Abstract
BACKGROUND
Microfluidic devices recreate the hemodynamic conditions of thrombosis.
METHODS
Whole blood inhibited with PPACK was treated ex vivo with inhibitors and perfused over collagen for 300 s (wall shear rate = 200 s−1) using a microfluidic flow assay. Platelet accumulation was measured in the presence of COX-1 inhibitor (aspirin, ASA), P2Y1 inhibitor (MRS 2179), P2Y12 inhibitor (2MeSAMP) or combined P2Y1 and P2Y12 inhibitors.
RESULTS
High dose ASA (500 μM), 2MeSAMP (100 μM), MRS 2179 (10 μM),or combined 2MeSAMP and MRS 2179 decreased total platelet accumulation by 27.5%, 75.6%, 77.7%, and 87.9% (p < 0.01), respectively. ASA reduced secondary aggregation rate between 150 and 300 s without effect on primary deposition rate on collagen from 60 to 150 s. In contrast, 2MeSAMP and MRS 2179 acted earlier and reduced primary deposition to collagen between 60 and 105 s and secondary aggregation between 105 and 300 s. RCOX and RP2Y (defined as a ratio of secondary aggregation rate to primary deposition rate) demonstrated 9 of 10 subjects had RCOX < 1 or RP2Y < 1 following ASA or 2MeSAMP addition, while 6 of 10 subjects had RP2Y < 1 following MRS 2179 addition. Combined MRS 2179 and 2MeSAMP inhibited primary platelet deposition rate and platelet secondary aggregation beyond that of each individual inhibitor. Receiver-Operator Characteristic area under the curve (AUC) indicated the robustness of RCOX and RP2Y to detect inhibition of secondary platelet aggregation by ASA, 2MeSAMP, and MRS 2179 (AUC of 0.874 0.966, and 0.889, respectively).
CONCLUSIONS
Microfluidic devices can detect platelet sensitivity to antiplatelet agents. The R-value can serve as a self-normalized metric of platelet function for a single blood sample.
Keywords: platelet, cyclooxygenase, ADP, thromboxane, hemodynamic
INTRODUCTION
Antiplatelet therapies are used in a variety of clinical settings from management of unstable angina to risk reduction of myocardial infarction or stroke. Aspirin is used by over 50 million patients in the United States to reduce the risk of cardiovascular events [1]. Aspirin irreversibly acetylates serine 529 of cyclooxygenase-1 (COX-1), blocking the enzyme active site for arachidonic acid and inhibiting the generation of prostaglandin H2 and thus thromboxane A2 (TXA2) production from platelets [2]. Inhibition of platelet TXA2 synthesis prevents platelet activation through the TXA2 receptor (TP), a receptor encoded by the TBXA2R gene.
In addition to TXA2, adenosine disphosphate (ADP) receptors are another target of antiplatelet therapies. The platelet plasma membrane contains two ADP receptors, P2Y1 and P2Y12, which are purinergic G protein coupled receptors. P2Y1 is linked to Gq and ADP signaling through this pathway results in rapid Ca2+ mobilization and platelet shape change [3,4]. P2Y12 is linked to a Gi protein. ADP binding to P2Y12 inhibits adenylate cyclase and stabilizes secondary platelet aggregation. Current therapies that target the P2Y12 receptor vary from prodrugs that irreversibly antagonize the P2Y12 receptor to direct, reversible antagonists [4]. Thienopyridines clopidogrel and prasugrel are examples of the former, while ticagrelor is an example of the latter. Currently, no P2Y1 antagonists are on the market, however, combined P2Y1 and P2Y12 antagonists are in development [4, 5]. To mimic the action of P2Y1 and P2Y12 antiplatelet therapies ex vivo, 2′-deoxy-N6-methyl adenosine 3′,5′-diphosphate (MRS 2179) and 2-methylthioadenosine 5-monophosphate (2MeSAMP) are used in this study as highly selective P2Y1 and P2Y12 antagonists, respectively.
Targeting signaling pathways such as TXA2 production and ADP/P2Y12 signaling reduces secondary platelet aggregation while not severely altering primary haemostasis. However, the delicate balance between preventing excessive clotting and increasing bleeding risks requires careful monitoring of antiplatelet therapies. The evaluation of the effect of pharmacological agents on platelet function often rely on tests with poorly defined fluid mechanics and flow fields (eg. aggregometry) that fail to replicate platelet adhesive mechanisms under realistic and defined hemodynamic conditions. Under flow conditions, the efficacy of pharmacological agents greatly depend on granule release, platelet-platelet contacts, and convective removal of autocrinic agonists from the injury site. Microfluidic devices can recreate the hemodynamic conditions required to study anti-platelet agents. These devices offer spatially controlled focal injuries with collagen or collagen with tissue factor bearing surfaces [6,7,8]. Microfluidic devices have also been used to study clot contraction and clot permeability with precise control of wall shear stress and transthrombus pressure gradients [8,10]. In fact, the core-shell hierarchy of clots observed in vivo following laser injury [9] can be replicated in vitro with such devices [10].
Here we continue the development of microfluidic assay metrics found previously [11] and extend these metrics to examine two ADP antagonists and validate this assay for detection of anti-platelet therapies through Receiver-Operator Characteristic (ROC) analysis. For flow assays to become a relevant clinical tool a large cohort of healthy donors must be tested with respect to response to antiplatelet agents. Toward that goal, we tested healthy subject platelet function with 38 donors and 66 independent blood draws (2 combined studies) after ex vivo addition of ASA. While coagulation assays can rely on stable pooled plasma for calibration, live platelet function assays have no available standard to calibrate the assay. We sought to define a self-normalized parameter, the R-value, to score the accumulation of platelets on the surface for a single blood sample test without reference to a prior test value or calibration fluid.
MATERIALS AND METHODS
Blood collection, labeling, and antiplatelet agents
Blood was collected via venipuncture from 11 healthy subjects who self-reported as non-smoking, free of oral medication, and abstained from alcohol 48 hr prior to donation. All subjects were free from illness or bleeding disorders. Blood samples were drawn into H-D-Phe-Pro-Arg-chloromethylketone (100 μM PPACK final concentration, Haematologic Technologies). All volunteers provided informed consent in accordance with IRB approval and the Declaration of Helsinki. Whole blood was treated with Phycoerythrin (PE) Mouse Anti-Human CD61 (αIIbβ3) antibody (BD Biosciences) in a ratio of (1:50) 7 min prior to perfusion. This antibody has no effect on platelet αIIbβ3 function in the assay. PPACK-treated whole blood was perfused through the device within 25 min of phlebotomy. Blood was incubated with vehicle (0.1% DMSO final concentration) or indicated concentrations of ASA, 2MeSAMP, MRS 2179, or combined 2MeSAMP and MRS 2179 20 min prior to the assay. This time scale is consistent with previous studies done with these compounds [11,12,13]. Acetylsalicylic acid (ASA, Sigma Aldrich) was dissolved in DMSO at 500 mM, 2-methylthioadenosine 5′-monophosphate triethylammonium salt hydrate (2MeSAMP, Sigma Aldrich) was dissolved in HEPES buffered saline (HBS, 20 mM HEPES, 160 mM NaCl, pH 7.5) at 1 mM/L, and 2′-deoxy-N6-methyladenosine 3′, 5′-bisphosphate ammonium salt (MRS 2179, Tocris Bioscience) at 0.1 mM in HBS. A dilution of ASA was then made to the desired final concentration in HBS within 1 h of the test. Final concentrations of antiplatelet therapies used were: 500 μM ASA, 100 μM 2MeSAMP, and 10 μM MRS 2179, well in excess of their IC50’s in the assay of 10-20 μM ASA, 2.56 μM 2MeSAMP, and 0.23 μM MRS 2179 [11,13]. Antiplatelet agent concentrations were used well in excess of their respective IC50 values to completely antagonize ADP receptors or abate COX-1 derived TXA2.
Microfluidic devices and real time platelet deposition imaging
Microfluidic devices were fabricated in polydimethylsiloxane (PDMS, Sylgard 184, Ellsworth Adhesives) according to previously described techniques [11,13]. The microfluidic device has 8 parallel channels (250 μm wide by 60 μm high) that converge to a common outlet (Figure 1A). The device was fed by 8 distinct wells, with perfusion achieved by withdrawal into a single syringe pump (Harvard Apparatus). The channels run perpendicularly over a 250 μm long stripe of patterned equine fibrillar collagen type 1 (Chronopar, Chronolog). Channels were spaced in close proximity to allow for all channels to be imaged simultaneously with a 2X objective lens using an inverted microscope (IX81, Olympus America Inc) equipped with a CCD camera (ORCAER, Hamamatsu). A custom stage insert held 3 microfluidic devices allowing 24 simultaneous thrombi to be imaged in 15 sec intervals. Prior to microfluidic assay, all channels were blocked with 0.5% bovine serum albumin (BSA) in HBS buffer. Blood samples were perfused at venous wall shear rate of 200 s−1 for 5 min (2 μL/min per channel).
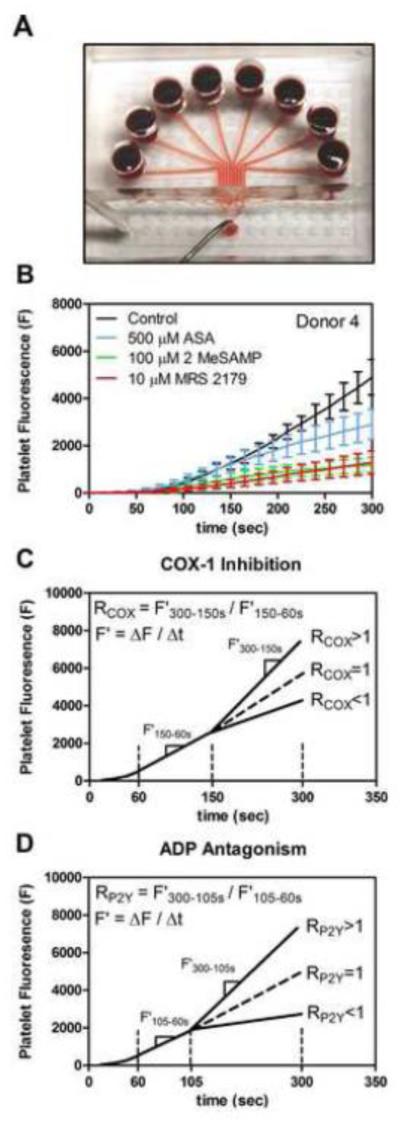
Fig. 1. 8-channel microfluidic device, measured platelet fluorescence dynamics, and RCOX, RP2Y schematic summaries.
(A), Photograph of the 8-channel microfluidic device, the device is fed by 8 wells converging to a single outlet. (B), Platelet fluorescence values over time in the presence of aspirin, 2MeSAMP, and MRS 2179. Error bars indicate standard deviation from 6 measurements at each time point. (C), Graphic representation of RCOX based on a ratio of deposition rates (F’) of secondary platelet aggregation to initial platelet adhesion to collagen. (D), Graphic representation of RP2Y.
Platelet accumulation analysis
Background corrected fluorescence values were measured using MATLAB (MathWorks) as previously described [13]. Briefly, all eight platelet deposits on a single microfluidic device were identified and corrected with their corresponding background regions downstream of each platelet deposit by a custom MATLAB script. To calculate overall percent inhibition after a 300 s perfusion, fluorescence values were normalized to each donor’s control platelet adhesion to collagen (FI300s(Drug)/FI300s). Additionally, an R-value for ASA (RCOX) was defined as a ratio of platelet deposition rates that normalized the late stage platelet deposition rate (F’300-150s) to early stage deposition rate (F’150-60s) for all ex vivo ASA additions, as previously established [11]. The RCOX value is an internal intradonor standard to score secondary aggregation due to TXA2 secretion, with values of RCOX > 1 indicating when secondary platelet aggregation was prominent and RCOX < 1 when secondary aggregation was not detected (Figure 1C). In a previous work [11], a dose dependent decrease in the rate of secondary platelet aggregation was found with increasing ex vivo ASA concentrations whereas the initial platelet deposition rate on collagen was unaffected by ASA. We now define an additional intradonor normalization metric RP2Y as a ratio of platelet deposition rates normalizing late stage secondary platelet aggregation rate due to ADP release (F’300-105s) to earlier stage platelet deposition rate (F’105-60s) (Figure 1D). The temporal onset for RP2Y was based on previous work [12] establishing that the effect of ADP antagonism occurred after 100 sec, as was further confirmed in kinetic traces for ADP antagonists for the 11 donors in the present study (Figure 1B).
Finally, sensitivity and specificity for detection of ASA, 2MeSAMP, or MRS 2179 was found through Receiver Operator Characteristic (ROC) analysis [14]. ROC curves were generated to detect the presence of each drug in the microfluidic assay through evaluation of true positive and false positive fractions and the area under the curve (AUC) [14]. In this analysis, for each antiplatelet therapy tested, ROC curves were generated comparing 10 average R-values, one from each of the 10 subjects with no ex vivo inhibition to 10 average measurements of the R-value for each anti-platelet drug.
RESULTS
Ex vivo testing of anti-platelet agents occurred in duplicate on 3 separate microfluidic devices run simultaneously. For 11 subjects, a total of 432 individual thrombi were formed under flow on collagen, resulting in 108 events each for 3 conditions (control, 2MeSAMP, and MRS 2179), 60 events for ex vivo ASA addition, and 48 events for ex vivo addition of 2MeSAMP and MRS 2179. Statistical analysis was conducted to evaluate intradonor variations for duplicate control conditions on a single microfluidic device and 3 devices run simultaneously (6 conditions). Total platelet accumulation (FI300s) and secondary aggregation [RCOX(0)] values for whole blood perfusion with vehicle buffer were tabulated from 11 blood samples and the coefficient of variation for each donor defined as standard deviation/mean was found (Supplemental Table S1, S2). Coefficients of variation for FI300s ranged from 5.3% to 35.8% (mean = 13.1%) for replicate control channels on a single device while values varied from 5.3% to 24.4% for triplicate microfluidic testing (mean = 16.3%). For RCOX (0), coefficient of variation values were all < 20% for a single microfluidic device or triplicate device testing with the exception of donor 4 and donor 10. In 3 comparisons evaluating ex vivo ASA efficacy (Figure 5), prior supplementary data for 28 individuals [11] obtained in the same manner as this study was combined with data from 10 subjects from this work. Only 1 subject partook in both ASA studies.
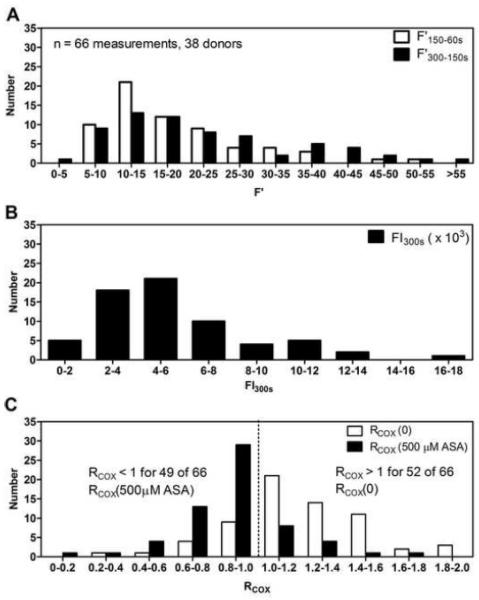
Fig. 5. Distribution of microfluidic metrics in the assessment of platelet adhesion to collagen and ASA’s inhibition on platelet function under flow.
(A), Binning of primary and secondary platelet deposition rates. (B), Binning of final platelet accumulation as measured by platelet fluorescence at t = 300s (FI300s). (C), Binning of RCOX values in assessment of the effect of ASA on secondary platelet aggregation in the microfluidic perfusion assay.
Platelets adhere substantially to the localized collagen surface within 1 minute and subsequent secondary platelet accumulation via platelet-platelet interactions over the 300 s perfusion time has been established in previous studies [12,13]. Growth in platelet coverage occurs in two dimensions on the collagen surface between 60 -105 sec. After 105 sec, the thrombus growth continues in the third dimension and becomes significant under flow due to secondary platelet-platelet aggregation mediated by soluble agonists such as ADP and TXA2 at approximately 105 sec and 150 sec, respectively.
Platelet inhibition by ASA, 2MeSAMP, and MRS 2179
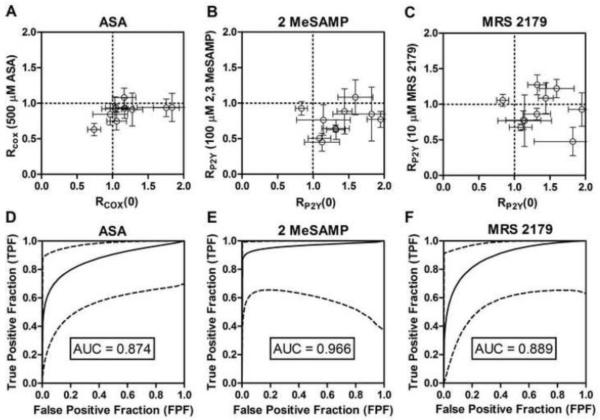
Platelet adhesion and subsequent secondary platelet aggregation were significantly reduced upon ex vivo addition of ASA, 2MeSAMP, or MRS 2179 (Figure 1B). Using RCOX or RP2Y (Figure 1C, 1D), 8 out of 10 subjects displayed significant secondary platelet aggregation [R(0 drug) > 1] before ex vivo drug addition (Figure 2A-C). In contrast, 9 out of 10 subjects had RCOX < 1 upon ex vivo treatment with ASA (Figure 2A). Furthermore, 9 out of 10 subjects had RP2Y < 1 with ex vivo 2MeSAMP addition, demonstrating that most subjects had secondary platelet aggregation and primary platelet deposition that was sensitive to P2Y12 antagonism (Figure 2B). RP2Y was less reliable in scoring MRS 2179 inhibition of P2Y1 with only 6 out of 10 subjects having RP2Y < 1 (Figure 2C), in part (ironically) due to the high potency of MRS 2179 in reducing Ca2+ mobilization through P2Y1 signaling. Hence MRS 2179 drastically reduces primary platelet deposition in comparison to 2MeSAMP, thus the associated difficulty of taking a ratio of two small numbers since both primary deposition and secondary aggregation rate were affected by MRS 2179.
Fig. 2. Microfluidic assay sensitivity and specificity of RCOX and RP2Y to detect inhibition of primary platelet adhesion or secondary platelet aggregation.
(A), Rcox for ex vivo ASA addition. (B), RP2Y for ex vivo 2MeSAMP addition. (C), RP2Y for ex vivo MRS 2179 addition. (D) ROC curve for detection of ex vivo ASA addition. (E), ROC curve for detection of ex vivo 2MeSAMP addition. (F), ROC curve for detection of ex vivo MRS 2179 addition. Error bars indicate standard deviation from 6 measurements of RCOX or RP2Y for each donor. Dashed lines are 95% confidence intervals.
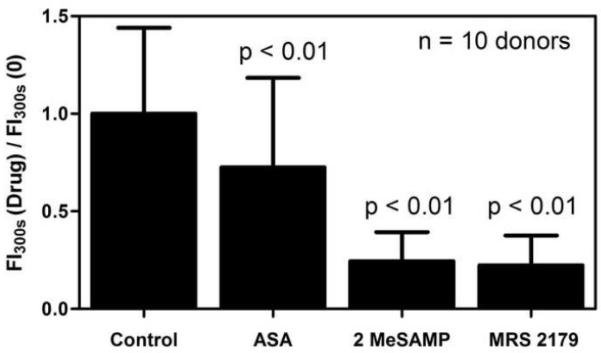
ROC curves were generated to examine the inhibitory effects of ASA, 2MeSAMP, and MRS 2179 in this assay (Figure 2D-F). In comparing 10 measurements of RCOX(0) (true full COX-1 activity, full P2Y12 and P2Y1 function) to 10 measurements of RCOX (500 μM ASA) (true full COX-1 inhibition), the ROC curve had an AUC of 0.874 (Figure 2D). Comparison of 10 RP2Y values at baseline to 10 RP2Y(100 μM 2MeSAMP) (true full P2Y12 antagonism) gave an ROC curve AUC of 0.966 (Figure 2E). Finally, in comparing the same 10 baseline RP2Y values to RP2Y(10 μM MRS 2179) an ROC curve AUC of 0.889 was obtained. Calculated ROC AUC values indicate excellent diagnostic discrimination between populations upon ex vivo treatment with ASA, 2MeSAMP, or MRS 2179. ASA, 2MeSAMP, and MRS 2179 all decreased total platelet accumulation at 300 sec when normalized against baseline collagen response reaching statistical significance for the entire cohort (p < 0.01, n = 10 subjects) and each antiplatelet agent (Figure 3). The % inhibition of platelet function was much greater for 2MeSAMP and MRS 2179 than ASA. However, the effect of ex vivo 2 MeSAMP and MRS 2179 were not statistically different from each other. While the % inhibition is very useful for measuring drug potency following ex vivo drug addition, it requires reference to a separate untreated blood sample which may not necessarily be available clinically with patients. In contrast, the R-value is self-normalized and can be used for a single blood sample obtained from a patient without the need to reference to a prior blood test to obtain % inhibition.
Fig. 3. Effect of ex vivo ASA, 2MeSAMP, MRS 2179 on final platelet aggregate size normalized by baseline platelet aggregate size formed over collagen.
Ex vivo addition of ASA, 2MeSAMP, MRS 2179 resulted in smaller final platelet aggregate size compared to response measured with no drug (p < 0.01, n = 10 donors).
Platelet inhibition by combined 2MeSAMP and MRS 2179 ex vivo addition
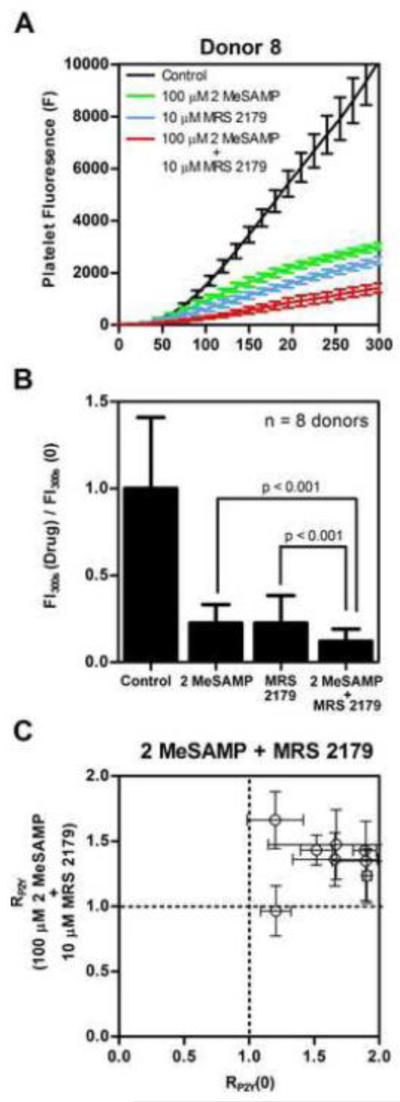
To investigate the effects of combined P2Y1 and P2Y12 inhibition by MRS 2179 and 2MeSAMP respectively, 7 of the 10 subjects from the above study of ex vivo addition of either P2Y1, P2Y12, or COX-1 inhibitor alone were re-enrolled along with new subject #11. Platelet fluorescence traces over time representing thrombus buildup during the microfluidic assay were reduced in the case of combined P2Y1 and P2Y12 inhibition of platelet function in comparison to ex vivo P2Y1 or P2Y12 inhibition alone (Figure 4A). Using MRS 2179 and 2MeSAMP together, both primary deposition rate and secondary aggregation rates were strongly reduced, compared to control or when MRS 2179 or 2MeSAMP were used individually (Figure 4A).
Fig. 4. Effect of combined ex vivo addition of 2MeSAMP and MRS 2179 on platelet function in comparison to ex vivo addition of 2MeSAMP or MRS 2179 alone.
(A) Platelet fluorescence dynamics over time in the presence of ex vivo 2MeSAMP, MRS 2179, or combined 2MeSAMP and MRS 2179. (B) Ex vivo addition of 2MeSAMP and MRS 2179 reduced final thrombus size more significantly than 2MeSAMP or MRS 2179 treatment alone when normalized against final control thrombus size over collagen (p < 0.01, n = 8 donors). (C) RP2Y for combined ex vivo 2MeSAMP and MRS 2179 addition. Error bars indicate standard deviation from 6 measurements of RP2Y for each donor.
Combined ex vivo addition of 2MeSAMP and MRS 2179 dramatically reduced total platelet accumulation at 300 sec when normalized against controlled platelet response to collagen (Figure 4B). The platelet mass at the end of 300 sec due to combined P2Y1 and P2Y12 inhibition was significantly smaller than the platelet masses due to single P2Y1 or P2Y12 inhibition (p < 0.001, n = 8 subjects) (Figure 4B). Because the primary deposition rate was so strongly attenuated, RP2Y was not suited to score combined P2Y1 and P2Y12 inhibition (Figure 4C). This is due to the high efficacy of combined MRS 2179 and 2MeSAMP treatment on the primary platelet deposition rate on collagen in this microfluidic thrombosis model. The primary deposition rate becomes exceedingly low once ADP is completely attenuated under flow conditions by knocking out both P2Y12 and P2Y1 signaling pathways. ADP is a potent modulator of platelet recruitment to the initial monolayer of platelets adherent to collagen. The R-value was originally intended to score secondary aggregation, hence the difficulties in using RP2Y to detect a ratio of platelet deposition rates over the two temporal ranges.
Statistics of platelet phenotyping with microfluidics using ASA
Finally, we incorporate and quantify historical supplementary data from a previous work [11] into this continuing study with intent to show the full distribution of microfluidic platelet assay metrics (total platelet accumulation, primary and secondary platelet deposition rates, RCOX). Distribution of these metrics is optimal with maximum data in assessing the capabilities of this microfluidic assay to detect ex vivo addition of ASA.
Microfluidic phenotyping of 37 donors, 10 from the current study and 28 from a previous study [11] showed a broad distribution of primary platelet deposition rates on collagen with no ex vivo drug treatment (Figure 5A). Late stage secondary platelet aggregation also distributed broadly (Figure 5A). Binning of total platelet accumulation showed varied inter-subject response to collagen with FI300s values ranging from 2,000 to 16,000 (Figure 5B). Interestingly, the 10 healthy subjects from this study exhibited FI300s values all below 6000 while the larger unique subset of 28 subjects from the previous study showed a wider range of FI300s values. Gaussian-like distribution of RCOX(0) values centered above 1 indicated that RCOX was a robust measurement of secondary aggregation response in untreated whole blood (Figure 5C). The use of RCOX = 1 as the decision value was previously reported as the microfluidic assay for detection of in vivo and ex vivo ASA had high discrimination (high true positive rate with low false positive rate) at this anchoring point [11]. In a total of 66 determinations of RCOX(0), 52 measurements were above the RCOX = 1 cutoff demonstrating a majority of donors had a strong secondary aggregation response in the assay. Observation of 14 values of RCOX < 1 indicate some donors lack detectable secondary aggregation by this assay, a phenomena potentially attributable to low levels of released ADP and TXA2 in the boundary layer formed above the platelet deposit [12]. In contrast, with 500 μM ASA added ex vivo, 49 of 66 determinations of RCOX < 1 demonstrated most subjects had platelets that were sensitive to ASA inhibition of platelet TXA2 (Figure 5C). The 17 determinations of RCOX where RCOX > 1 with 500 μM ASA added ex vivo could be due to non COX-1 mediated ASA effects on platelets when they are activated by collagen with autocrinic ADP present [15]. Such blood samples may be worthy of further study with respect to the phenotype of functional insensitivity to ASA despite COX-1 acetylation. A notable left shift of the distribution away from RCOX(0) was evident for the 66 determinations of RCOX(500 μM ASA) (Figure 5C).
DISCUSSION
We demonstrated the utility of an 8-channel microfluidic device to assess various anti-platelet agents on platelet function. We extended from a previous study [11] the RCOX value, a normalized metric to detect reduction in secondary aggregation due to ex vivo ASA addition (Figure 1C). We now define RP2Y a ratio of secondary platelet aggregation rate to primary platelet deposition rate (with a different temporal profile than RCOX) to quantify P2Y1 and P2Y12 antagonists (Figure 2). Examination of RP2Y and ROC curve testing establish 2MeSAMP and MRS 2179 as potent anti-platelet drugs that target initial platelet adhesion to collagen and the secondary wave of platelet recruitment by attenuating the autocrine ADP pathway (Figure 6). The effect of ASA on secondary platelet-platelet interactions has been well characterized in platelet aggregometry [16]. Under flow, ADP and TXA2 are complex and interacting modulators since both can become elevated in a concentration boundary layer [12] . Additionally, RP2Y was shown to be unsuited for scoring the potency of combined P2Y1 and P2Y12 antagonism of platelet function because primary deposition rate was so strongly inhibited in this microfluidic thrombosis model. Measured platelet fluorescence traces and normalization of final platelet mass against control platelet masses formed over collagen for 8 healthy donors showed ex vivo dual treatment with P2Y12 and P2Y1 inhibitors to be significantly more potent than single ex vivo P2Y1 or P2Y12 antagonism (Figure 4).
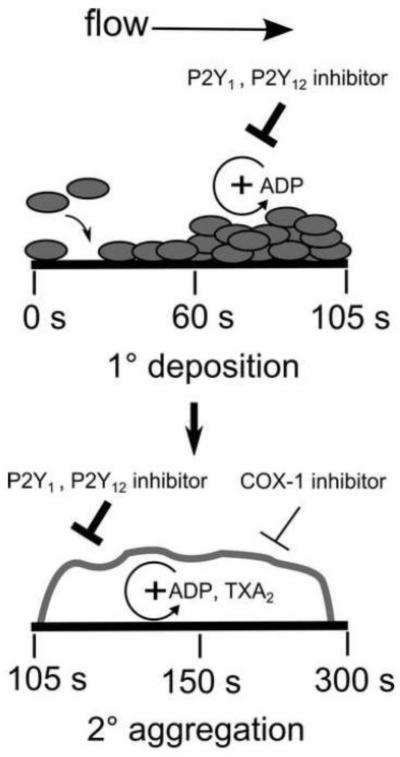
Fig. 6.
Effect of P2Y1, P2Y12 antagonists and ASA on primary platelet adhesion to collagen and secondary platelet aggregation due to ADP and thromboxane autocrine signaling. COX-1 inhibitor ASA reduced secondary aggregation between 150 and 300 s without an effect on primary platelet deposition to collagen from 60 to 150 s. 2MeSAMP and MRS 2179 reduced primary deposition between 60 and 105 s and secondary platelet aggregation between 105 and 300 s. Time intervals marked are indicators for RCOX and RP2Y, 60 - 150 sec & 150 sec - 300 sec and 60 - 105 sec & 105 sec - 300 sec respectively. X-axis is not to scale.
We report findings with some similarities and differences to the prior flow studies of Lucitt et al. [17] and Menolicchio et al. [18]. Lucitt et al. found no effect on the rate of platelet coverage of the collagen surface with in vitro ASA addition at 1500 s−1. However, percent surface coverage may be a less sensitive measure of secondary aggregation which also increases the height of the deposit. Menolicchio et al. also reported a limited reduction of platelet aggregate growth above the layer of platelets adherent to collagen with in vitro addition ASA at 1500 s−1. Since there was no thrombin/fibrin generation allowed in Lucitt [17] who used 300 ATU hirudin or Menolicchio [18] who used 68 USP heparin, arterial shear rates of 1500 s−1 may limit the detection of ASA action because thrombin/fibrin dramatically stabilize the platelet deposit at arterial conditions [7]. As a deposit grows in height in a flow channel, the shear rates become quite high during a constant flow rate perfusion and embolization is likely, especially at an initial arterial wall shear rate, with or without fibrin present [7]. At the venous shear rate used with antiplatelet agents in the present study, partially occlusive deposits formed in the absence of thrombin/fibrin are more reliably measured since there is no embolization, even under constant flow conditions. In prior work, we have shown that the IC50 of ASA measured at venous shear rates was quite similar to that measured at arterial shear rates [11]. In addition, the IC50 of 2MeSAMP and MRS 2179 at venous shear rates were also on the same order of magnitude to that found at arterial shear rates (1000s−1) (Supplemental Figure S3).
Lucitt et al. also reported an effect of in vitro 2MeSAMP on initial platelet recruitment on collagen delaying the time to reach 2.5% platelet surface coverage to 56 sec as compared to 33 sec for the control case in an 8 min assay at 1500 s−1. Lucitt et al. found that in vitro ASA had no effect on this initial stage of platelet adhesion. We report findings consistent with Lucitt et al. but at 200 s−1. We found that ASA does not affect primary platelet deposition to collagen (F’150-60s), while 2MeSAMP and MRS 2179 inhibit primary platelet response to collagen (F’105-60s) but more significantly affects secondary platelet aggregation (F’300-105s) requiring RP2Y as a new internally normalized metric to characterize platelet response to ADP antagonists under flow. ADP antagonists were found to inhibit platelet function by ~ 105 sec as compared to ~ 150 sec due to ASA inhibition of TXA2 release. Also, Lucitt et al. determined 2MeSAMP significantly reduced the rate of platelet aggregation formation on collagen by impairing recruitment of additional platelets. Menolicchio et al. report marked reduction of platelet aggregation above the initial platelet surface on collagen due to in vitro addition of 2MeSAMP. Both report these results at 1500s−1. This is consistent with our findings at 200s−1 with RP2Y and ROC curves detecting significant impairment of secondary platelet aggregation due to both ADP antagonists tested.
Monitoring of P2Y12 inhibition by clopidogrel or other P2Y12 antagonists can be achieved through assays such as vasodilator-stimulated phosphoprotein phosphorylation (VASP), turbidometric platelet aggregometry, and the VerifyNow P2Y12 test. Although platelet aggregometry remains the gold standard for platelet function testing, several disadvantages exist such as poor reproducibility, high sample volume, and complex sample preparation [20]. Turbidometric platelet aggregometry testing uses ADP induced platelet aggregation to measure the effect of clopidogrel. However, ADP can illicit platelet aggregation through P2Y1 while VASP requires flow cytometry and an experienced technician [20]. Point of care assays are especially advantageous in clinical settings as they enable immediate decision making for dosing of antiplatelet drugs. The VerfiyNOW P2Y12 is the only device that meets the various constraints to be considered a point of care assay. Interestingly, in comparing the clinical utility of this microfluidic assay to the VerifyNow P2Y12 system, ROC curve AUC values were strikingly similar. A ROC curve value of 0.929 was found in the assessment of the VerifyNow P2Y12 assay to detect antiplatelet effects during clopidogrel therapy, comparable to the 0.966 value found for 2MeSAMP in this study [21].
Often, microfluidic chambers utilize a single flow path comprised of a millimeter length collagen coated cover slip or capillary tube enabling platelets to tether, activate, and re-adhere along the entire length [17]. With such long zones available for adhesion, the platelet coverage is often a function of distance along the collagen. The current microfluidic injury model exposes platelets to a narrow 250 μm long collagen strip with no dependency on distance down the “injury” site as the image capture zone is the entire prothrombotic surface. Furthermore, the type of collagen used as the adhesive substrate impacts platelet-surface interactions. Previous reports utilize bovine soluble collagen type I, porcine type I collagen, or equine tendon fibrillar type I collagen [7, 18, 22]. The equine fibrillar type I collagen used in this study is a thoroughly established prothrombogenic surface as a recent study examined sources of variability over this collagen type in microfluidic flow assays for n = 104 healthy donors [23].
Several previous flow systems have been examined for the detection of P2Y1, P2Y12 antagonists and COX-1 effects under various shear rates [17, 18, 22]. However, distinct in our studies are the eight channels comprising this single microfluidic device capable of performing dose-response experiments for 8 different concentrations on a single antiplatelet agent or high throughput examination of three antiplatelet agents in duplicate with simultaneous testing across three devices. Single channel platelet function measurements make dose response testing and high replicate testing of multiple drugs particularly cumbersome. Dose dependent antiplatelet therapy testing on platelet function is crucial in narrowing the therapeutic window for these drugs towards personalized medicine in a clinical setting.
While antiplatelet agents are used for the prevention of arterial thrombosis, the monitoring of antiplatelet agents in anticoagulated whole blood (that lacks fibrin stabilization) is perhaps most precisely detected at venous shear rate, especially when constant flowrate perfusion is used. The action of antiplatelet agents on thrombus buildup in the presence of fibrin generation at arterial shear rates remains an area of active investigation. Such arterial thrombosis studies will be best conducted under microfluidic conditions of constant pressure drop (not constant flow rate) where channel occlusion is a natural and important endpoint to the experiment [7]. Further work would be required to examine whether the presence of thrombin and fibrin in this microfluidic thrombosis model affects the inhibitory capabilities of 2MeSAMP and MRS 2179 on platelet function and as well as the compounds’ respective IC50 values at venous and arterial wall shear rates. A constant pressure drop microfluidic thrombosis model at initial arterial wall shear rates would be more physiologically relevant. This could be a future avenue to investigate the efficacy of P2Y1 and P2Y12 inhibitors over increasingly complex surface triggered prothrombogenic surfaces such as tissue factor and collagen [6].
The 8-channel microfluidic phenotyping of anti-platelet therapies in this study was able to reproduce the biochemical signaling pathways and transport processes under which antiplatelet therapies must act. Quantification through deposition rates, total platelet fluorescence and the all encompassing R value self-normalizes donors without requiring donor specific measurements of vWF levels, platelet count, and hemoatocrit. However, quantification of secondary platelet aggregation using RP2Y or RCOX has not been tested with patients on clopidogrel or combined clopidogrel and aspirin regiments. Translation of these values to quantify patients treated for coronary heart disease remains an area of future study. This work represents another step towards a functional high throughput point of care platelet function assay to detect patient specific response to various antiplatelet therapies.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Thomas V. Colace for his advice and guidance in microfluidic platelet assays and Mei Yan Lee for her custom MATLAB script automating calculations from the microfluidic platelet assays. This study was supported by National Institutes of Health, NIH R01 HL103419 (Dr. Diamond)
Abbreviations
- (ASA)
Aspirin
- (COX-1)
cyclooxygenase 1
- (TXA2)
thromboxane A2
- (ADP)
Adenosine diphosphate
Footnotes
Disclosure of Conflict of Interests The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Campbell CL, Smyth S, Montalescot G, et al. Aspirin Dose for the Prevention of Cardiovascular Disease. JAMA. 2007;297:2018–2024. doi: 10.1001/jama.297.18.2018. [DOI] [PubMed] [Google Scholar]
- [2].Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110:255–258. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- [3].Brass LF, Stalker TJ, Zhu L, et al. Signal Transduction During Platelet Plug formation. In: Michelson AD, editor. Platelets. 2nd ed Academic Press; Boston: 2007. pp. 367–397. [Google Scholar]
- [4].Michelson AD. Antiplatelet therapies for treatment of cardiovascular disease. Nat. Rev. Drug Discovery. 2010;9:154–169. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- [5].Chang H, Yanachkov IB, Dix EJ, et al. Modified diadenosine tetraphosphates with dual specificity for P2Y1 and P2Y12 are potent antagonists of ADP-induced platelet activation. J Thromb Haemost. 2012;12:2573–2580. doi: 10.1111/jth.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Colace TV, Jobson J, Diamond SL. Relipidated Tissue Factor Linked to Collagen Surfaces Potentiates Platelet Adhesion and Fibrin Formation in a Microfluidic Model of Vessel Injury. Bioconjugate Chem. 2011;22:2104–109. doi: 10.1021/bc200326v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Colace TV, Muthard RW, Diamond SL. Thrombus Growth and Embolism on Tissue Factor-Bearing Collagen Surfaces Under Flow: Role of Thrombin With and Without Fibrin. Arterioscler Thromb Vasc Biol. 2012;32:1466–1476. doi: 10.1161/ATVBAHA.112.249789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Muthard RW, Diamond SL. Blood clots are rapidly assembled hemodynamic sensors: Flow arrest triggers intraluminal thrombus contraction. Arterioscler Thromb Vasc Biol. 2012;12:2938–2945. doi: 10.1161/ATVBAHA.112.300312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stalker TJ, Traxler EA, Wu J, et al. Hierarchical organization in the hemostatic response and its relationship to the platelet signaling network. Blood. 2013;121:1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Muthard RW, Diamond SL. Side view thrombosis microfluidic device with controllable wall shear rate and transthrombus pressure gradient. Lab Chip. 2013;13:1883–1891. doi: 10.1039/c3lc41332b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li R, Fries S, Li X, et al. Microfluidic Assay of Platelet Deposition on Collagen by Perfusion of Whole Blood from Healthy individuals taking aspirin. Clin Chem. 59:1195–1204. doi: 10.1373/clinchem.2012.198101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Flamm MH, Colace TV, Chatterjee MS, et al. Multiscale prediction of patient specific platelet function under flow. Blood. 2012;120:190–198. doi: 10.1182/blood-2011-10-388140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maloney SF, Brass LF, Diamond SL. P2Y12 or P2Y1 inhibitors reduce platelet deposition in a microfluidic model of thrombosis while apyrase lacks efficacy under flow conditions. Integr Biol. 2010;2:183–92. doi: 10.1039/b919728a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Eng J. Receiver Operating Characteristic Analysis. Academic Radiology. 2005;12:909–916. doi: 10.1016/j.acra.2005.04.005. [DOI] [PubMed] [Google Scholar]
- [15].Gurbel PA, Bliden KP, DiChia J, et al. Evaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation. 2007;115:3156–3164. doi: 10.1161/CIRCULATIONAHA.106.675587. [DOI] [PubMed] [Google Scholar]
- [16].Rinder CS, Student LA, Bonan JL, et al. Aspirin does not inhibit adenosine diphosphate-induced platelet alpha-granule release. Blood. 1993;82:505–512. [PubMed] [Google Scholar]
- [17].Lucitt MB, O’Brien S, Cowman J, et al. Assaying the efficacy of dual-antiplatelet therapy: use of a controlled-shear-rate microfluidic device with a well-defined collagen surface to track dynamic platelet adhesion. Anal Bioanal. 2013;14:4823–4834. doi: 10.1007/s00216-013-6897-y. [DOI] [PubMed] [Google Scholar]
- [18].Mendolicchio GL, Zavalloni D, Bacci M, et al. Variable effect of P2Y12 inhibition on platelet thrombus volume in flowing blood. J Thromb Haemost. 2012;9:373–382. doi: 10.1111/j.1538-7836.2010.04144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bhatt D, Stone GW, Mahaffey KW, et al. Effect of Platelet Inhibition with Cangrelor during PCI on Ischemic Events. N Engl J Med. 2013;368:1303–1313. doi: 10.1056/NEJMoa1300815. [DOI] [PubMed] [Google Scholar]
- [20].Michelson AD. Platelet Function Testing in Cardiovascular Diseases. Circulation. 2004;110:489–493. doi: 10.1161/01.CIR.0000147228.29325.F9. [DOI] [PubMed] [Google Scholar]
- [21].Jeong Y, Bliden KP, Antonino MJ, et al. Usefulness of the VerifyNow P2Y12 assay to evaluate the antiplatelet effects of ticagrelor and clopidogrel therapies. Am Heart J. 2012;164:35–42. doi: 10.1016/j.ahj.2012.03.022. [DOI] [PubMed] [Google Scholar]
- [22].Hosokawa K, Ohnishi T, Fukasawa M, et al. A novel automated microchip flow chamber system to quantitatively evaluate thrombus formation and antithrombotic agents under blood flow conditions. Microvasc Res. 2011;83:154–61. doi: 10.1111/j.1538-7836.2011.04464.x. [DOI] [PubMed] [Google Scholar]
- [23].Neeves KB, Onasogoa AA, Hansen RR, et al. Sources of Variability in Platelet Accumulation on Type 1 Fibrillar Collagen in Microfluidic Flow Assays. PLoS One. 2013;8:e54680. doi: 10.1371/journal.pone.0054680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.