Abstract
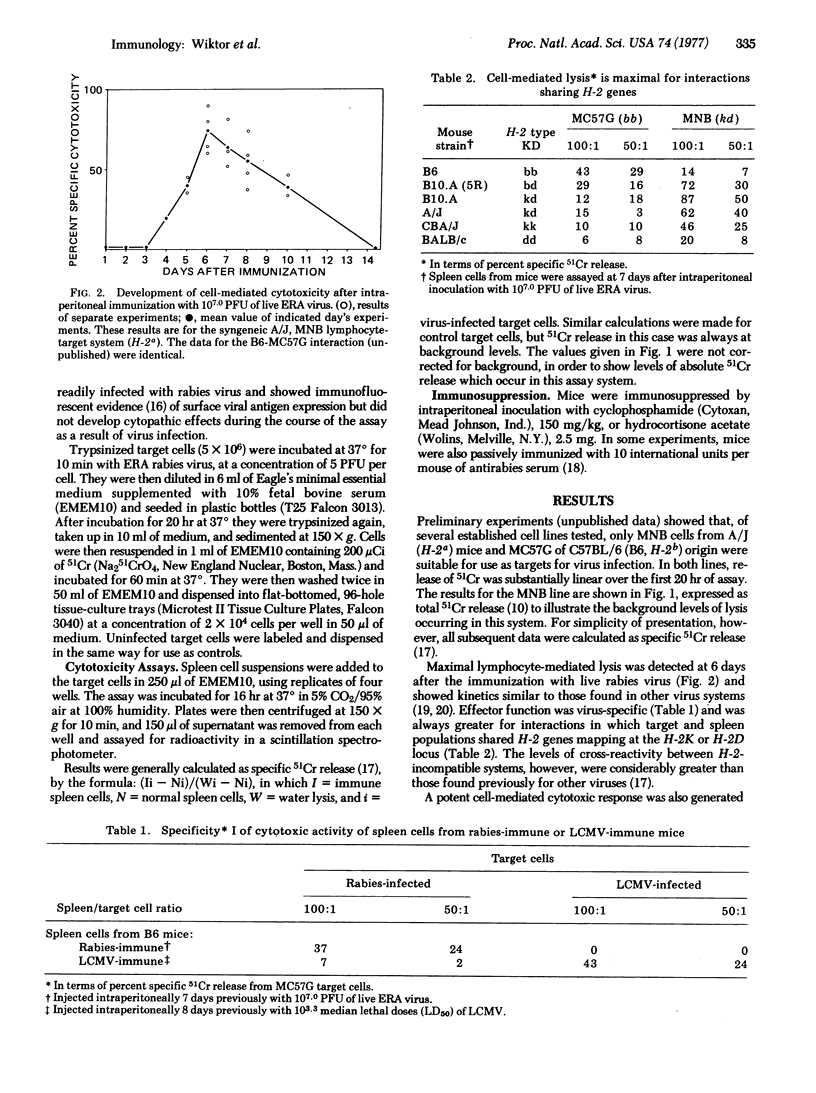
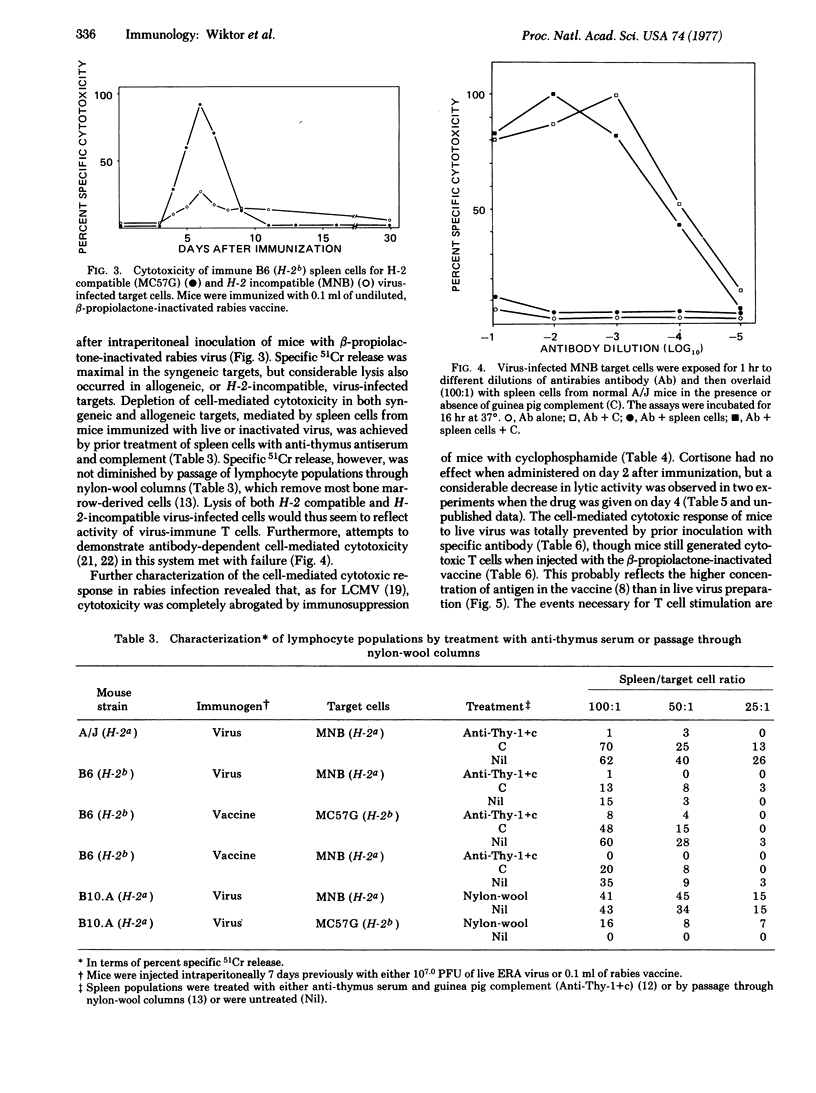
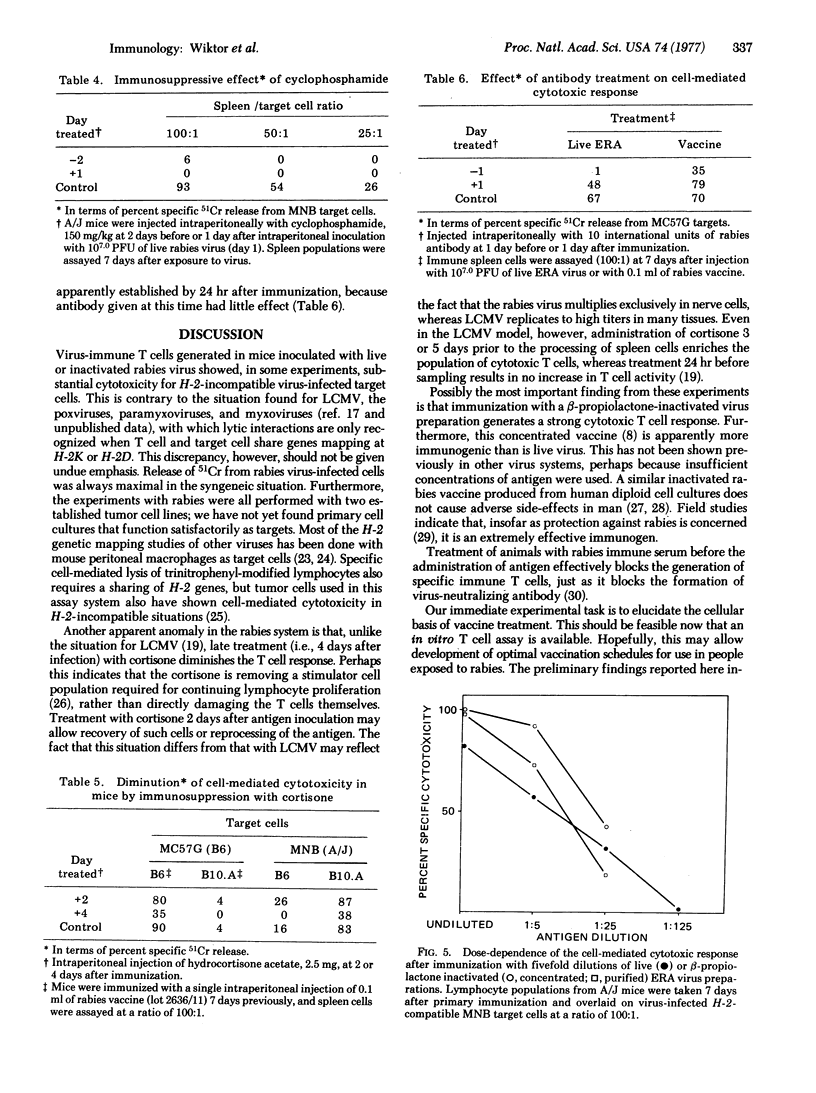
Mice exposed to live or beta-propiolactone-inactivated rabies virus generated a strong, specific cell-mediated cytotoxic response which was generally maximal 6 days after inoculation. Release of 51Cr was apparently a function of immune thymus-derived lymphocytes (T cells) because it was abrogated by prior incubation of spleen cells with anti-thymus antiserum and complement but was undiminished by passage of spleen cells through nylon-wool columns. Cytotoxicity was always maximal for interactions in which thymus-derived cells and targets shared H-2 genes but, unlike the situation found in other assays of this type, considerable lysis of allogeneic, virus-infected target cells may also occur. Perhaps the most significant finding from these experiments is that an inactivated virus has been shown to stimulate a potent cytotoxic thymus-derived cell response. Manipulation of this experimental model may allow analysis of the antigens required for stimulation of cell-mediated immunity. A more practical consequence may be the development of more rational protocols for postexposure vaccination against rabies. Prior treatment of mice with antirabies antibody severely depressed the generation of cell-mediated immunity.
Full text
PDF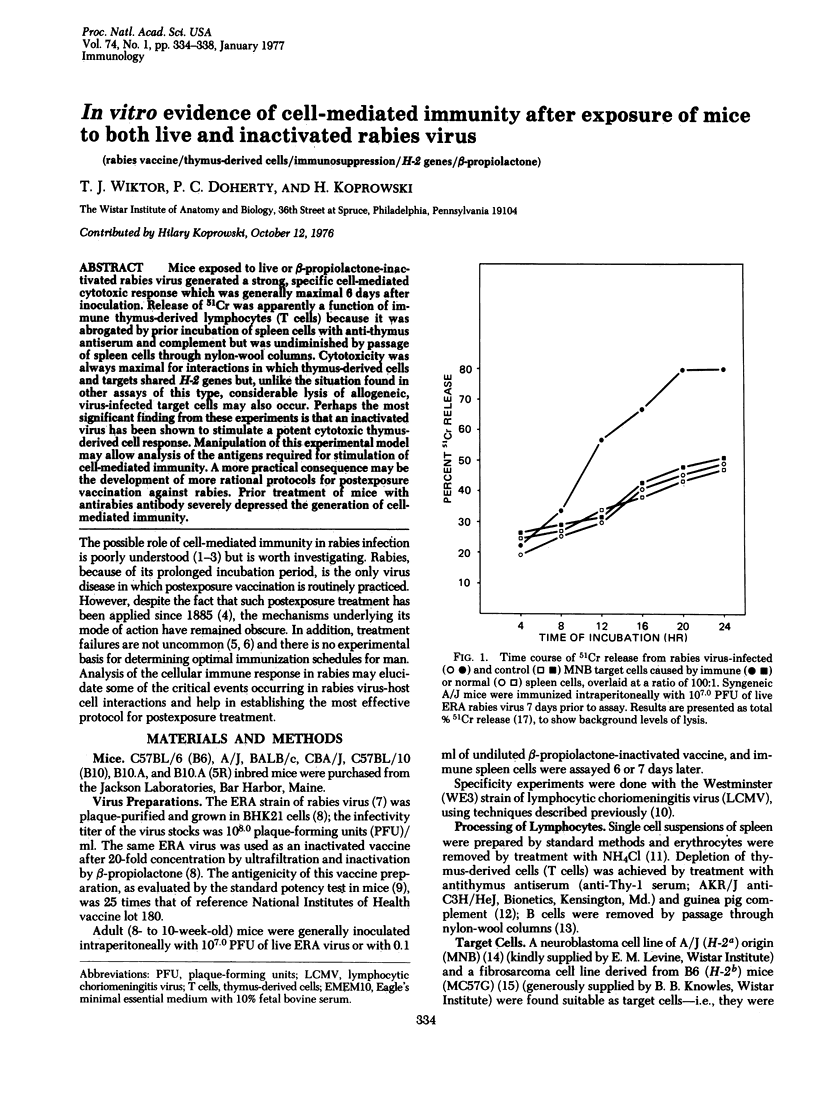

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. A., Daly F. T., Jr, Kidd J. C. Human rabies after antiserum and vaccine postexposure treatment. Case report and review. Ann Intern Med. 1966 Jun;64(6):1297–1302. doi: 10.7326/0003-4819-64-6-1297. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Doherty P. C., Dunlop M. B., Gardner I. D., Zinkernagel R. M., David C. S. Genes required for cytotoxicity against virus-infected target cells in K and D regions of H-2 complex. Nature. 1975 Mar 20;254(5497):269–270. doi: 10.1038/254269a0. [DOI] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Blanden R. V., Zinkernagel R. M. Specificity of virus-immune effector T cells for H-2K or H-2D compatible interactions: implications for H-antigen diversity. Transplant Rev. 1976;29:89–124. doi: 10.1111/j.1600-065x.1976.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M., Ramshaw I. A. Specificity and development of cytotoxic thymus-derived lymphocytes in lymphocytic choriomeningitis. J Immunol. 1974 Apr;112(4):1548–1552. [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. Specific immune lysis of paramyxovirus-infected cells by H-2-compatible thymus-derived lymphocytes. Immunology. 1976 Jul;31(1):27–32. [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. T-cell-mediated immunopathology in viral infections. Transplant Rev. 1974;19(0):89–120. doi: 10.1111/j.1600-065x.1974.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Hattwick M. A., Hochberg F. H., Landrigan P. J., Gregg M. B. Skunk-associated human rabies. JAMA. 1972 Oct 2;222(1):44–47. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Wiktor T. J., Koprowski H. Pathogenesis of rabies in immunodeficient mice. J Immunol. 1975 Jun;114(6):1761–1765. [PubMed] [Google Scholar]
- MacLennan I. C. Antibody in the induction and inhibition of lymphocyte cytotoxicity. Transplant Rev. 1972;13:67–90. doi: 10.1111/j.1600-065x.1972.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Miller C. A., Levine E. M. Effects of aluminum salts on cultured neuroblastoma cells. J Neurochem. 1974 May;22(5):751–758. doi: 10.1111/j.1471-4159.1974.tb04290.x. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Perlmann H., Wigzell H. Lymphocyte mediated cytotoxicity in vitro. Induction and inhibition by humoral antibody and nature of effector cells. Transplant Rev. 1972;13:91–114. doi: 10.1111/j.1600-065x.1972.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Plotkin S. A., Wiktor T. J., Koprowski H., Rosanoff E. I., Tint H. Immunization schedules for the new human diploid cell vaccine against rabies. Am J Epidemiol. 1976 Jan;103(1):75–80. doi: 10.1093/oxfordjournals.aje.a112207. [DOI] [PubMed] [Google Scholar]
- Schlumberger H. D., Wiktor T. J., Koprowski H. Antigenic and immunogenic properties of components contained in rabies virus-infected tissue culture fluids. J Immunol. 1970 Aug;105(2):291–298. [PubMed] [Google Scholar]
- Turner G. S. Humoral and cellular immune responses of mice to rabies and smallpox vaccines. Nat New Biol. 1973 Jan 17;241(107):90–92. doi: 10.1038/newbio241090a0. [DOI] [PubMed] [Google Scholar]
- Wiktor T. J., Kamo I., Koprowski H. In vitro stimulation of rabbit lymphocytes after immunization with live and inactivated rabies vaccines. J Immunol. 1974 Jun;112(6):2013–2019. [PubMed] [Google Scholar]
- Wiktor T. J., Kuwert E., Koprowski H. Immune lysis of rabies virus-infected cells. J Immunol. 1968 Dec;101(6):1271–1282. [PubMed] [Google Scholar]
- Wiktor T. J., Lerner R. A., Koprowski H. Inhibitory effect of passive antibody on active immunity induced against rabies by vaccination. Bull World Health Organ. 1971;45(6):747–753. [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Plotkin S. A., Grella D. W. Human cell culture rabies vaccine. Antibody response in man. JAMA. 1973 May 21;224(8):1170–1171. [PubMed] [Google Scholar]
- Wiktor T. J., Sokol F., Kuwert E., Koprowski H. Immunogenicity of concentrated and purified rabies vaccine of tissue culture origin. Proc Soc Exp Biol Med. 1969 Jul;131(3):799–805. doi: 10.3181/00379727-131-33981. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Characteristics of the interaction in vitro between cytotoxic thymus-derived lymphocytes and target monolayers infected with lymphocytic choriomeningitis virus. Scand J Immunol. 1974;3(3):287–294. doi: 10.1111/j.1365-3083.1974.tb01259.x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. H-2 compatability requirement for T-cell-mediated lysis of target cells infected with lymphocytic choriomeningitis virus. Different cytotoxic T-cell specificities are associated with structures coded for in H-2K or H-2D;. J Exp Med. 1975 Jun 1;141(6):1427–1436. doi: 10.1084/jem.141.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Peritoneal macrophages as target cells for measuring virus-specific T cell mediated cytotoxicity in vitro. J Immunol Methods. 1975 Sep;8(3):263–266. doi: 10.1016/0022-1759(75)90120-9. [DOI] [PubMed] [Google Scholar]



