Abstract
Background
Lesch-Nyhan disease (LND) is a rare, X-linked, neurodevelopmental metabolic disorder that results from a near-complete lack of hypoxanthine phosphoribosyl-transferase enzyme activity. LND is characterized by hyperuricemia, motor neurological abnormalities, recurrent self-injury, and cognitive impairment, but its neural substrates remain poorly understood.
Methods
In this cross-sectional study, we measured gray matter abnormalities in 21 persons with LND, 17 with an attenuated variant of the phenotype (LNV), and 33 healthy controls using voxel-based morphometry. We conducted an analysis of covariance to identify group differences in regional gray matter volume (GMV), followed by six pair-wise post-hoc group comparisons.
Findings
Patients with LND showed 20% smaller intracranial volumes (17% gray and 26% white matter) than healthy adults. The largest differences were found in basal ganglia, frontotemporal, and limbic regions, with sparing of parieto-occipital regions. The gray matter volumes of LNV participants invariably fell between those of patients with classical LND and healthy controls. Compared to healthy adults, patients with LND showed additional GMV reductions in the temporal lobe and left lateralized structures, and patients with LNV showed additional reductions in lingual and precuneus regions with sparing of right frontal and temporal regions. LND participants showed reductions in the ventral striatum and prefrontal areas relative to LNV.
Interpretation
This study of brain morphology reveals regional abnormalities associated with known neurological and behavioral deficits in persons with LND. It also revealed that patients with LNV show milder gray matter abnormalities in many of the same brain regions and preservation of GMV in other regions which could provide important clues to the neural substrates of differences between thephenotypes.
INTRODUCTION
Lesch-Nyhan disease (LND) is a rare, genetic disorder caused by mutations affecting the gene encoding the purine salvage enzyme hypoxanthine-guanine phosphoribosyltransferase (HPRT).1 Residual HPRT activity almost always is less than 1.5% of normalin classical LND, which manifests with hyperuricemia, dystonia, cognitive dysfunction, self-injury, and disturbing interpersonal behaviors.2–5 Mutations that permit activity greater than 1.5% of normal typically yield variant (LNV) phenotypesin which motor and cognitive difficulties vary with enzyme function, and behavioral abnormalities are attenuated or absent.6
Brain regions responsible for the neurological and behavioral abnormalities remain unclear. Previous studies focused on the basal ganglia(BG) because cardinal features of LND implicate dysfunction of the BG and its circuits.7 Motor abnormalities have been linked with a circuit involving the putamen, thalamus, and motor cortex. Cognitive deficits have been associated with a circuit involving the caudate, thalamus, and dorsolateral prefrontal cortex. Unusual behaviors may be related to circuits involving the ventral striatum, thalamus, and limbic regions. Overt structural brain abnormalities typically are not seen on routine clinical neuroimaging. Only one quantitative MRI study of LND has been reported, which found reductions of select BG volumes.8 PET and autopsy studies of LND brains have found a reduction indopamine and related measures.7, 9 These findings contribute to current views that LND involves a developmental defect of BG dopamine pathways. Other brain regions have received less attention but warrant further investigation due to the complex phenotype of the disease. We used voxel-based morphometry to conduct whole brain analyses of regional volumes across the HPRT spectrum to determine affected brain regions, and regions discriminating LND from LNV.
METHOD
Participants
Twenty males and one female with classic LND, 17 males with LNV, and 33 male healthy controls (HC) participated in this study. The clinical diagnosis of LND was based on the presence of hyperuricemia, characteristic motor neurological abnormalities, a history of self-injurious behavior, and cognitive impairment. Diagnoses were confirmed by fibroblast assays showing residual HPRT enzyme activity levels ≤1.5% of normal or a mutation that predicted null enzyme activity. All LNV participants hadhyperuricemia, similar (but generally milder) motor abnormalities, and cognitive dysfunction, but no reported history of self-injury. As in our previous work ,10 the diagnosis of LNV was based on documentation of hyperuricemia and the absence of self-injury. The clinical diagnosis was supported by evidence of reduced HPRT enzyme activity, a mutation in the HPRT1 gene, or both in most cases. Patients were recruited through clinics, physician referral, the Lesch-Nyhan Disease Patient Registry, and the Matheny School and Hospital in Peapack, New Jersey. Healthy controls were recruited from the Baltimore metropolitan area and had no reported history of substance abuse, severe mental illness or neurological disorder.
Procedure
Each LND participant gave written informed consent to participate if competent to do so, or oral informed assent if not (in which case a legal guardian gave written informed consent). All LNV and HC participants gave written informed consent to participate, as did each informant who rated participants. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Johns Hopkins Medicine and Emory University Institutional Review Boards.
In addition to a brain magnetic resonance imaging (MRI) scan, each participant underwent neurological examination by a neurologist (H.A.J.) who specializes in movement disorders and LND, and cognitive testing supervised by a neuropsychologist (D.J.S.) with experience in LND. One or more informants (typically a family member or caregiver) rated each patient’s behavior, adaptive functioning, and personality. MRI: Each participant received a three-dimensional, T1-weighted brain scan. For scans conducted before 2009, we used a 1.5 Tesla GE Genesis Signa machine (TR=35ms, TE=2ms, FOV=256mm, flip angle 45°, voxel size=1.0×1.0×1.5mm). For those obtained in 2009 or later we used a Siemens 3T Trio (TR=2300ms, TE=2.9ms, FOV=256mm, flip angle=9°, voxel size=1.0×1.0×1.2mm), which replaced the GE machine. All LND and some LNV patients were administered alprazolam prior to the MRI (to limit movement in the scanner), scanned while drowsy or asleep, and then monitored in a waiting area until they awoke.
Imaging and Statistical Analyses
Voxel-based morphometric (VBM) analyses were performed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) within SPM8 (Wellcome Department of Imaging Neuroscience Group, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Because the patients’ intracranial volumes were smaller than those of healthy controls, the brain images of both patient groups were adjusted for VBM analyses. Specifically, the T1-weighted images were manually aligned to the anterior commissure posterior commissure line prior to automated segmentation and modulation within the VBM8 toolbox. This manual step was conducted in response to concern that their smaller brains would prevent the accurate automated realignment of LND and LNV images. Thereafter, segmentation and normalization were completed following the optimized protocol. Jacobian modulation was applied to the volumes in order to account for differences in relative brain size because entering TICV into the statistical model would confound the results. The resulting GMVs were smoothed with a 6mm isotropic Gaussian kernel and compared using an ANCOVA in SPM8. In order to account for potential hardware effects, we included the scanner as a covariate and controlled for GMV variations due to age. Additionally, we carried out a contrast modeling GMV effects due to scanner type in each group individually and in the principle analysis comparing LND to HC to test for an interaction between scanner type and diagnosis. Roughly equal percentages of the participants in each diagnostic group were scanned on each machine. That is, 13 patients with LND, 10 with LNV, and 18 HC were scanned on the 1.5T machine, and 8 patients with LND, 7 with LNV, and 15 HC were scanned on the 3T machine.
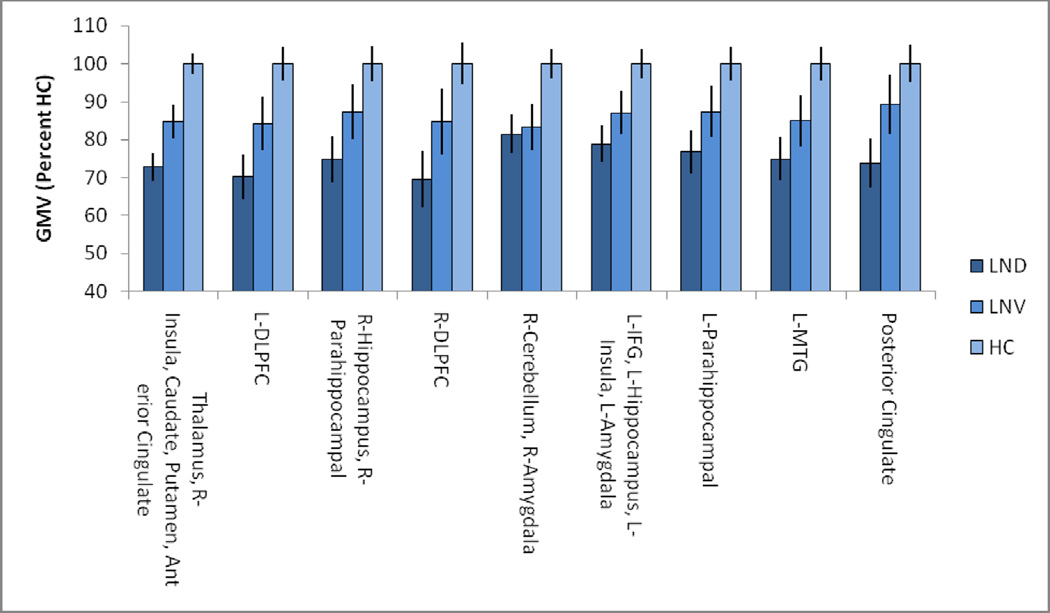
Our primary analysis identified group differences in regional GMV by ANCOVA using a clusterlevel family-wise error correction of p<.01 and a cluster-forming threshold of p<.0005.Masks of each cluster that passed our threshold criteria for the F contrast were created using xjview (http://www.alivelearn.net/xjview). The GMVs for each identified cluster were extracted and presented for qualitative comparison in Figure 2. Six pairwise post-hoc t-tests were conducted within SPM8 to identify spatially distinct regions of GMV that distinguished each pair of participant groups. For these analyses we used slightly more liberal significance levels(family-wise error correction of p<.05 and cluster-forming threshold of p<.001).To assess volumetric associations of dystonia we created a mask of the basal ganglia using the WFU pickatlas,11 and correlated the extracted volumes with BFM dystonia ratings as well as total intracranial volume controlling for age.
Figure 2.
Gray matter volumes in clusters significant for the F-contrast across all three groups.
Role of the funding source
The study sponsors had no role in study design, collection, analysis, or interpretation of data, preparation of the report, or decision to submit the paper for publication.
RESULTS
As shown in Table 1, the three groups did not differ in age or sex. Nor did the LNV and HC groups differ in years of schooling. Compared to healthy controls, participants with LNV and LND showed stepwise decreases in IQ, dystonia severity ratings, total intracranial volume (TICV), total brain volume (TBV), GMV, and white matter volume (WMV). Post-hoc analyses showed that the two patient groups also differed from each other in IQ, dystonia severity, and WMV. Compared to healthy adults, patients with LND showed a mean TICV reduction of 20%. Patients with LNV showed a reduction of 14%.Patients with LND showed larger reductions of WMV than GMV (26.2% vs. 17%). Patients with LNV showed slightly larger reductions of GMV than WMV (15.6% vs. 14.3%) No group differences were found for the ratio of brain-to-intracranial volume (TBV/TICV). Age correlated inversely with TBV/TICV ratio among healthy controls (r = −0.55; p=0.001) and patients (r = −0.57; p=0.001).
Table 1.
Demographic characteristics, dystonia severity, IQ and brain volumes (in cm3) by group1
| Characteristic | LND | LNV | HC | Statistic | p |
|---|---|---|---|---|---|
| Age, years | 25.0 ± 9.2 | 35.2 ± 16.8 | 28.9 ± 12.9 | F (2,68) = 3.01 | 0.06 |
| Sex, male/female (n) | 20/1 | 17/0 | 33/0 | Χ2 (2,68) = 2.42 | 0.30 |
| Education, years | NA | 11.1 ± 3.4a | 14.0 ± 2.2b | t (34) = 2.41 | 0.05 |
| Race, white/black (n) | 19/2 | 15/1 | 28/6 | Χ2 (2,68) = 1.80 | 0.41 |
| BFM dystonia rating2 | 73.3 ± 13.4a | 23.8 ± 24.5b | 1.4 ± 1.5c | F (2,19) = 48.0 | 0.001 |
| KBIT-2 Composite IQ3 | 54.3 ± 9.4a | 84.7 ± 38.4b | 112.7 ± 19.8c | F (2,43) = 26.0 | 0.001 |
| Intracranial volume4 | 1197 ± 178a | 1286 ± 106a | 1495± 130b | F (2,68) = 31.1 | 0.001 |
| Total brain volume5 | 1003 ±155a | 1077 ±107a | 1267 ±120b | F (2,68)= 29.9 | 0.001 |
| Gray matter volume | 601 ± 106a | 611 ± 78a | 724 ± 71b | F (2,68) = 17.4 | 0.001 |
| White matter volume | 402 ± 63a | 466 ± 40b | 543 ± 68c | F (2,68) = 34.7 | 0.001 |
| CSF volume | 193 ± 49a | 208 ± 32 | 227 ± 32b | F (2,68) = 5.25 | 0.008 |
| TBV/TICV ratio | 0.84 ± 0.03 | 0.84 ± 0.03 | 0.85 ± 0.03 | F (2,68) = 1.29 | 0.282 |
Except for sex and race, all values expressed as means ± standard deviations. Because patients with LND received special education services that differed greatly in nature and intensity, it would be misleading to list the number of years of schooling they completed. The presence of different subscripts denote groups that differed (p< 0.05) based on post-hoc comparisons, identical subscripts indicate lack of a significant difference.
LND = classic Lesch-Nyhan disease; LNV = Lesch-Nyhan variant; HC = healthy control.
Burke-Fahn-Marsden Dystonia Rating Scale31
Kaufman Brief Intelligence Test, 2nd Edition32
Sum of gray matter, white matter and CSF volumes (TICV)
Sum of gray matter and white mattervolumes (TBV)
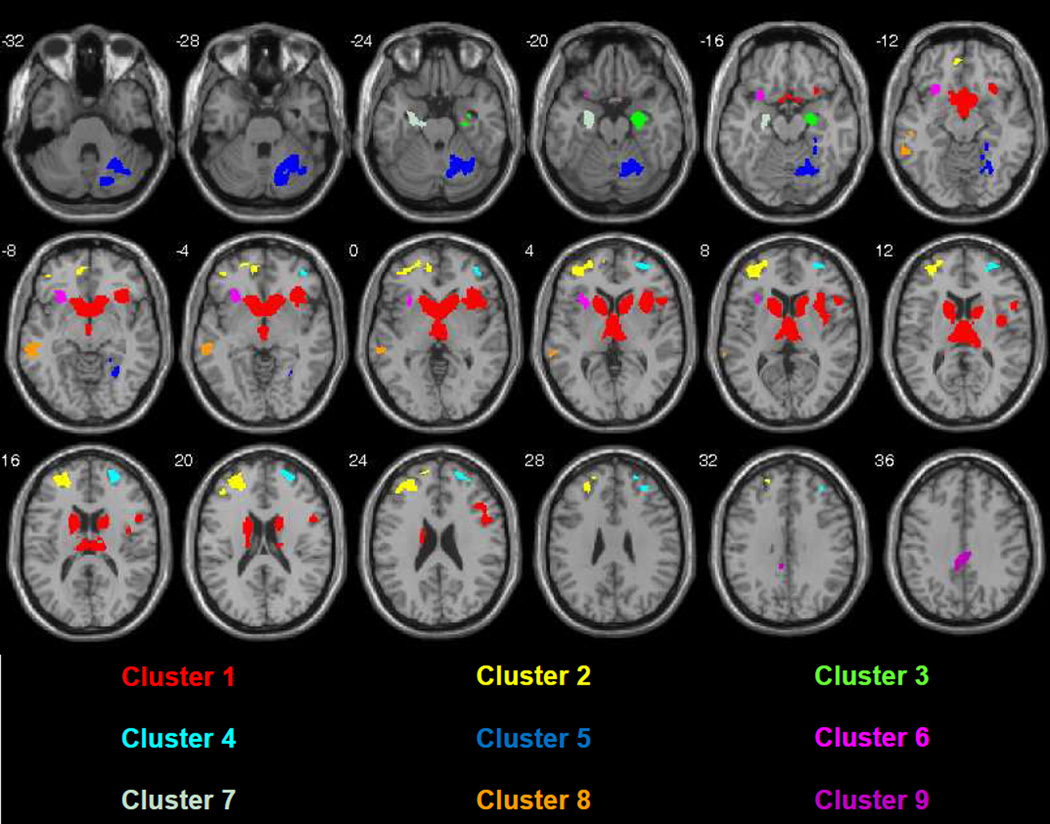
To identify brain regions contributing to TICV reductions, we next conducted a whole brain VBM analysis of GMV using a three-group ANCOVA, controlling for age and scanner as nuisance variables. To control for false positives, we used a family-wise cluster-level error correction of p<0.01. This analysis revealed diagnostic group differences in 9 clusters of cortical and subcortical GMV.Table 2 shows the structures for which group differences in GMV were found, with labels from the Wake Forest University (WFU) Pick Atlas.11Results of the ANCOVA also are shown in Figure 1. Gray matter volume abnormalities were most prominent in the caudate, thalamus and anterior putamen, but also were found in limbic, temporal and frontal regions. The occipital and parietal lobes were nearly completely spared, and only one region of abnormal GMV was found in the cerebellum.
Table 2.
Regions in which gray matter volumes of persons with LND or LNV were significantly smaller than those of healthy controls based on ANCOVA contrast
| Hemisphere & Structure | # voxels | Cluster | MNI Coordinates |
|---|---|---|---|
| Frontal Lobe | |||
| Left DLPFC | 1530 | 22 | −27,54,1.5 |
| Right DLPFC | 810 | 41 | 28.5,52.5,18 |
| Left inferior frontal gyrus | 340 | 62 | −39,42,7.5 |
| Left orbital frontal cortex | 306 | 21 | −24,55.5, −1.5 |
| Right orbital frontal cortex | 343 | 41 | 40.5,48, −3 |
| Temporal Lobe | |||
| Left middle temporal gyrus | 573 | 82 | −60, −31.5, −6 |
| Right parahippocampal gyrus | 364 | 32 | 28.5, −31.5, −16.5 |
| Left parahippocampal gyrus | 369 | 72 | −22.5, −21, −19.5 |
| Right hippocampus | 79 | 31 | 27, −10.5, −18 |
| Left hippocampus | 56 | 61 | −27, −7.5, −19.5 |
| Right Amygdala | 63 | 51 | 27, −4.5, −19.5 |
| Left Amygdala | 79 | 61 | −27.5, −3.5, −19.5 |
| Sub-Lobar | |||
| Bilateral thalamus | 1630 | 12 | 10.5, −12,9 and −7.5, −10.5,10.5 |
| Left Insula | 441 | 61 | −31.5,21,6 |
| Right Insula | 1149 | 12 | 36,24,3 |
| Bilateral caudate | 2957 | 12 | 13.5,19.5, −3 and −10.5,19.5, −4.5 |
| Bilateral Putamen | 559 | 12 | 19.5,9,9 and −18,12,9 |
| Cingulate gyrus | 481 | 91 | −6, −37.5,36 |
| Anterior cingulate | 555 | 12 | 0.5,9, −9.5 |
| Parietal Lobe | |||
| None | |||
| Occipital Lobe | |||
| None | |||
| Cerebellum | |||
| Right cerebellum | 2043 | 52 | 22.5, −60, −30 |
Superscripts denote differentiation of cluster volume based on qualitative comparison of post-hoc tests.
Volumetric reductions only in LND relative to healthy controls,
Volumetric reductions in both LND and LNV relative to healthy controls.
Figure 1.
Axial slice view of the results of ANCOVA. Note that the greatest abnormalities involve the basal ganglia, frontal, temporal and limbic regions with near complete sparing of the occipital and parietal lobes. (Neurological Convention, p<.0005, FWE <.01, single subject T1 template image)
To verify that our results were not significantly affected by machine-related differences, we modeled the effects of which scanner was used, basing the analysis on a liberal (uncorrected) threshold of p<0.001 to identify any machine-related differences in GMV. As shown in the supplementary content (Figure S1), this revealed negligible effects. No areas of GMV abnormality remained significant after applying the correction for false positives used to examine our main effects of interest. As further evidence that the results were not affected by differences in the scanner used, we compared regional GMV differences between participant groups separately for each scanner. Despite the fact that different participants were scanned on each machine, these analyses revealed similar regions of GMV abnormality in patients with LND compared to healthy adults. These results are shown in Figure S2 of the supplementary content.
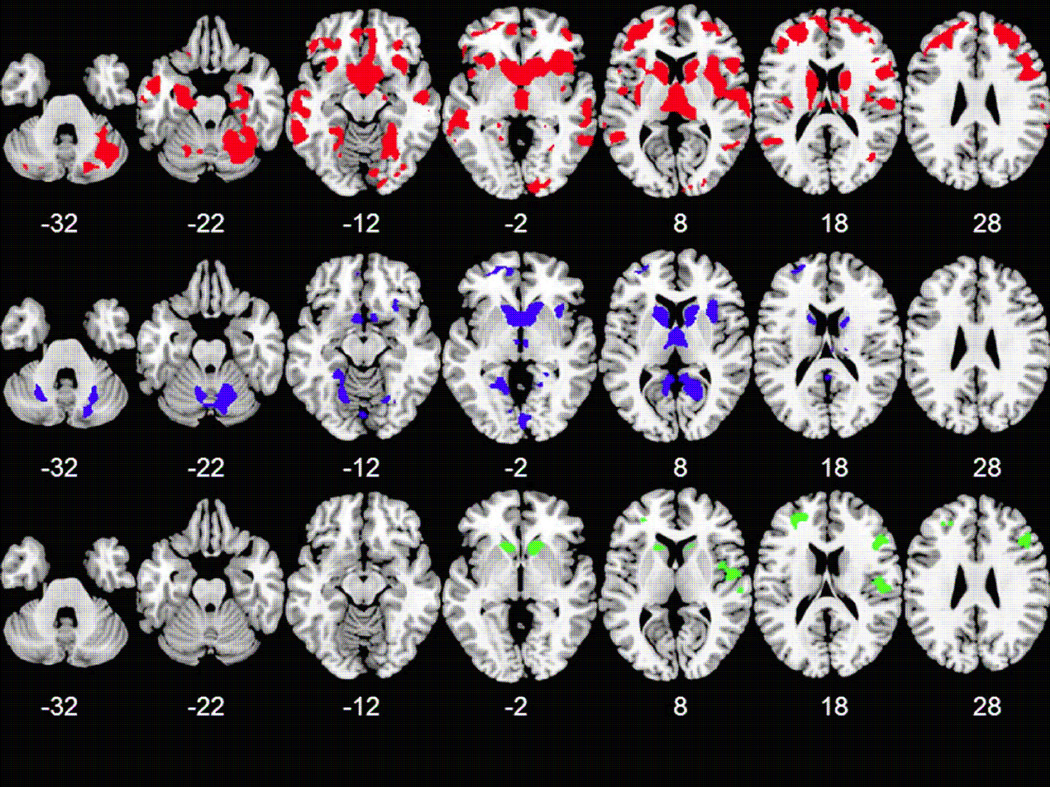
The results of pair wise comparisons in SPM largely echoed the findings of the ANCOVA and qualitative comparisons shown in Figure 2. Compared to healthy controls, patients with LND showed additional volumetric reductions in lateral portions of the temporal lobe bilaterally, left fusiform gyrus, left cerebellum and left insula. Compared to healthy controls, patients with LNV showed additional volumetric reductions in the lingual gyrus bilaterally and the precuneus bilaterally with relative sparing of the right frontal and temporal regions and the cingulate. Relative to patients with LNV, those with LND showed reduced GMV in the ventral striatum, parietal operculum and prefrontal areas. Contrasts of LND > HC, LNV > HC and LND >LNV showed no significant results passing our statistical thresholds. Results are shown in figure 3.
Figure 3.
Axial slice view gray matter volume abnormalities identified by post-hoc comparisons, number indicates z value. (red HC > LND; blue HC > LNV; green LNV > LND) (Neurological Convention, p<.001, FWE <.05, skull stripped average template)
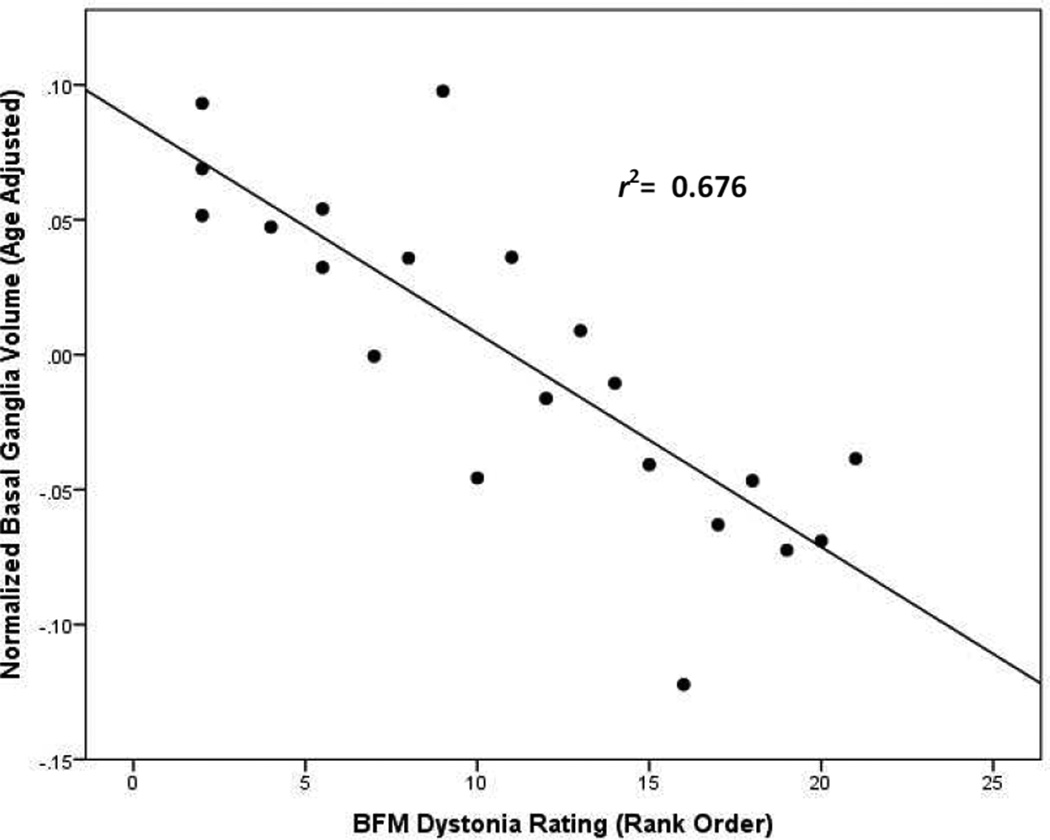
Finally, we conducted a series of partial correlations (controlling for age) between BFM dystonia ratings and selected brain measures in the combined patient groups. Dystonia severity showed the strongest partial correlation with BG volume (r = −0.82; p< 0.001), followed by TICV (r = −0.76; p< 0.001) and total GMV (r = −0.69; p< 0.001). A graph depicting the relationship between BFM dystonia ratings and the BG volumes of patients with LND and LNV is shown in Figure 4. A similar graph of the relationship between BFM ratings and TICV is provided in the supplementary content (Figure S3).
Figure 4.
Scatterplot showing partial correlation (controlling for age) of Burke-Fahn-Marsden (BFM) dystonia severity ratings and basal ganglia volumes for patients with LND and LNV.
DISCUSSION
These studies reveal significant but regionally selective brain volume abnormalities across the spectrum of HPRT deficiency. Compared to healthy controls, patients with the classical complete syndrome of LND showed 20% smaller intracranial volumes, with 17% less GMV, and 26% less WMV. Corresponding abnormalities in the more mildly affected patients with LNV were less severe, with 14% smaller intracranial volumes, 15% less GMV and 14% less WMV. Both patient groups showed GMV abnormalities in similar brain regions, but the abnormalities of those with LNV were milder than those with LND in every affected region identified by the ANCOVA. This both strengthens the plausibility of the findings, and might account for some of the differences in the clinical phenotypes observed in the two patient groups.
The brain volume abnormalities were not associated with enlarged ventricles or sulci, or with decreased ratios of brain-to-intracranial volume. Therefore, the smaller brain volumes likely reflect a developmental defect, rather than a degenerative one. The correlations between age and TBV/TICV ratio were nearly identical for patients (r = −0.57) and healthy controls (r = −0.55). Thus, patients with LND/LNV do not show evidence of accelerated brain aging.
Correspondence with prior studies
Considering the magnitude of brain volume abnormalities in LND, it is surprising that they have not been described previously. Among 11 patients (9 LND, 2 LNV) in which the results of head CT scans have been reported, two investigators noted cerebral atrophy.12, 13 A third described enlarged Sylvian fissures,14 and the rest were described as normal.15–20 Clinical brain MRI studies of 25 patients (23 LND, 2 LNV) also have been reported. Twenty-two were described as normal.7, 9, 17, 21 The other three showed mild atrophy, sulcal prominence, or decreased T2 signal in the BG.8, 22 The only quantitative MRI study published to date compared manually traced regions of interest in 7 patients with LND and found their total cerebral volumes to be 17% smaller than age-matched healthy controls.8 The fact that the relatively large brain abnormalities found here have been largely overlooked likely reflects the fact that variation among normal individuals is large, and that clinical radiologists rarely interpret reduced brain size in the absence of cerebral atrophy.
The brain volume abnormalities in LND and LNV clearly show that the consequences of HPRT deficiency are not restricted to the nigrostriatal dopamine pathways, which are emphasized in many prior studies.23–25 (See research in context panel) On the other hand, neither is the brain globally affected, as the occipital and parietal cortices are essentially spared. The most prominent volume abnormalities were found in the caudate and putamen, followed by their downstream targets: the thalamus, cerebral cortex and limbic brain. Thus, GMV was markedly reduced in large portions of the dorsolateral prefrontal cortex (DLPFC), orbital frontal cortex (OFC), inferior frontal gyrus (IFG), and portions of the temporal lobes. Decreased GMV also was observed in the amygdala, insula, hippocampus, and parahippocampal region. All of these cortical and subcortical regions are intimately connected with the basal ganglia and serve different functions.23
Relevance of imaging findings to the clinical phenotype
Most features of the clinical phenotype show graded severity across the spectrum of HPRT deficiency. For example, we previously found that the IQ scores of patients with both LND (mean = 59) and LNV (mean = 72) were lower than those of healthy controls (mean = 108), but not significantly different from each other.2 However, patients with LNV scored between patients with LND and healthy controls in virtually every domain of cognitive function. The motor disorder also shows a graded spectrum where patients with LND are more severely affected than those with LNV.5, 10 These findings argue that analyzing some aspects of the phenotype as continuous rather than categorical variables might prove more useful for elucidating pathophysiological relationships for some aspects of the disease. On the other hand, the defining clinical feature that distinguishes LND from LNV is the presence of self-injurious behavior. The attenuated variants may show some other behavior problems, such as inattentiveness and impulsivity, but self-injury is absent.3
In both LND and LNV, abnormalities of the circuit involving the caudate and DLPFC may account for cognitive impairments involving attention, working memory, and executive functions.2 Similarly, abnormalities of the left temporal regions may contribute to impairments of verbal learning/memory.2 The temporal lobes also contribute to the joining of perceptions with emotional responses.26 Almost by definition, our patient population shows some derangement of these processes.
The movement disorder, which is dominated by dystonia in both LND and LNV, likely is due to abnormalities of the BG circuit involving the putamen and motor cortex.23 In fact, severity scores for dystonia correlated more highly (r = −0.82) with basal ganglia volume than with either TICV or total GMV. However, direct involvement of the motor cortex may also explain the mild spasticity shown by approximately 25% of patients.
Given the role of the OFC in reward evaluation, decision-making and emotion,27 and inhibitory control,28 it seems reasonable to posit that the deficits found in this region contribute to impulsivity and disinhibition.2, 3 Persons with conduct disorder have been found to show reduced insula volume,29 a finding that may explain the aggressive, impulsive, and other disturbing behaviors found in LND and to a lesser extent in LNV.3
Brain regions responsible for self-injurious behavior, the key feature that distinguishes LND from LNV, remain unknown. Comparisons of brain regions affected in the two groups revealed one fairly large cluster (481voxels) including the posterior cingulate that trended toward distinguishing patients with LND from those with LNV (Table 2 and Figure 3). In humans, the cingulate gyrus has been found to play a key role in the experience of fear, anxiety, and pain.30 It is possible that when an HPRT-related abnormality in this region of the cingulate reaches some threshold, self-injurious behavior results. However, the posterior cingulate did not emerge as significantly different between the LND and LNV groups, possibly due to heterogeneity in the LNV group that prevented GMV abnormalities in this region from passing statistical thresholds in a whole brain analysis. In fact, when we relaxed the statistical threshold slightly (to a cluster forming threshold p<.01, and FWE =.004), the posterior cingulate did appear abnormal in the LND group. Another region that did distinguish LND from LNV involved the ventral striatum and orbitofrontal cortex. This region previously was postulated to play a role in self-injurious behavior based on its known role in reward mechanisms of the brain, and animal studies showing that manipulations of this region can cause self-injury.23 In fact, deep brain stimulation targeting the anteromedial portion of the internal segment of globus pallidus, part of the circuit thought to connect the ventral striatum with the orbitofrontal cortex, can suppress self-injury in patients with LND.21 This area therefore also deserves further scrutiny.
Relationship between HPRT and regional brain changes
The biological basis for apparently selective vulnerability of BG circuits among patients with HPRT deficiency remains unclear. It seems feasible that a primary, developmental insult involving nigrostriatal dopamine neurons leads to early atrophy of downstream structures in the basal ganglia, thalamus and cortex. It also is feasible that a primary insult involving cortical structures leads to retrograde dysfunction of these circuits. While the concept of selective neuronal vulnerability in both developmental and degenerative diseases has received considerable attention, selective dysfunction of whole circuits has not. Thus, just as LND helped define the concept of behavioral phenotype, it also might provide a model for understanding the consequences of a developmental insult that causes dysfunction of widely distributed neural circuits.
Limitations
The major limitation of this study was the use of two different scanners to acquire MRI data. However, when the scanner used was statistically modeled as a nuisance variable, the effects on GMV were minimal and unrelated to brain regions that distinguished the LND, LNV and HC groups. Thus, while this limitation should be kept in mind, we doubt that it contributed significantly to the findings. Moreover, nearly identical results were obtained when data acquired on each scanner were analyzed separately. Further, combining data acquired on two machines enabled us to analyze the largest sample of LND and LNV patients ever reported, and to discover heretofore unknown structural brain abnormalities in this devastating disease. Finally, while LND is a rare disease, self-injurious behavior characterizes many different neuropsychiatric disorders, and the findings reported here may provide some clues about the neural substrates of self-injury.
RESEARCH IN CONTEXT
Systematic review
We searched PubMed using the term “Lesch-Nyhan” in conjunction with “brain imaging,” “MRI,” “neuroimaging,” and “computed tomography” without restricting the publication date. We also reviewed the reference lists of articles identified using these terms and of our own publications. These searches confirmed that no whole-brain volumetric comparison of classical or variant Lesch-Nyhan disease has been reported to date. One MRI study of basal ganglia structures found that 7 patients with Lesch-Nyhan disease had smaller caudate nuclei and total cerebral volumes than 7 age-matched healthy controls, but other regions were not examined8. Another review of neural circuitry and Lesch-Nyhan disease postulated connections between behavioral features of the disease and circuits containing the basal ganglia and higher order brain structures, but no comprehensive volumetric studies were then available to address these relationships23.
Interpretation
Since the identification of Lesch-Nyhan disease (LND) in 1964, most experts have assumed that its primary neural defect involves the basal ganglia. Although all individuals with LND have severe motor neurological impairment, the classical behavioral phenotype includes features that cannot easily be explained by basal ganglia dysfunction alone. This study shows that, compared to healthy adults, patients with classical LND and attenuated variants demonstrate neuroanatomic abnormalities that are most pronounced in the basal ganglia, but extend to limbic and frontotemporal structures whose known functions are consistent with many neuropsychiatric aspects of the LND phenotype. These heretofore unrecognized brain abnormalities extend our understanding of the neural consequences of this single-gene disorder of purine metabolism.
Supplementary Material
Acknowledgments
Funding Sources:
This work was supported by the National Institute on Child Health and Human Development (R01 HD053312), the Therapeutic Cognitive Neuroscience Fund, and the Benjamin and Adith Miller Family Endowment on Aging, Alzheimer’s, and Autism Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions:
Dr. Schretlen: study concept and design, analysis and interpretation of data, drafting/ revising manuscript for content, statistical analysis, study supervision, secured funding
Mr. Varvaris: drafting/revising manuscript for content, analysis and interpretation of data, statistical analysis, acquisition of data
Ms. Ho: drafting/revising manuscript for content, interpretation of data, study coordination, acquisition of data
Dr. Vannorsdall: drafting/revising manuscript for content, study concept and design, interpretation of data, acquisition of data
Dr. Gordon: revising manuscript for content, secured funding
Dr. Harris: revising manuscript for content, study concept, acquisition of data
Dr. Jinnah: drafting/revising manuscript, study concept and design, interpretation of data, acquisition of data, study coordination, secured funding
Conflicts of Interest:
All authors have no conflicts of interest related to the content of this manuscript.
Contributor Information
Mark Varvaris, Email: mvarvar2@jhmi.edu.
Tiffany E. Ho, Email: tho7@jhmi.edu.
Tracy D. Vannorsdall, Email: tvannor1@jhmi.edu.
Barry Gordon, Email: bgordon@jhmi.edu.
James C. Harris, Email: jharrisd@jhmi.edu.
H. A. Jinnah, Email: hjinnah@emory.edu.
REFERENCES
- 1.Lesch M, Nyhan WL. Familial Disorder of Uric Acid Metabolism + Central Nervous System Function. Am J Med. 1964;36(4):561. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- 2.Schretlen DJ, Harris JC, Park KS, Jinnah HA, Del Pozo NO. Neurocognitive functioning in Lesch-Nyhan disease and partial hypoxanthine-guanine phosphoribosyl transferase deficiency. Journal of the International Neuropsychological Society. 2001;7(7):805–812. doi: 10.1017/s135561770177703x. [DOI] [PubMed] [Google Scholar]
- 3.Schretlen DJ, Ward J, Meyer SM, Yun J, Puig JG, Nyhan WL, et al. Behavioral aspects of Lesch-Nyhan disease and its variants. Developmental Medicine and Child Neurology. 2005;47(10):673–677. doi: 10.1017/S0012162205001374. [DOI] [PubMed] [Google Scholar]
- 4.Anderson LT, Ernst M. Self-injury in Lesch-Nyhan disease. J Autism Dev Disord. 1994;24(1):67–81. doi: 10.1007/BF02172213. [DOI] [PubMed] [Google Scholar]
- 5.Jinnah HA, Visser JE, Harris JC, Verdu A, Larovere L, Ceballos-Picot I, et al. Delineation of the motor disorder of Lesch-Nyhan disease. Brain. 2006;129(Pt 5):1201–1217. doi: 10.1093/brain/awl056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu R, Ceballos-Picot I, Torres RJ, Larovere LE, Yamada Y, Nguyen KV, et al. Genotypephenotype correlations in neurogenetics: Lesch-Nyhan disease as a model disorder. Brain. doi: 10.1093/brain/awt202. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong DF, Harris JC, Naidu S, Yokoi F, Marenco S, Dannals RF, et al. Dopamine transporters are markedly reduced in Lesch-Nyhan disease in vivo. Proc Natl Acad Sci. 1996;93(11):5539–5543. doi: 10.1073/pnas.93.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris JC, Lee RR, Jinnah HA, Wong DF, Yaster M, Bryan RN. Craniocerebral magnetic resonance imaging measurement and findings in Lesch-Nyhan syndrome. Archives of Neurology. 1998;55(4):547–553. doi: 10.1001/archneur.55.4.547. [DOI] [PubMed] [Google Scholar]
- 9.Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Hardy K, et al. Presynaptic dopaminergic deficits in Lesch-Nyhan disease. New England Journal of Medicine. 1996;334(24):1568–1572. doi: 10.1056/NEJM199606133342403. [DOI] [PubMed] [Google Scholar]
- 10.Jinnah HA, Ceballos-Picot I, Torres RJ, Visser JE, Schretlen DJ, Verdu A, et al. Attenuated variants of Lesch-Nyhan disease. Brain. 2010;133(Pt 3):671–689. doi: 10.1093/brain/awq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 12.Holdeigel M. Cranial computerized tomography in incomplete Lesch-Nyhan syndrome. Radiologe. 1987;27(3):127–129. [PubMed] [Google Scholar]
- 13.Saito Y, Takashima S. Neurotransmitter changes in the pathophysiology of Lesch-Nyhan syndrome. Brain and Development. 2000;22(Suppl 1):S122–S131. doi: 10.1016/s0387-7604(00)00143-1. [DOI] [PubMed] [Google Scholar]
- 14.Hara K, Kashiwamata S, Ogasawara N, Ohishi H, Natsume R, Yamanaka T, et al. A female case of the Leach-Nyhan syndrome. Tohoku Journal of Experimental Medicine. 1982;137(3):275–282. doi: 10.1620/tjem.137.275. [DOI] [PubMed] [Google Scholar]
- 15.Hatanaka T, Higashino H, Woo M, Yasuhara A, Sugimoto T, Kobayashi Y. Lesch-Nyhan syndrome with delayed onset of self-mutilation: hyperactivity of interneurons at the brainstem and blink reflex. Acta Neurologica Scandinavica. 1990;81(2):184–187. doi: 10.1111/j.1600-0404.1990.tb00960.x. [DOI] [PubMed] [Google Scholar]
- 16.Fattal A, Spirer Z, Zoref-Shani E, Sperling O. Lesch-Nyhan syndrome: biochemical characterization of a case with attenuated behavioral manifestation. Enzyme. 1984;31(1):55–60. [PubMed] [Google Scholar]
- 17.Lynch BJ, Noetzel MJ. Recurrent coma and Lesch-Nyhan syndrome. Pediatric Neurology. 1991;7(5):389–391. doi: 10.1016/0887-8994(91)90073-t. [DOI] [PubMed] [Google Scholar]
- 18.Salman RA, Glickman RS, Super S. Lesch-Nyhan syndrome: report of two cases. Journal of Oral Medicine. 1987;42(1):10–13. 66. [PubMed] [Google Scholar]
- 19.van Bogaert P, Ceballos I, Desguerre I, Telvi L, Kamoun P, Ponsot G. Lesch-Nyhan syndrome in a girl. Journal of Inherited Metabolic Disease. 1992;15(5):790–791. doi: 10.1007/BF01800022. [DOI] [PubMed] [Google Scholar]
- 20.Watts RW, Spellacy E, Gibbs DA, Allsop J, McKeran RO, Slavin GE. Clinical, post-mortem, biochemical and therapeutic observations on the Lesch-Nyhan syndrome with particular reference to the Neurological manifestations. Quarterly Journal of Medicine. 1982;51(201):43–78. [PubMed] [Google Scholar]
- 21.Taira T, Kobayashi T, Hori T. Disappearance of self-mutilating behavior in a patient with leschnyhan syndrome after bilateral chronic stimulation of the globus pallidus internus. Case report. Journal of Neurosurgery. 2003;98(2):414–416. doi: 10.3171/jns.2003.98.2.0414. [DOI] [PubMed] [Google Scholar]
- 22.Jankovic J, Caskey TC, Stout JT, Butler IJ. Lesch-Nyhan syndrome: a study of motor behavior and cerebrospinal fluid neurotransmitters. Annals of Neurology. 1988;23(5):466–469. doi: 10.1002/ana.410230507. [DOI] [PubMed] [Google Scholar]
- 23.Visser JE, Baer PR, Jinnah HA. Lesch-Nyhan disease and the basal ganglia. Brain Research Reviews. 2000;32(2):449–475. doi: 10.1016/s0165-0173(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 24.Baumeister AA, Frye GD. The biochemical basis of the behavioral disorder in the Lesch-Nyhan syndrome. Neurosci Biobehav Rev. 1985;9(2):169–178. doi: 10.1016/0149-7634(85)90043-0. [DOI] [PubMed] [Google Scholar]
- 25.Nyhan WL. Dopamine function in Lesch-Nyhan disease. Environ Health Perspect. 2000;108(Suppl 3):409–411. doi: 10.1289/ehp.00108s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130(Pt 7):1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- 27.Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10(3):284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 28.Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127(Pt 5):1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- 29.Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37(1):335–342. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 30.Ochsner KN, Ludlow DH, Knierim K, Hanelin J, Ramachandran T, Glover GC, et al. Neural correlates of individual differences in pain-related fear and anxiety. Pain. 2006;120(1–2):69–77. doi: 10.1016/j.pain.2005.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35(1):73–77. doi: 10.1212/wnl.35.1.73. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. 2nd Edition. Circle Pines, MN: American Guidance Services, Inc; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.