Abstract
There has been significant interest in understanding how interactions between the host immune system and the gut microbiota regulate intestinal homeostasis. Recent data suggest that the Nod-like receptor family of pattern recognition receptors regulate both the composition of the gut microbiota and innate immune signaling pathways that prevent pathologic intestinal inflammation and tumorigenesis. In this review, we will focus on NLRP6 and NLRP12, two members of the Nod-like receptor family that have emerged as important players in the maintenance of intestinal homeostasis, and discuss the signaling pathways engaged by these receptors as well as the current models of how these receptors protect against the development of colitis and tumorigenesis.
Introduction
Since the discovery that host bacterial recognition pathways are critical for maintaining intestinal homeostasis [1], there have been numerous studies demonstrating how members of the Nod-like receptor (NLR) family play an important role in both promoting host defense against invasive pathogens and reducing host susceptibility to chemically-induced colitis and subsequent tumorigenesis [2–9]. NLRs have been traditionally considered as pattern-recognition receptors (PRRs), in that they are activated in response to conserved structural motifs found in microbes or pathogen-associated molecular patterns (PAMPs), such as peptidoglycan or flagellin [10, 11]. More recently, NLRs, particularly NLRP3, have been implicated in recognizing endogenous stimuli related to cellular injury, or damage-associated molecular patterns (DAMPs), which can result in sterile inflammation[12]. NLRs are characterized by a tripartite structure consisting of i) a variable N-terminal protein-protein interaction domain, ii) a central nucleotide-binding oligomerization (NOD) domain that mediates the self-oligomerization occurring during activation [13], and iii) a C-terminal leucine-rich repeat involved in ligand specificity. The N-terminal domain of an NLR can be defined as a caspase recruitment domain (CARD), pyrin domain (PYD), acidic, transactivating domain or baculovirus inhibitor repeat (BIR). There are at least 23 identified human NLRs, and 34 NLRs have been identified in mice. A standardized nomenclature system [14] categorizes the NLR family into four subfamilies based on the type of N-terminal domain. Two NLRs in particular (NLRP6 and NLRP12), both highly expressed in the intestine, act as negative regulators of intestinal inflammation and tumorigenesis [7–9, 15, 16]. Nlrp6 and Nlrp12 belong to the subfamily of NLRs that contain an N-terminal (PYD), which can interact with other PYD-containing proteins that are important for downstream signaling events. Multiple members of the NLR family, including NOD1, NOD2, NLRC4, and NLRP3, have been implicated in maintaining intestinal homeostasis [2, 4, 15, 17, 18]. These members are relatively well-characterized and the nature of their upstream ligands have been identified. In this review, we will focus on the recently recognized roles of NLRP6 and NLRP12, two NLRs whose upstream agonist has not yet been identified, in the protection against intestinal inflammation and tumorigenesis.
NLRP6 and NLRP12 participate in multiple signaling pathways
Early in vitro studies have implicated both NLRP6 and NLRP2 in inflammasome formation [19, 20]. Inflammasomes are multiprotein complexes whose assembly is mediated by the adaptor protein apoptosis-associated speck-like protein (ASC). ASC possesses both a carboxy terminal, CARD, and a PYD, and therefore is capable of interacting with NLRs that also contain a PYD domain through homophilic protein-protein interactions. The current model of inflammasome assembly hypothesizes that NLR activation by its agonist results in oligomerization through the NOD domain of the receptor. Subsequently, through CARD-CARD and PYD-PYD protein-protein interactions, a large macromolecular complex is assembled, which serves as a platform for procaspase-1 recruitment. After recruitment to the inflammasome complex, procaspase-1 self-cleaves into active caspase-1 [21]. Caspase-1 then cleaves pro-IL-1β and pro-IL-18 into their mature and active forms [22].
Both NLRP6 and NLRP12 have been demonstrated to co-localize with ASC in a characteristic speckled pattern within the cytoplasm. Co-localization is dependent on the presence of the PYD in both NLRP6 and NLRP12 [19, 20]. Thus, both NLRP6 and NLRP12 have been considered as members of the inflammasome. However, in these studies, which employed overexpression of NLR proteins, a direct physical interaction between ASC and either NLRP6 or NLRP12 could not be demonstrated. This may reflect the poor solubility of ASC upon oligomerization after activation [23]. Alternatively, the interaction between ASC and NLRP6 or NLRP12 may be highly transient in nature. Nonetheless, coexpression of ASC and NLRP6 in COS-7L cells in vitro resulted in increased IL-1β production that was dependent on the availability of caspase-1 and the NLRP6 PYD [20]. Similarly, NLRP12 has been demonstrated to cooperate with ASC to promote caspase-1 activation and production of IL-1β in a PYD-dependent manner [19]. Consistent with a role for NLRP12 in the production of IL-1β and IL-18, NLRP12-deficient mice have increased mortality to infection by an attenuated strain of Yersinia pestis in comparison to WT mice [24]. In this model, IL-1β production by bone marrow-derived macrophages (BMDMs) and serum IL-18 levels are decreased in NLRP12-deficient mice. However, IL-1β and IL-18 production are not completely abolished, suggesting redundancy in cytokine production pathways. Reduced IL-18 production was also observed in NLRP6-deficient mice, which was associated with increased susceptibility to chemically-induced colitis and colitis-associated tumorigenesis (discussed further below) [7–9]. Evidence for the involvement of NLRP6 and NLRP12 in inflammasome formation and activation remains largely derived from in vitro studies in which these proteins are overexpressed. To date, the ligand that stimulates NLRP6 or NLRP12 activity remains unknown. Thus, direct evidence that NLRP6 or NLRP12 activate the inflammasome under physiologic conditions in vivo has been difficult to obtain.
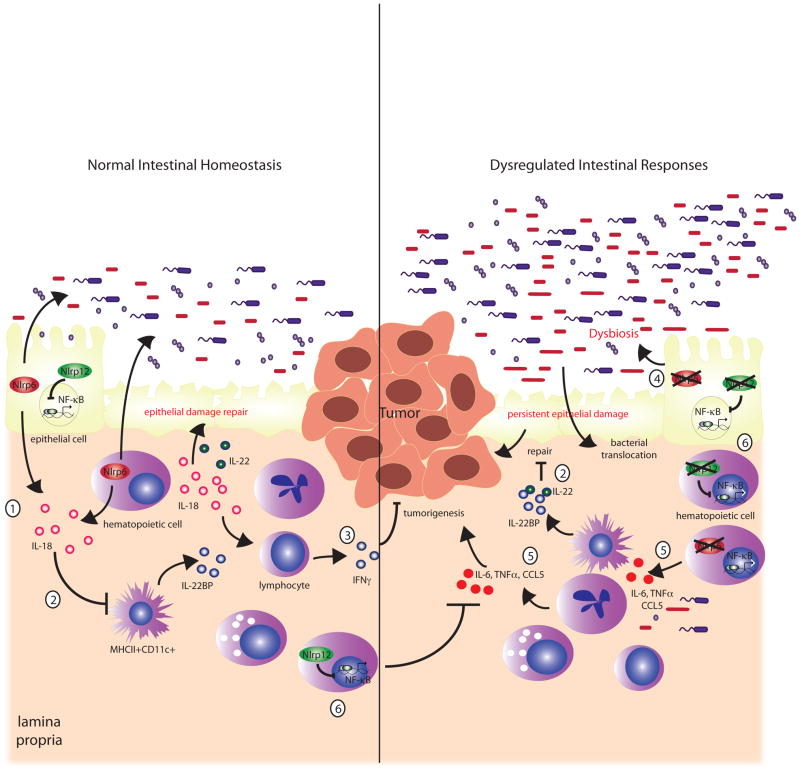
In addition to a potential role in inflammasome signaling, in vitro studies have also suggested that NLRP6 and NLRP12 are positioned upstream of NF-κB and MAPK in cell signaling pathways (Figure 1). Although NLRP6 and NLRP12 are both unable to activate NF-κB alone in luciferase reporter assays, there is synergistic activation of NF-κB together with ASC when either NLRP6 or NLRP12 were co-transfected with ASC in 293 T cells [19, 20]. More recently, NLRP6 was shown to be a negative regulator of canonical NF-κB signaling. Specifically, stimulation of NLRP6-deficient BMDMs with bacterial Toll-like receptor (TLR)-2 and TLR4 ligands, results in significantly upregulated NF-κB and MAPK (ERK) signaling, culminating in increased cytokine production such as TNF-α and IL-6 [25]. Consequently, NLRP6-deficient mice are resistant to systemic bacterial infection, resulting from greater induction of proinflammatory cytokines, increased bacterial clearance and survival [25]. However, whether NLRP6 also participates in host defense against gastrointestinal infections remains to be investigated.
Figure 1. Multiple mechanisms involved in suppressing inflammation and tumorigenesis by NLRP6 and NLRP12.
NLRP6 regulates IL-18 production, which helps maintain a healthy microbiome and promotes epithelial repair (1). IL-18 also downregulates IL-22BP (2), thereby allowing IL-22 to participate in epithelial proliferation and repair of epithelial damage. NLRP6-deficiency is also associated with persistent IL-22BP production by primarily MCHII+CD11c+ cells [35], which results in inhibition of the reparative activity of IL-22 (2). IL-18 potentially upregulates IFN-γ production, which promotes anti-tumor responses (3). In the absence of Nlrp6, the microbiome is altered, leading to the accumulation of potentially colitogenic bacteria, or dysbiosis (4), which can upregulate inflammatory factors, including CCL5 to promote inflammation and tumorigenesis especially in the presence of a damaged epithelial barrier (5). The persistence of epithelial damage allows bacterial translocation into the colonic mucosa to stimulate an aberrant inflammatory response by immune cells that promote inflammation and tumorigenesis (5). Similarly, NLRP12 negatively regulates NF-κB responses (6) although there are discordant observations regarding canonical versus non-canonical NF-kB pathways. NLRP12 deficiency is also associated with increased inflammation followed by tumorigenesis related to uncontrolled NF-κB signaling in either hematopoietic and/or nonhematopoietic cells (6).
NLRP12 has also been shown to downregulate NF-κB responses to TLR agonists, and its expression is reduced in response to TLR stimulation in vitro in THP-1 cells [16, 26]. However, it is unclear whether NRLP12 acts primarily on the non-canonical versus canonical pathway. Early in vitro studies implementing siRNA knockdown of NLRP12 expression or protein overexpression suggested that NLRP12 negatively regulates the non-canonical NF-κB pathway. More specifically, NLRP12 directly associates with the NF-κB-inducing kinase (NIK) through its NOD and LRR domains, leading to proteasome-mediated degradation of NIK [27]. NIK induces NF-κB activation by inducing the phosphorylation of the non-canonical NF-κB effector p100, which is then processed into the p52 subunit that transcriptionally activates p52-regulated genes. Other in vitro protein overexpression studies show that NLRP12 also downregulates TLR-mediated pathways through association with IRAK-1 [26]. Furthermore, knockdown of NLRP12 expression by siRNAs in THP-1 cells results in higher expression of IL-6 in response to TLR ligands as well as to Mycobacterium tuberculosis [26]. However, these in vitro results were not reproduced in in vivo models of pulmonary infection with Mycobacterium tuberculosis or Klebsiella pneumonia [28]. Specifically, although TNF-α and IL-6 production in bone marrow-derived dendritic cells (BMDCs) were significantly elevated in NLRP12-deficient cells compared with that in WT cells, NLRP12-deficient mice did not exhibit any differences in disease outcome compared with WT mice [28], suggesting that other NLRs may be able to substitute for NLRP12. In the gastrointestinal tract, NLRP12 negatively regulates inflammatory responses during chemically-induced colitis [15, 16](discussed further below). Although these studies point to a role for NLRP12 in regulating NF-κB, at least one study has demonstrated no significant difference in NF-κB activation in response to Yersinia or TNF-α [24]. It is therefore possible that the nature of the stimulus and the specific context in which it is given is important in revealing a significant function for NLRP12 in NFκB regulation.
Nlrp6 maintains intestinal homeostasis by regulating the gut microbiome
A significant role for the gut microbiota in dictating host susceptibility to intestinal disease has emerged. This is highlighted by studies showing transmissibility of intestinal disease by co-housing of laboratory mice [7, 17, 29, 30]. Dextran sulfate sodium (DSS) can be used to induce a disease model of colitis, characterized by mucosal ulceration and disruption of the epithelial barrier that results in commensal-driven inflammation arising from bacterial translocation into the mucosa. Mice deficient in several of the NLRs have increased susceptibility to DSS-induced colitis associated with microbiome changes distinct from that in WT mice [7, 17, 31], including NLRP6. NLRP6-deficient mice developed more severe DSS-induced colitis compared with WT mice; however, after co-housing WT and NLRP6-deficient mice together for 4 weeks, WT mice acquire the same phenotype as NLRP6-deficient mice [7]. Microbiome profiles obtained by 16s rRNA sequencing revealed distinct microbial communities in WT and NLRP6-deficient mice [7]. In fact, NLRP6-deficient and ASC-deficient mice exhibit similar bacterial community profiles, suggesting an inflammasome-dependent regulation of the composition of the gut microbiota. Interestingly, mice deficient in other inflammasome-related NLRs, such as NLRC4, NLRP10, NLRP12, and AIM2, do not affect the susceptibility of WT mice to colitis after co-housing [7]. Notably, bacterial phylotypes that are significantly different between WT and NLRP6-deficient mice include Prevotella, and a member of the family Prevotellaceae as well as the phylum TM7 [7]. Antibiotic treatment of Nlrp6−/− mice results in the disappearance of both Prevotellaceae and TM7 and is associated with the amelioration of DSS-induced colitis [7]. IL-18-deficient mice, but not IL-1R-deficient mice, are also able to transmit increased susceptibility to colitis to WT mice. Moreover, the Nlrp6−/− mice used in this study have decreased levels of IL-18 in the serum and colon at baseline, suggesting that the altered microbiome in NLRP6-deficient mice is related to defective IL-18 production [7]. Changes in the microbiome not only affect susceptibility to colitis, but also to inflammation-associated tumorigenesis under the AOM/DSS model, in which mice are given an intraperitoneal injection of the carcinogen azoxymethane followed by multiple rounds of DSS treatment [7, 30]. This phenotype is shared by both NLRP6-deficient and ASC-deficient mice and is transmissible to WT mice. The Nlrp6−/− mice used in both colitis and tumor development studies have elevated levels of CCL5 at baseline, associated with abnormal crypt hyperplasia and increased immune cell infiltration. When CCL5-deficient mice are co-housed with Nlrp6−/− mice, CCL5-deficient mice do not develop increased colitis or tumors, suggesting that the dysbiotic microbiota promotes both inflammation and tumorigenesis that is mediated by CCL5 (Figure 1) [7, 30].
The above results are exciting in that they suggest that certain bacteria within the micriobiota are potentially colitogenic or tumorigenic and the accumulation of these bacteria is regulated by NLRP6 and the inflammasome. This indicates possible therapeutic targets for the treatment or prevention of inflammatory bowel disease and colitis-associated colorectal cancer. However, the results of these studies should also be interpreted with some caution. First, the community structure of the gut microbiome in IL-18-deficient mice remains distinct from that of NLRP6-deficient mice, and therefore it is likely that additional factors contribute to the microbiome of Nlrp6-deficient mice. In addition, Asc−/− mice cross-fostered with WT mothers do not develop more severe colitis compared with non-cross-fostered Asc−/− mice, suggesting that ASC deficiency alone is insufficient for the development of a dysbiotic microbiome capable of increasing disease severity, and that environmental rather than genetic factors contribute to microbiome composition. Finally, alterations in colon crypt morphology and impaired serum levels of IL-18 were not observed in colonies of NLRP6-deficient mice at other institutions [8], suggesting that there may be colony-dependent effects. As previous studies evaluating TLR-deficient mice determined that the composition of the microbiome is largely dependent on maternal transmission of the microbiota from isolated mouse colonies rather than on any TLR defect [32], it remains possible that the observed microbiome changes in NLRP6-deficient mice [7] are related more to differences in ancestry rather than to an immune defect. Additional experiments with germfree WT and Nlpr6−/− mice may provide additional insight into the relationship between NLRP6 and the gut microbiome.
NLRP6 has also been implicated in the regulation of the gut microbiome to affect stress-induced small intestinal disease in the water-avoidance stress (WAS) model of irritable bowel syndrome [32], in which mice are regularly placed on a platform surrounded by water for 10 days. Inflammation, interestingly, is limited to the small intestine and is associated with a reduction in NLRP6 expression in the epithelium and decreased IL-1β and IL-18 production [33]. This phenotype is also transmissible to co-housed WT mice not subjected to water avoidance stress, and 16s rRNA sequencing revealed distinct microbiome changes in the luminal contents of the small intestine with changes in relative abundance in multiple bacterial families. Pretreatment of mice with a Lactobacillus-containing probiotic significantly reduced the severity of WAS-induced intestinal inflammation and also ‘normalized’ the microbiome. It remains to be determined whether the observed microbiome changes are mediated by NLRP6 signaling and directly cause intestinal inflammation, rather than are a consequence of it.
NLRP6 maintains intestinal homeostasis by regulating epithelial repair and proliferation
As discussed above, impaired IL-18 production may be associated with the development of an altered microbiota, or dysbiosis, that predisposes to colitis and tumorigenesis. However, IL-18 also promotes epithelial repair by i) MyD88 signaling [1, 34]; and ii) downregulating the expression of IL-22 binding protein (IL-22BP) [35] (Figure 1). IL-22BP interacts with IL-22, a cytokine which promotes epithelial proliferation during wound healing, to prevent it from binding to its cognate receptor [36, 37]. Indeed, IL-18-deficient mice have more severe DSS-induced colitis, which is associated with impaired epithelial repair [4]. Consistently, mice deficient in caspase-1 and ASC also have decreased IL-18 production and greater severity of colitis compared with WT mice, a condition which can be rescued by the administration of recombinant IL-18 to caspase-1−/− mice [4]. Caspase-1 and IL-18-deficent mice also develop more inflammation-associated tumors, with the former exhibiting decreased IFN-γ and STAT1 signaling, both of which are important for anti-tumor responses [5]. NLRP6-deficient mice also have decreased IL-18 levels in the serum and in the colon tissue during the acute inflammatory response to DSS in the AOM/DSS model, which may explain defects in epithelial restitution observed in these mice [8]. Consistent with this hypothesis, NLRP6-deficient mice also fail to downregulate IL-22BP after DSS-induced injury in the AOM/DSS tumor model [35]. Interestingly, although studies suggest that NLRP6 is expressed primarily in the epithelium [7], bone marrow chimeric experiments suggest that Nlrp6 functions in the hematopoietic cell compartment rather than in non-hematopoietic cells [8]. Consistent with this observation, NLRP6 has been implicated in negatively regulating inflammatory responses in immune cells (discussed further below). In addition, Nlrp6 mRNA expression is inducible such as by PPAR-γ agonists [33, 38], and whether this occurs in hematopoietic cells in response to an as yet unidentified ligand to suppress tumorigenesis remains to be determined.
As a consequence of poor intestinal repair of DSS-induced epithelial damage, the epithelium barrier remains compromised in NLRP6-deficient mice, resulting in significantly elevated levels of proinflammatory, protumorigenic cytokines and chemokines, likely in response to translocated bacteria [8]. Gene expression profiling of tumor and normal tissue in NLRP6-deficient and WT mice also demonstrated that NLRP6 is important for the negative regulation of factors involved in epithelial proliferation such as Wnt and Notch target genes [9]. Thus, although NRLP6-deficient mice exhibit increased inflammatory responses, consistent with a negative regulatory role in NF-κB and MAPK signaling as demonstrated in in vivo infection models [25], its role within the intestine is likely more related to the regulation of IL-18 production.
NLRP12 maintains intestinal homeostasis by negatively regulating inflammatory responses
Similar to NLRP6, NLRP12 also plays an important role in protecting against the development of DSS-induced colitis and inflammation-associated tumorigenesis with AOM/DSS [15, 16] (Figure 1). In contrast to NLRP6, NLRP12-deficient mice are unable to transmit colitis susceptibility to WT mice after co-housing, suggesting that a mechanism distinct from microbiome regulation is responsible for its protective function [7]. Indeed, consistent with a role as a negative regulatory of NF-κB and MAPK responses, NLRP12-deficient mice develop more severe colitis than WT mice. However, the precise mechanism by which NLRP12 negatively regulates NF-κB to protect against colitis and colitis-associated tumorigemnesis remains to be determined as the two groups that reported this phenotype suggest disparate mechanisms [15, 16].. In both studies, the colitis in Nlrp12−/− mice is associated with significantly increased extent of disease, the production of proinflammatory cytokines and/or chemokines and the activation of NF-κB and ERK signaling in the colon [15, 16]. However, bone marrow chimeric experiments performed in the study by Zaki et al. [16] suggest that NLRP12 functions in the hematopoietic cell compartment to suppress tumorigenesis (Figure 1). Moreover, the increased inflammatory responses are related to increased canonical and not non-canonical NFκB activation. This is consistent with the observation that NLRP12-deficient BMDMs exhibit increased canonical, but not non-canonical, NF-κB and ERK activation in response to TLR ligands since excessive inflammatory responses to commensal bacteria in the setting of a breached epithelial barrier from DSS-induced colitis contributes to increased colitis and tumorigenesis. However, bone marrow chimeric experiments by Allen et al. [15]suggest that although NLRP12 function in both the hematopoietic and non-hematopoietic compartments is important for early disease manifestations of DSS-induced colitis, the non-hematopoietic compartment is ultimately important for limiting tumor numbers [15]. Furthermore, disease severity in NLRP12-deficient mice is predominantly associated with dysregulation of the non-canonical rather than canonical NF-κB pathway, consistent with previous studies by the same group demonstrating that NLRP12 interacts with NIK to regulate the p52 subunit of NF-κB [26]. The differences between studies can be partially reconciled by the fact that the study by Allen et al. also observed increased canonical NF-κB signaling in BMDCs after TLR ligand exposure, in NLRP12-defiicent mice; moreover, non-canonical NF-κB signaling can influence both the canonical pathway and MAPK signaling [39–41]. The discrepancy in findings regarding whether NLRP12 is important in the hematopoietic or non-hematopoietic compartments is more difficult to explain. The analysis of NF-κB activation was in different cell types (BMDMs vs BMDCs), and there may have been differences in the colonies of Nlrp12−/− mice (e.g., the microbiota) that may have influenced the outcome of the bone marrow chimeric experiments. Regardless, taken together, these studies suggest an important role for NLRP12 in limiting inflammatory responses within the colon.
Conclusions
Exciting progress has been made in understanding how host immune receptors promote intestinal health. However, as demonstrated above, more than one mechanism may be important. Both NLRP6 and NLRP12 play protective roles in limiting the development of chemically-induced injury and carcinogenesis through regulation of IL-18 production, NF-κB and MAPK-dependent inflammatory responses, and prevention of dysbiosis. However, additional confirmatory experiments are needed to clarify the relative contributions of these different mechanisms in the regulation of intestinal homeostasis and to determine whether other mechanisms are involved. Discrepancies on the identity of the cell type(s) important for NLRP6 or NLRP12 function within the intestine and the differential impact of NLRP12 on canonical versus canonical NF-κB signaling remain. Moreover, our understanding of NRLP6 and NLRP12 function in intestinal homeostasis has been based entirely on one model of colitis involving DSS-induced epithelial injury. As additional mechanisms can be involved in the development of inflammatory bowel disease in humans, it would be important to also determine the effect of NLRP6 or NLRP12 deficiency in other models of colitis. A role for NLRP6 or NLRP12 in host defense against infections involving the intestine also remains to be investigated. Further advances in understanding the mechanism by which these two NLRs protect the intestine will be aided by the identification of the ligand or stimulus recognized by each NLR. This will also facilitate the development of therapeutics that modulate NLRP6 and NLRP12 function within the intestine to prevent or treat inflammation and cancer.
Acknowledgments
Many thanks to Gabriel Nunez and Luigi Franchi for thoughtful discussions and critical reading of the manuscript. The author’s research is supported by the American Cancer Society Research Scholar Grant, National Institutes of Health R01 CA166879, and a GI SPORE Developmental Research Project Grant
Footnotes
The author declares no commercial or financial conflicts of interest.
References
- 1.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Chen GY, Shaw MH, Redondo G, Nunez G. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 2008;68:10060–10067. doi: 10.1158/0008-5472.CAN-08-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R, Huot L, Grandjean T, Bressenot A, Delanoye-Crespin A, Gaillot O, Schreiber S, Lemoine Y, Ryffel B, Hot D, Nunez G, Chen G, Rosenstiel P, Chamaillard M. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. 2013 doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol. 2010;185:4912–4920. doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, Ting JP. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen GY, Liu M, Wang F, Bertin J, Nunez G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J Immunol. 2011;186:7187–7194. doi: 10.4049/jimmunol.1100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, Peyrin-Biroulet L, Lemoine Y, Hot D, Chamaillard M. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc Natl Acad Sci U S A. 2011;108:9601–9606. doi: 10.1073/pnas.1100981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 11.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 12.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inohara N, Koseki T, Lin J, del Peso L, Lucas PC, Chen FF, Ogura Y, Nunez G. An induced proximity model for NF-kappa B activation in the Nod1/RICK and RIP signaling pathways. J Biol Chem. 2000;275:27823–27831. doi: 10.1074/jbc.M003415200. [DOI] [PubMed] [Google Scholar]
- 14.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, Hoffman HM, Hugot JP, Inohara N, Mackenzie A, Maltais LJ, Nunez G, Ogura Y, Otten LA, Philpott D, Reed JC, Reith W, Schreiber S, Steimle V, Ward PA. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, Woodford RM, Davis BK, Uronis JM, Herfarth HH, Jobin C, Rogers AB, Ting JP. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity. 2012;36:742–754. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaki MH, Vogel P, Malireddi RK, Body-Malapel M, Anand PK, Bertin J, Green DR, Lamkanfi M, Kanneganti TD. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20:649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R, Huot L, Grandjean T, Bressenot A, Delanoye-Crespin A, Gaillot O, Schreiber S, Lemoine Y, Ryffel B, Hot D, Nunez G, Chen G, Rosenstiel P, Chamaillard M. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. 2013;123:700–711. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C, Eisenbarth SC, Flavell RA. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci U S A. 2010;107:21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, Lora JM, Geddes BJ, Briskin M, DiStefano PS, Bertin J. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem. 2002;277:29874–29880. doi: 10.1074/jbc.M203915200. [DOI] [PubMed] [Google Scholar]
- 20.Grenier JM, Wang L, Manji GA, Huang WJ, Al-Garawi A, Kelly R, Carlson A, Merriam S, Lora JM, Briskin M, DiStefano PS, Bertin J. Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-kappaB and caspase-1. FEBS Lett. 2002;530:73–78. doi: 10.1016/s0014-5793(02)03416-6. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem. 2002;277:21119–21122. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 22.Lamkanfi M, Kanneganti TD, Van Damme P, Vanden Berghe T, Vanoverberghe I, Vandekerckhove J, Vandenabeele P, Gevaert K, Nunez G. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics. 2008;7:2350–2363. doi: 10.1074/mcp.M800132-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandes-Alnemri T, Alnemri ES. Assembly, purification, and assay of the activity of the ASC pyroptosome. Methods Enzymol. 2008;442:251–270. doi: 10.1016/S0076-6879(08)01413-4. [DOI] [PubMed] [Google Scholar]
- 24.Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, Aune MH, Conlon JE, Burbage JJ, Proulx MK, Liu Q, Reed G, Mecsas JC, Iwakura Y, Bertin J, Goguen JD, Fitzgerald KA, Lien E. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity. 2012;37:96–107. doi: 10.1016/j.immuni.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anand PK, Malireddi RK, Lukens JR, Vogel P, Bertin J, Lamkanfi M, Kanneganti TD. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature. 2012;488:389–393. doi: 10.1038/nature11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams KL, Lich JD, Duncan JA, Reed W, Rallabhandi P, Moore C, Kurtz S, Coffield VM, Accavitti-Loper MA, Su L, Vogel SN, Braunstein M, Ting JP. The CATERPILLER protein monarch-1 is an antagonist of toll-like receptor-, tumor necrosis factor alpha-, and Mycobacterium tuberculosis-induced pro-inflammatory signals. J Biol Chem. 2005;280:39914–39924. doi: 10.1074/jbc.M502820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lich JD, Williams KL, Moore CB, Arthur JC, Davis BK, Taxman DJ, Ting JP. Monarch-1 suppresses non-canonical NF-kappaB activation and p52-dependent chemokine expression in monocytes. J Immunol. 2007;178:1256–1260. doi: 10.4049/jimmunol.178.3.1256. [DOI] [PubMed] [Google Scholar]
- 28.Allen IC, McElvania-TeKippe E, Wilson JE, Lich JD, Arthur JC, Sullivan JT, Braunstein M, Ting JP. Characterization of NLRP12 during the in vivo host immune response to Klebsiella pneumoniae and Mycobacterium tuberculosis. PLoS One. 2013;8:e60842. doi: 10.1371/journal.pone.0060842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, Gordon JI, Onderdonk AB, Glimcher LH. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu B, Elinav E, Huber S, Strowig T, Hao L, Hafemann A, Jin C, Wunderlich C, Wunderlich T, Eisenbarth SC, Flavell RA. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc Natl Acad Sci U S A. 2013;110:9862–9867. doi: 10.1073/pnas.1307575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirota SA, Ng J, Lueng A, Khajah M, Parhar K, Li Y, Lam V, Potentier MS, Ng K, Bawa M, McCafferty DM, Rioux KP, Ghosh S, Xavier RJ, Colgan SP, Tschopp J, Muruve D, MacDonald JA, Beck PL. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. 2011;17:1359–1372. doi: 10.1002/ibd.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ubeda C, Lipuma L, Gobourne A, Viale A, Leiner I, Equinda M, Khanin R, Pamer EG. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J Exp Med. 2012;209:1445–1456. doi: 10.1084/jem.20120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y, Zhang M, Chen CC, Gillilland M, 3rd, Sun X, El-Zaatari M, Huffnagle GB, Young VB, Zhang J, Hong SC, Chang YM, Gumucio DL, Owyang C, Kao JY. Stress-induced corticotropin-releasing hormone-mediated NLRP6 inflammasome inhibition and transmissible enteritis in mice. Gastroenterology. 2013;144:1478–1487. 1487 e1471–1478. doi: 10.1053/j.gastro.2013.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O’HUigin C, Marincola FM, Trinchieri G. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O’Connor W, Jr, Murphy AJ, Valenzuela DM, Yancopoulos GD, Booth CJ, Cho JH, Ouyang W, Abraham C, Flavell RA. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491:259–263. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witte E, Witte K, Warszawska K, Sabat R, Wolk K. Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev. 2010;21:365–379. doi: 10.1016/j.cytogfr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Kempster SL, Belteki G, Forhead AJ, Fowden AL, Catalano RD, Lam BY, McFarlane I, Charnock-Jones DS, Smith GC. Developmental control of the Nlrp6 inflammasome and a substrate, IL-18, in mammalian intestine. Am J Physiol Gastrointest Liver Physiol. 2011;300:G253–263. doi: 10.1152/ajpgi.00397.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhawan P, Richmond A. A novel NF-kappa B-inducing kinase-MAPK signaling pathway up-regulates NF-kappa B activity in melanoma cells. J Biol Chem. 2002;277:7920–7928. doi: 10.1074/jbc.M112210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adli M, Merkhofer E, Cogswell P, Baldwin AS. IKKalpha and IKKbeta each function to regulate NF-kappaB activation in the TNF-induced/canonical pathway. PLoS One. 2010;5:e9428. doi: 10.1371/journal.pone.0009428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarnegar B, Yamazaki S, He JQ, Cheng G. Control of canonical NF-kappaB activation through the NIK-IKK complex pathway. Proc Natl Acad Sci U S A. 2008;105:3503–3508. doi: 10.1073/pnas.0707959105. [DOI] [PMC free article] [PubMed] [Google Scholar]