and Soil. 2010;328:27–34. [Google Scholar]
- Sun GX, Williams PN, Carey AM, Zhu YG, Deacon C, Raab A, Feldmann J, Islam RM, Meharg AA. Inorganic arsenic in rice bran and its products are an order of magnitude higher than in bulk grain. Environmental Science and Technology. 2008;42:7542–7546. doi: 10.1021/es801238p. [DOI] [PubMed] [Google Scholar]
- Sun GX, Williams PN, Zhu YG, Deacon C, Carey AM, Raab A, Feldmann J, Meharg AA. Survey of arsenic and its speciation in rice products such as breakfast cereals, rice crackers and Japanese rice condiments. Environment international. 2009;35:473–475. doi: 10.1016/j.envint.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Valette-Silver NJ, Riedel GF, Crecelius EA, Windom H, Smith RG, Dolvin SS. Elevated arsenic concentrations in bivalves from the southeast coasts of the USA. Marine environmental research. 1999;48:311–333. [Google Scholar]
- Williams PN, Price AH, Raab A, Hossain SA, Feldmann J, Meharg AA. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environmental Science and Technology. 2005;39:5531–5540. doi: 10.1021/es0502324. [DOI] [PubMed] [Google Scholar]
- Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environmental Science and Technology. 2007;41:6854–6859. doi: 10.1021/es070627i. [DOI] [PubMed] [Google Scholar]
- Xu XY, McGrath SP, Meharg AA, Zhao FJ. Growing rice aerobically markedly decreases arsenic accumulation. Environmental Science and Technology. 2008;42:5574–5579. doi: 10.1021/es800324u. [DOI] [PubMed] [Google Scholar]
- Yamaji N, Ma JF. A transporter at the node responsible for intervascular transfer of silicon in rice. Plant Cell. 2009;21:2878–2883. doi: 10.1105/tpc.109.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye WL, Wood BA, Stroud JL, Andralojc PJ, Raab A, McGrath SP, Feldmann J, Zhao FJ. Arsenic speciation in phloem and xylem exudates of castor bean. Plant Physiology. 2010;154:1505–1513. doi: 10.1104/pp.110.163261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao FJ, Ma JF, Meharg AA, McGrath SP. Arsenic uptake and metabolism in plants. New Phytologist. 2009;181:777–794. doi: 10.1111/j.1469-8137.2008.02716.x. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, McGrath SP, Meharg AA. Arsenic as a Food Chain Contaminant: Mechanisms of Plant Uptake and Metabolism and Mitigation Strategies. Annual Review of Plant Biology. 2010;61:535–559. doi: 10.1146/annurev-arplant-042809-112152. [DOI] [PubMed] [Google Scholar]
- Zheng MZ, Cai C, Hu Y, Sun GX, Williams PN, Cui HJ, Li G, Zhao FJ, Zhu YG. Spatial distribution of arsenic and temporal variation of its concentration in rice. New Phytologist. 2011;189:200–209. doi: 10.1111/j.1469-8137.2010.03456.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Mean total concentrations of rubidium (Rb) and strontium (Sr) in flag leaf and grain, for rice panicles exposed to 333 μM of 1 of 5 arsenic treatments together with 1 mM Rb and Sr, or, in the case of the zero exposure controls, ultrapure, MilliQ water only.
Fig. S2 Dose response curves showing mean arsenic concentrations in grain and flag leaf for DMA treated panicles and for arsenite treated panicles under increasing levels of exposure.
Fig. S3 Mean total solution uptake for panicles treated with 0, 33, 133 and 333 μM arsenite or DMA. [Author, please note that there is no reference to this figure in the text. Please insert it.]
Fig. S4 Chromatograms showing arsenic speciation in fresh leaf extracts of arsenite and arsenate fed flag leaves together with a standard mix.
Fig. S5 Chromatograms showing arsenic speciation in leaf feeding vials of arsenite and arsenate.
Fig. S6 Fluorescence microtomography images showing distributions of arsenic for virtual cross sections of rice grain.
Fig. S7 Mean total concentrations of Rb and Sr in rice grain, husk and flag leaf for excised rice panicles subjected to a stem girdling treatment and hydroponically fed, over a 48 h period, nutrient solution.
Methods S1 Phloem transport of arsenic species from flag leaf to grain during grain filling. [Author, please insert a reference to Methods S1 in the text]