Abstract
Background
Estrogen receptor alpha (ERα) and cyclin D1 are frequently co-expressed in human breast cancer. Some, but not all, studies link tamoxifen resistance to co-expression of cyclin D1 and ERα. In mice over-expression of either cyclin D1 or ERα in mammary epithelial cells is sufficient to induce mammary hyperplasia. Cyclin D1 over-expression in mice leads to mammary adenocarcinoma associated with activated estrogen signaling pathways. ERα over-expression in mice leads to mammary hyperplasia and cancer. Significantly, disease development in these mice is abrogated by loss of cyclin D1.
Methods
Genetically engineered mouse models were used to determine whether or not ERα over-expression demonstrated cooperativity with cyclin D1 over-expression in cancer development, reaction to the chemical carcinogen DMBA, or tamoxifen response.
Results
Adding ERα over-expression to cyclin D1 over-expression increased the prevalence of hyperplasia but not cancer. Single dose DMBA exposure did not increase cancer prevalence in any of the genotypes although cyclin D1 over-expressing mice demonstrated a significant increase in hyperplasia. Tamoxifen treatment was initiated at both young and older ages to test for genotype-specific differences in response. Although normal ductal structures regressed in all genotypes at both younger and older ages, tamoxifen did not significantly reduce the prevalence of either hyperplasia or cancer in any of the genotypes. All of the cancers that developed were hormone receptor positive, including those that developed on tamoxifen, and all showed expression of nuclear-localized cyclin D1. In summary, development of tamoxifen resistant hyperplasia and cancer was associated with expression of ERα and cyclin D1.
Conclusion
These preclinical models will be useful to test strategies for overcoming tamoxifen resistance, perhaps by simultaneously targeting cell cycle regulatory pathways associated with cyclin D1.
Keywords: ERα; Cyclin D1; Breast cancer; Hyperplasia, Tamoxifen; Mouse models
Introduction
Estrogen Receptor alpha (ERα) and cyclin D1 are frequently although not invariably co-expressed in invasive human breast cancers [1–5]. Increased levels of ERα and cyclin D1 expression are reported in precursor lesions and non-invasive breast cancers [6–9]. The two proteins share a molecular interplay. Cyclin D1 lies downstream of estrogen signaling through ERα [10–13] but cyclin D1 also lies upstream of ERα and can activate ERα driven transcription through cyclin dependent kinase (CDK) independent mechanisms [14–16]. ERα and cyclin D1 also activate the same set of genes linked to breast cancer progression [17].
Combination estrogen and progesterone hormone replacement therapy in post-menopausal women significantly increases breast cancer risk [18–20]. Cyclin D1 positively regulates progesterone receptor expression levels and this has been postulated to be a factor that contributes to the increased risk of cancer development following hormonal exposure [21]. In ERα positive breast cancer, coincident cyclin D1 expression in untreated women has been repeatedly presented, although not always, as a positive prognostic factor [1,2,4,5]. Some studies have linked expression of cyclin D1 in ERα positive breast cancers to an impaired response to tamoxifen [22–28] while others show no statistically significant effects [29,30].
Genetically engineered ERα over-expression in mammary epithelial cells in transgenic mice leads to development of ductal hyperplasia and ductal carcinoma in situ (DCIS) lesions by four months of age [31], hyperplastic alveolar nodules (HANs) by eight months of age [32], and both ERα positive and ERα negative invasive mammary cancers by 12 months of age following single-dose treatment with the chemical carcinogen 12-dimethylbenz[a]anthracene (DMBA) [33]. Cyclin D1 is found in the ductal hyperplasias, DCIS, and invasive cancers. Loss of cyclin D1 in this mouse model, referred to as Conditional Estrogen Receptor in Mammary tissue (CERM) mice, leads to a DNA damage response in response to pubertal estrogen stimulation that causes apoptotic death of the mammary epithelial cells and abrogates normal mammary gland development [34]. ERα over-expressing CERM mice express ERα at approximately two-fold higher levels than normal [31,32,35,36], comparable to what is described in human preneoplastic breast tissue [9,37]. This results in increased Erbb2, Efgr, Pgr, Tnsfs11 RNA expression, and phosphorylated mitogen-activated protein kinase 3/1 (ERK1/2), mitogen-activated protein kinase 8 (JNK), signal transducer and transactivator (STAT)3, and STAT5 protein expression in normal-appearing mammary tissue [32,35]. High percentages of precancerous and cancer cells exhibit increased cyclin D1, antigen identified by monoclonal antibody (Ki67), proliferating cell nuclear antigen (PCNA), phosphorylated ERK1/2 and phosphorylated STAT5 [31–33,35].
Genetically engineered cyclin D1 over-expression targeted to the mammary epithelial cells of transgenic mice leads to development of preneoplasia in young mice followed by cancer development when mice are older than one year [21,38]. Cyclin D1 expression levels in these mice are comparable to those found in cultured human breast cancer cells [38]. Increased expression of cyclin D1-regulated genes including progesterone receptor A, cyclin A2, immediate early response 3, small stress protein 1, and tumor necrosis factor-associated factor-interacting protein starts in preneoplasia and is believed to contribute to carcinogenic transformation [17,21].
Mice over-expressing cyclin D1 were crossed with CERM mice to determine if ERα and cyclin D1 would demonstrate cooperativity in vivo in the development of hyperplasia and cancer. Cohorts of mice were exposed to single-dose DMBA [33,39] to test if the combination of over-expressed cyclin D1 with ERα increased susceptibility to this carcinogenic insult. The response to tamoxifen was tested at two different ages (four and ten months of age) to test if tamoxifen could cause regression of preneoplasia and cancer initiated by over-expression of ERα and cyclin D1 either alone or in combination.
Materials and Methods
Mouse models
Bi-transgenic tet-op-ERαMouse Mammary Tumor Virus (MMTV) (termed Conditional Estrogen Receptor in Mammary tissue or CERM) mice carrying tet-op-ERα and MMTV-rtTA transgenes [31–36] were crossed with MMTV-cyclin D1 (termed D1) transgenic mice [21,38] to generate female CERM (n=30), CERM/D1 (n=52) and D1 (n=42) mice on a C57Bl/6 background. C57Bl/6 wild-type (WT) mice were used as controls in the no-intervention and tamoxifen-intervention studies (n=13). Detection of the MMTV-rtTA, tet-op-ERα and MMTV-cyclin D1 transgenes was performed from tails by polymerase chain reaction (PCR) (Transnetyx, Cordova, TN). Mice were maintained on a 12-hour light/dark cycle with a doxycycline-containing diet (Bio-Serv, Frenchtown, NJ) with water ad libitum. All genotypes demonstrated equivalent growth, activity, and fertility, and the Georgetown University Animal Care and Use Committee and Institutional Biosafety Committee approved all procedures.
Time-course and intervention studies
A no-intervention time-course study without any treatment was performed to determine if there were any genotype-specific differences in prevalence of hyperplastic alveolar nodules (HANs) at one year of age or development of mammary cancer by one year of age in CERM (n=16), CERM/D1 (n=25) and D1 (n=20) mice. A smaller control cohort of WT (n=4) mice was followed to provide non-transgenic mammary gland whole mounts (WMs) for comparative examination with the experimental mice. An interventional time-course study following a single intragastric 1 mg dose of the chemical carcinogen 12-dimethylbenz[a]anthracene (DMBA) (D3254, Sigma) at four months of age [33,39] was performed to determine if there were any genotype-specific differences in prevalence of HANs or development of mammary cancer at one year of age in CERM (n=4), CERM/D1 (n=10) and D1 (n=9) mice following exposure to this chemical carcinogen. An interventional time-course study following tamoxifen treatment initiated at ten months of age was performed to determine if there were any genotype-specific differences in prevalence of HANs by one year of age or development of mammary cancer by one year of age in CERM (n=10), CERM/D1 (n=17) and D1 (n=13) mice. A control cohort of WT (n=9) mice was followed to provide non-transgenic mammary gland whole mounts for comparative examination with the experimental mice. An interventional time-course study following tamoxifen treatment initiated at four months of age was performed to determine if there were any genotype-specific differences in prevalence of HANs at six months of age or development of mammary cancer by six months of age in CERM (n=16), CERM/D1 (n=6) and D1 (n=4) mice. A control cohort of WT mice (n=19) was followed to provide non-transgenic mammary gland whole mounts for comparative examination with the experimental mice. Mice were implanted subcutaneously with a 25 mg 60-day constant release tamoxifen pellet (Innovative Research of America, Sarasota, FL). All mice were followed with weekly clinical examination until necropsy timepoints at age six or 12 months, or when tumor reached 1 cm3 or other health issues required euthanasia.
Histology and mammary gland whole mount studies
At the time of necropsy one inguinal mammary gland was taken for mammary gland whole mount analyses and palpable tumors were isolated from surrounding mammary tissue, formalin-fixed, embedded and sectioned for histological analyses. Hematoxylin and eosin (H&E)-stained sections were used to evaluate histology of the tumors. All tumors were classified as invasive adenocarcinomas. For immunohistochemistry, paraffin-embedded mammary gland sections were deparaffinized in three successive five minute xylene incubations, and rehydrated in two successive three minute each incubation steps of 100%, 95% and 75% followed by 50% ethanol for two minutes. Endogenous peroxidase was quenched with 3% hydrogen peroxide for 10 min and sections washed twice in dH2O for two minutes. Antigen retrieval was performed either in a decloaker using BORG retrieval solution (Biocare Medical, Concord, CA) or by immersion at 98°C for 20 minutes in 10 mM citrate buffer (pH 6.0) with 0.05% Tween. Primary antibodies: ERα (Santa Cruz, sc-542) (1:750 1 hr RT), PgR (Santa Cruz, sc-538) (1:250 1 hr RT), cyclin D1 (Neomarkers, RM-9104, Fremont, CA) (1:50-100 overnight 4°C or 1 hr RT), Ki67 (Novacastra, NCL-Ki67-MM1, Logan, UT) (1:25-1:100 1 hr RT), and ErbB2 (Santa Cruz, sc284). Immunohistochemical staining was performed using the rabbit VectaStain or the Mouse-On-Mouse kit (Vector Labs, Burlingame, CA). Slides were exposed to biotin-conjugated anti-rabbit or anti-mouse secondary antibodies, VectaStain ABC reagent and DAB chromate (Dako, Carpinteria, CA) to detect horseradish peroxidase, counterstained with Hematoxylin (Vector Labs), dehydrated, and mounted with VectaMount or Acrymount (Vector Labs). Mammary gland whole mounts were examined under low power magnification (0.5X, 1X, 4X) to identify and count HANs. The percentage of mice with at least one HAN per mammary gland and the percentage of mice with more than one (multiple) HANs per mammary gland were calculated and recorded for each genotype at the one-year timepoint. Digital images were captured using a Nikon Eclipse E800M microscope and DMX1200 camera with the ACT-1 Version 2.7 program (Nikon Corporation, Melville, NY).
Statistical analyses
Statistical analyses were performed with GraphPad Prism version 4 for Windows (La Jolla, CA). HAN, multiple HANs and adenocarcinoma prevalence were compared using Fisher’s Exact Test (one-tailed test). Statistical significance was reached when p ≤ 0.05.
Results
Addition of ERα over-expression to cyclin D1 over-expression increased prevalence of mammary hyperplasia but not adenocarcinoma
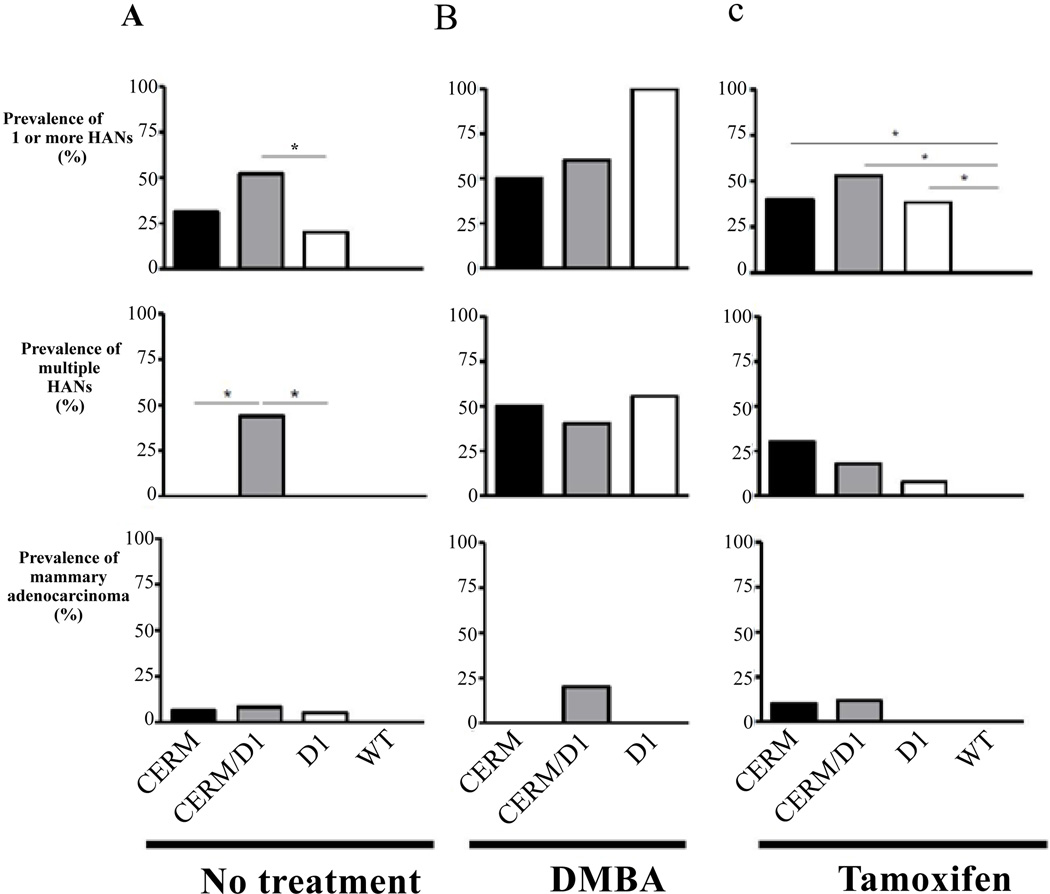
The addition of ERα over-expression to cyclin D1 over-expression in the CERM/D1 mice significantly increased the percentage of mice demonstrating one or more HANs at one year of age (Fisher’s exact, one tailed test, p≤0.05); however, it did not increase the number of mice developing mammary adenocarcinoma (Figure 1A). Notably, only mice over-expressing both ERα and cyclin D1 demonstrated multiple HANs (Figure 1A).
Figure 1. Prevalence of hyperplastic alveolar nodules (HANs) and invasive adenocarcinomas in one-year-old mice over-expressing ERα (CERM), cyclin D1 (D1), both ERα and D1 (CERM/D1) mice in no-treatment and DMBA and tamoxifen intervention groups.
(A) Bar graphs comparing the prevalence of at least one HAN, multiple HANs, and invasive cancers in one-year-old CERM, CERM/D1, D1 and WT mice that received no treatment intervention. (B) Bar graphs comparing the prevalence of at least one HAN, multiple HANs, and invasive cancers in one-year-old CERM, CERM/D1, and D1 mice that were exposed to a single-dose of DMBA intervention at four months of age. (C) Bar graphs comparing the prevalence of at least one HAN, multiple HANs, and invasive cancers in one-year-old CERM, CERM/D1, D1 and WT mice that received tamoxifen treatment intervention at ten months of age. * indicates statistically significant differences (Fisher’s exact test, one tailed, p≤0.05).
DMBA exposure increased prevalence of mammary hyperplasia but not adenocarcinoma in mice over-expressing cyclin D1
Single-dose DMBA exposure at four months of age was used to investigate if this chemical carcinogen could increase the percentage of mice demonstrating HANs or adenocarcinoma at age one year in CERM, CERM/D1 or D1 mice. Only D1 mice demonstrated a significant increase in HAN prevalence compared to the mice without any treatment (Fisher’s exact, one-tailed test, p≤0.05) but none developed adenocarcinoma (Figure 1B). While adenocarcinoma development following single-dose DMBA treatment was limited to the CERM/D1 mice (Figure 1B), the percentage found was not statistically significantly different from untreated mice.
Tamoxifen treatment at ten months of age did not reduce prevalence of mammary hyperplasia or adenocarcinoma induced by over-expression of ERα or cyclin D1
Tamoxifen treatment was initiated at ten months of age to determine if this established breast cancer preventive could reduce the percentage of mice demonstrating either HANs or adenocarcinoma in CERM, CERM/D1 or D1 mice by age one year. Notably, the percentage of mice demonstrating HANS or adenocarcinoma was not significantly different from untreated mice (Figure 1A and 1C). However, while none of the no-intervention CERM or D1 mice demonstrated multiple HANs, over 25% of the tamoxifen-treated CERM and 5% of the D1 mice exhibited more than one HAN, a significant increase from mice without any treatment (Fisher’s exact, one-tailed test, p ≤ 0.05) (Figure 1A and 1C). At the time tamoxifen treatment was initiated no mice demonstrated palpable tumors; however adenocarcinomas developed on tamoxifen treatment in CERM (n=1) and CERM/D1 (n=2) mice (Figure 1C).
Mammary hyperplasia following tamoxifen treatment was a time-dependent acquired phenotype at ten months of age and not directly associated with either ERα or cyclin D1 transgene expression
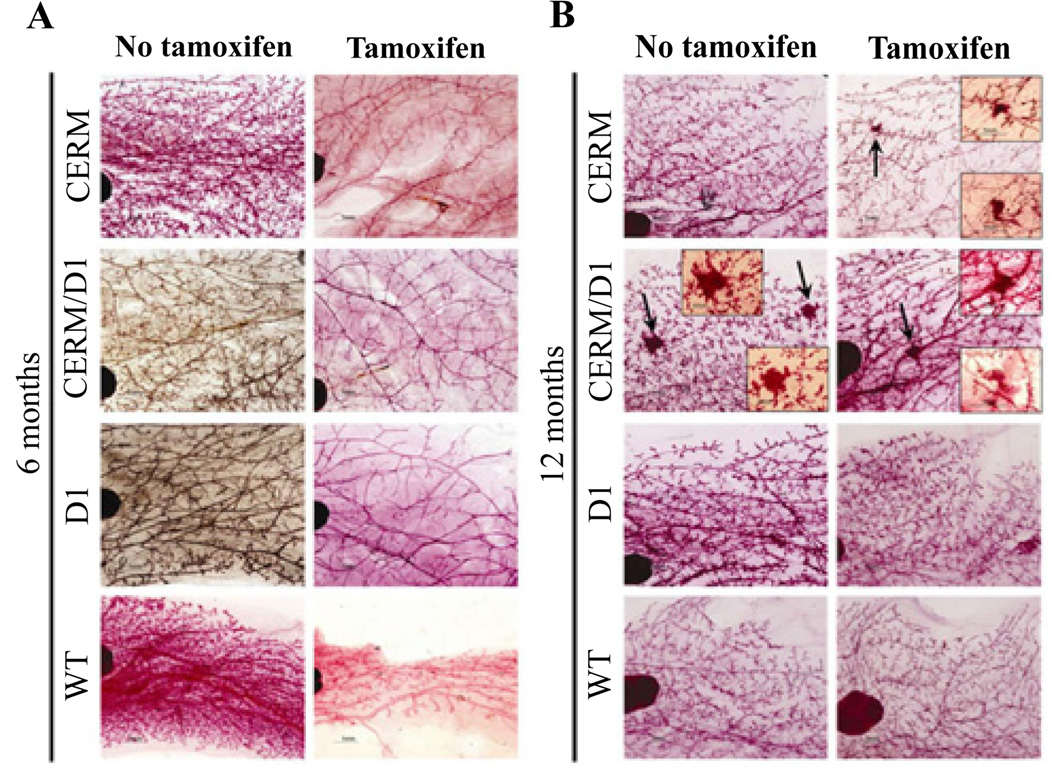
To test if resistance to tamoxifen-induced ductal regression was an acquired phenotype over time in the different models and not a direct result of ERα or cyclin D1 expression alone or in combination, the response to tamoxifen was also determined in four-month-old mice and compared to the results from the ten-month-old mice. WT control mice showed expected ductal regression at six months of age, two months after initiation of tamoxifen treatment. Similar patterns of ductal regression were seen in six-month-old CERM, CERM/D1 and D1 mice (Figure 2). HANs were found following tamoxifen treatment only in the CERM, CERM/D1 and D1 mice when the intervention was initiated at age ten months (Figures 1 and 2).
Figure 2. Representative mammary gland whole mounts without and with tamoxifen treatment in six-month and one-year old CERM, CERM/D1, D1 and WT mice.
Representative whole mount images of six-month and one-year old CERM, CERM/D1, D1 and WT mice treated with tamoxifen for two months demonstrating uniform ductal regression in all genotypes when treated at four months of age and presence of multiple HANs in CERM and CERM/D1 mice when treated at ten months of age (arrows and insets).
Mammary adenocarcinomas expressed similar expression levels of hormone receptors, cyclin D1, Ki67 and ErbB2 irrespective of genotype or treatment group
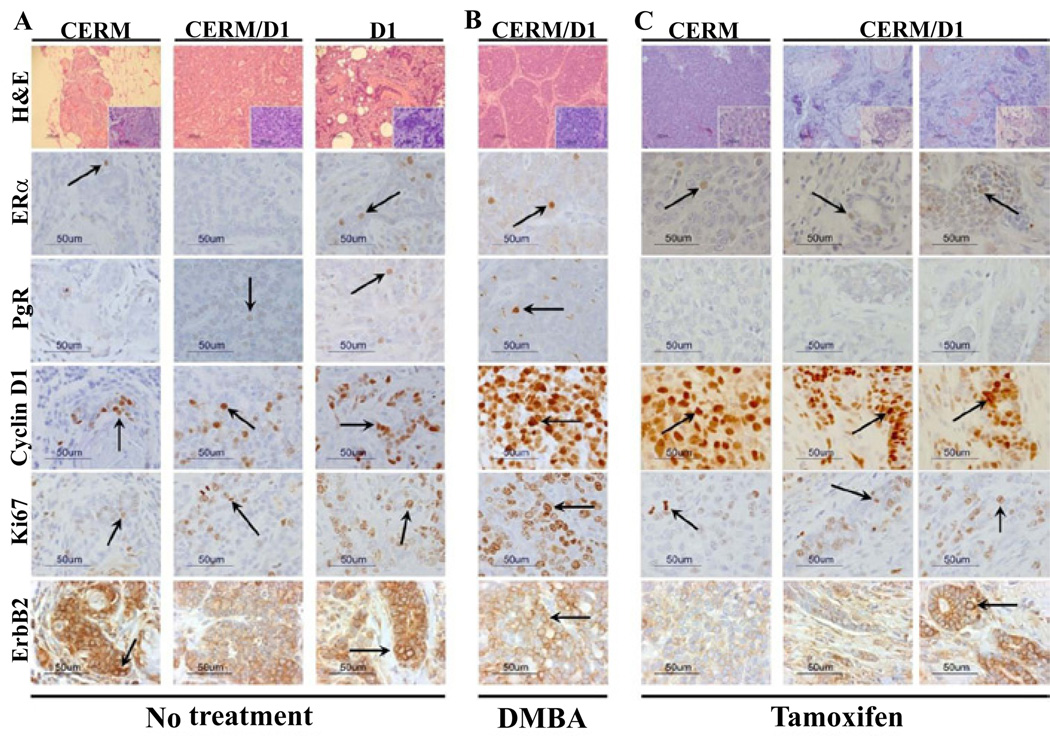
Immunohistochemistry was used to compare relative expression levels of the hormone receptors ERα and PgR, cyclin D1, Ki67 and ErbB2 in the mammary adenocarcinomas that developed in the different genotypes across treatment groups. Hormone receptor, cyclin D1, Ki67 and ErbB2 expression were detected in all the mammary adenocarcinomas without any significant differences between genotypes or treatment groups (Figure 3).
Figure 3. Invasive adenocarcinomas demonstrated expression of hormone receptors and cyclin D1.
Representative panels illustrating H&E staining and immunohistochemistry for ERα, PgR, cyclin D1, Ki67 and ErbB2 in mammary adenocarcinomas from CERM, CERM/D1 and D1 mice. Arrows indicate representative stained cells with either nuclear (ERα, PgR, cyclin D1, Ki67) or higher intensity 2+ membrane (ErbB2) staining. Size markers are indicated on all panels.
Discussion
The most significant finding of the study was the persistence of hyperplasia and development of adenocarcinomas on tamoxifen treatment when it was initiated at ten months of age. These findings are consistent with the appearance of tamoxifen resistance in these mice. It is possible that the cancers and persistent hyperplasias demonstrated preexisting molecular profiles pre-disposing them to tamoxifen resistance as has been suggested for women receiving tamoxifen treatment [40]. Consistent with this, primary prevention with tamoxifen in women was unable to reduce the incidence of ERα positive breast cancers developing on tamoxifen although it did decrease the incidence of ERα positive breast cancers post-treatment [41]. One caveat of this mouse study was that the tamoxifen intervention was relatively short (two months) whereas in women the current recommended duration is between two and five years. It is possible that a longer duration tamoxifen treatment would have been associated with a reduction in hyperplasia prevalence. However, this is balanced against the observation that cancer developed on tamoxifen in the CERM and CERM/D1 genotypes and this was associated with an increase in the percentage of mice with multiple hyperplastic foci in the CERM mice, results that are compatible with the notion that tamoxifen resistance is present in a subset of mammary epithelial cells in these mice by ten months of age.
Timing of interventions to prevent breast cancer may be critical. As discussed above primary prevention with tamoxifen in women was more effective post-treatment than during treatment [41]. In the study here tamoxifen administered at four months of age uniformly induced ductal regression and no resistant hyperplasias or cancers appeared. The different results following tamoxifen treatment at four and ten months of age suggests that time-dependent changes in the mammary epithelial cells following expression of the ERα and D1 transgenes may be required for the appearance of tamoxifen resistance. Time-dependent alterations in salivary epithelial cells following expression of an oncoprotein are known to be responsible for the differences in regression of hyperplasia following down-regulation of the initiating oncoprotein [42,43]. It is possible the similar molecular time-dependent changes occur in the mammary epithelium following ERα and D1 over-expression that can interrupt the response to tamoxifen.
In women, the majority of tamoxifen resistant breast cancers express ERα [44] with co-expression of cyclin D1 with ERα frequently found in cancers resistant to tamoxifen [22,24–28]. Down-regulation of cyclin D1 is associated with tamoxifen induced growth inhibition [45,46]. Cyclin D1 has been shown to enhance the growth response to estrogen and progesterone [21]. The adenocarcinomas that developed both on and off tamoxifen in this study demonstrated similar patterns of gene expression with both detectable hormone receptor expression, principally ERα, and cyclin D1 expression.
The genetically engineered transgenic models studied here demonstrate significant parallels with human breast cancer development both in the initiating pathways and the resultant pathophysiology. In women, estimates of DCIS progression to invasive cancer range between 14 and 50% [47]. Similarly, in the CERM model, whereas ~25% of the mice demonstrate hyperplasia at age one year, invasive cancer is found in less than five percent of the mice at the same timepoint. The higher prevalence of hyperplasia and DCIS as compared to invasive cancer in the CERM mice models the higher prevalence of benign breast disease as compared to invasive cancer in women [48]. Possible next steps in studies utilizing these genetically engineered mouse models would be to directly test if earlier intervention with tamoxifen could prevent emergence of tamoxifen-resistant hyperplasias and cancers as well as to investigate if adding a therapeutic agent such as a CDK4/6 inhibitor targeting the cyclin D1 signaling pathway [49] might limit development of tamoxifen resistance.
Acknowledgements
The work was supported by NCI, NIH RO1CA112176 (P.A.F, A.M.M, E.D.C), R01CA89041 (P.A.F), R01CA89041-1OS1 (M.C.C, P.A.F), P30CA051008 (P.A.F and Lombardi Comprehensive Cancer Center (LCCC) Animal Shared Resource (ASR), Histopathology and Tissue Shared Resources (HTSR), WCU (World Class University) program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R31-10069) (P.A.F), DOD Breast Cancer Research Program Predoctoral Traineeship Awards W81XWH-05-1-0271 (A.M.M) and W81XWH-05-1-0302 (M.C.C, S.M.F), and The Susan G. Komen Breast Cancer Foundation KG080359 (E.D.C) and PDF0503642 (M.T.S).
Abbreviations
- CERM
Conditional ERα in Mammary tissue
- ERα
Estrogen Receptor α
- PgR
Progesterone Receptor
- HAN
Hyperplastic Alveolar Nodule
- DMBA
7, 12-dimethylbenz [a] anthracene
- DCIS
Ductal Carcinoma In Situ
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- 1.Barnes DM, Gillett CE. Cyclin D1 in breast cancer. Breast Cancer Res Treat. 1998;52:1–15. doi: 10.1023/a:1006103831990. [DOI] [PubMed] [Google Scholar]
- 2.Hwang TS, Han HS, Hong YC, Lee HJ, Paik NS. Prognostic value of combined analysis of cyclin D1 and estrogen receptor status in breast cancer patients. Pathol Int. 2003;53:74–80. doi: 10.1046/j.1440-1827.2003.01441.x. [DOI] [PubMed] [Google Scholar]
- 3.Joe AK, Memeo L, McKoy J, Mansukhani M, Liu H, et al. Cyclin D1 overexpression is associated with estrogen receptor expression in Caucasian but not African-American breast cancer. Anticancer Res. 2005;25:273–281. [PubMed] [Google Scholar]
- 4.Utsumi T, Yoshimura N, Maruta M, Takeuchi S, Ando J, et al. Correlation of cyclin D1 MRNA levels with clinico-pathological parameters and clinical outcome in human breast carcinomas. Int J Cancer. 2000;89:39–43. doi: 10.1002/(sici)1097-0215(20000120)89:1<39::aid-ijc7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.Van Diest PJ, Michalides RJ, Jannink L, van der Valk P, Peterse HL, et al. Cyclin D1 expression in invasive breast cancer. Correlations and prognostic value. Am J Pathol. 1997;150:705–711. [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Fatah TM, Powe DG, Hodi Z, Reis-Filho JS, Lee AH, et al. Morphologic and molecular evolutionary pathways of low nuclear grade invasive breast cancers and their putative precursor lesions: further evidence to support the concept of low nuclear grade breast neoplasia family. Am J Surg Pathol. 2008;32:513–523. doi: 10.1097/PAS.0b013e318161d1a5. [DOI] [PubMed] [Google Scholar]
- 7.Gillett CE, Lee AH, Millis RR, Barnes DM. Cyclin D1 and associated proteins in mammary ductal carcinoma in situ and atypical ductal hyperplasia. J Pathol. 1998;184:396–400. doi: 10.1002/(SICI)1096-9896(199804)184:4<396::AID-PATH1259>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Hameed O, Ghali VS, Tartter PI, Mizrachi H. Immunohistochemical staining for cyclin D1 and Ki-67 aids in the stratification of atypical ductal hyperplasia diagnosed on breast core biopsy. Am J Clin Pathol. 2005;124:862–872. [PubMed] [Google Scholar]
- 9.Zhou CJ, Zhang QH, Zhang TG, Sun SZ, Li H, et al. Expression of ER, Ki-67 and cyclin D1 in the pre-cancerous breast of Chinese patients. Pathol Oncol Res. 2009;15:153–158. doi: 10.1007/s12253-008-9100-6. [DOI] [PubMed] [Google Scholar]
- 10.Hong J, Shah NN, Thomas TJ, Gallo MA, Yurkow EJ, et al. Differential effects of estradiol and its analogs on cyclin D1 and CDK4 expression in estrogen receptor positive MCF-7 and estrogen receptor-transfected MCF-10AEwt5 cells. Oncol Rep. 1998;5:1025–1033. doi: 10.3892/or.5.5.1025. [DOI] [PubMed] [Google Scholar]
- 11.Prall OW, Rogan EM, Musgrove EA, Watts CK, Sutherland RL. c-Myc or cyclin D1 mimics estrogen effects on cyclin E-Cdk2 activation and cell cycle reentry. Mol Cell Biol. 1998;18:4499–4508. doi: 10.1128/mcb.18.8.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prall OW, Rogan EM, Sutherland RL. Estrogen regulation of cell cycle progression in breast cancer cells. J Steroid Biochem Mol Biol. 1998;65:169–174. doi: 10.1016/s0960-0760(98)00021-1. [DOI] [PubMed] [Google Scholar]
- 13.Zhang D, Trudeau VL. Integration of membrane and nuclear estrogen receptor signaling. Comp Biochem Physiol A Mol Integr Physiol. 2006;144:306–315. doi: 10.1016/j.cbpa.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Bindels EM, Lallemand F, Balkenende A, Verwoerd D, Michalides R. Involvement of G1/S cyclins in estrogen-independent proliferation of estrogen receptor-positive breast cancer cells. Oncogene. 2002;21:8158–8165. doi: 10.1038/sj.onc.1206012. [DOI] [PubMed] [Google Scholar]
- 15.Neuman E, Ladha MH, Lin N, Upton TM, Miller SJ, et al. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol Cell Biol. 1997;17:5338–5347. doi: 10.1128/mcb.17.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zwijsen RM, Wientjens E, Klompmaker R, van der Sman J, Bernards R, et al. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88:405–415. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]
- 17.Yang C, Trent S, Ionescu-Tiba V, Lan L, Shioda T, et al. Identification of cyclin D1- and estrogen-regulated genes contributing to breast carcinogenesis and progression. Cancer Res. 2006;66:11649–11658. doi: 10.1158/0008-5472.CAN-06-1645. [DOI] [PubMed] [Google Scholar]
- 18.Bakken K, Fournier A, Lund E, Waaseth M, Dumeaux V, et al. Menopausal hormone therapy and breast cancer risk: Impact of different treatments. The European prospective investigation into cancer and nutrition (EPIC) Int J Cancer. 2011;128:144–156. doi: 10.1002/ijc.25314. [DOI] [PubMed] [Google Scholar]
- 19.Chlebowski RT, Kuller LH, Prentice RL, Stefanick ML, Manson JE, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360:573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 21.Yang C, Chen L, Li C, Lynch MC, Brisken C, et al. Cyclin D1 enhances the response to estrogen and progesterone by regulating progesterone receptor expression. Mol Cell Biol. 2010;30:3111–3125. doi: 10.1128/MCB.01398-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aaltonen K, Amini RM, Landberg G, Eerola H, Aittomaki K, et al. Cyclin D1 expression is associated with poor prognostic features in estrogen receptor positive breast cancer. Breast Cancer Res Treat. 2009;113:75–82. doi: 10.1007/s10549-008-9908-5. [DOI] [PubMed] [Google Scholar]
- 23.Hodges LC, Cook JD, Lobenhofer EK, Li L, Bennett L, et al. Tamoxifen Functions As a Molecular Agonist Inducing Cell Cycle Associated Genes in Breast Cancer Cells. Mol Cancer Res. 2003;1:300–311. [PubMed] [Google Scholar]
- 24.Jirstrom K, Stendahl M, Ryden L, Kronblad A, Bendahl PO, et al. Adverse effect of adjuvant tamoxifen in premenopausal breast cancer with cyclin D1 gene amplification. Cancer Res. 2005;65:8009–8016. doi: 10.1158/0008-5472.CAN-05-0746. [DOI] [PubMed] [Google Scholar]
- 25.Kenny FS, Hui R, Musgrove EA, Gee JM, Blamey RW, et al. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin Cancer Res. 1999;5:2069–2076. [PubMed] [Google Scholar]
- 26.Rudas M, Lehnert M, Huynh A, Jakesz R, Singer C, et al. Cyclin D1 expression in breast cancer patients receiving adjuvant tamoxifen-based therapy. Clin Cancer Res. 2008;14:1767–1774. doi: 10.1158/1078-0432.CCR-07-4122. [DOI] [PubMed] [Google Scholar]
- 27.Stendahl M, Kronblad A, Ryden L, Emdin S, Bengtsson NO, et al. Cyclin D1 overexpression is a negative predictive factor for tamoxifen response in postmenopausal breast cancer patients. Br J Cancer. 2004;90:1942–1948. doi: 10.1038/sj.bjc.6601831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zwart W, Rondaij M, Jalink K, Sharp ZD, Mancini MA, et al. Resistance to antiestrogen arzoxifene is mediated by overexpression of cyclin D1. Mol Endocrinol. 2009;23:1335–1345. doi: 10.1210/me.2008-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han S, Park K, Bae BN, Kim KH, Kim HJ, et al. Cyclin D1 expression and patient outcome after tamoxifen therapy in estrogen receptor positive metastatic breast cancer. Oncol Rep. 2003;10:141–144. [PubMed] [Google Scholar]
- 30.Michalides R, van Tinteren H, Balkenende A, Vermorken JB, Benraadt J, et al. Cyclin A is a prognostic indicator in early stage breast cancer with and without tamoxifen treatment. Br J Cancer. 2002;86:402–408. doi: 10.1038/sj.bjc.6600072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frech MS, Halama ED, Tilli MT, Singh B, Gunther EJ, et al. Deregulated estrogen receptor alpha expression in mammary epithelial cells of transgenic mice results in the development of ductal carcinoma in situ. Cancer Res. 2005;65:681–685. [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz-Cruz ES, Furth PA. Deregulated estrogen receptor alpha and p53 heterozygosity collaborate in the development of mammary hyperplasia. Cancer Res. 2010;70:3965–3974. doi: 10.1158/0008-5472.CAN-09-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miermont AM, Parrish AR, Furth PA. Role of ER{alpha} in the differential response of Stat5a loss in susceptibility to mammary preneoplasia and DMBA-induced carcinogenesis. Carcinogenesis. 2010;6:1124–1131. doi: 10.1093/carcin/bgq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frech MS, Torre KM, Robinson GW, Furth PA. Loss of cyclin D1 in concert with deregulated estrogen receptor alpha expression induces DNA damage response activation and interrupts mammary gland morphogenesis. Oncogene. 2008;27:3186–3193. doi: 10.1038/sj.onc.1210974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Díaz-Cruz ES, Sugimoto Y, Gallicano IG, Bruggemeier RW, Furth PA. Comparison of increased Aromatase versus ERα in the generation of mammary hyperplasia and cancer. Cancer Research. 2011;71:5477–5487. doi: 10.1158/0008-5472.CAN-10-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furth PA, Cabrera MC, Díaz-Cruz ES, Millman S, Nakles RE. Estrogen signaling aberrations in breast cancer risk. Ann NY Acad Sci. 2011;1229:147–155. doi: 10.1111/j.1749-6632.2011.06086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhandare D, Nayar R, Bryk M, Hou N, Cohn R, et al. Endocrine biomarkers in ductal lavage samples from women at high risk for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2620–2627. doi: 10.1158/1055-9965.EPI-05-0302. [DOI] [PubMed] [Google Scholar]
- 38.Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, et al. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 39.Keshava N, Mandava U, Kirma N, Tekmal RR. Acceleration of mammary neoplasia in aromatase transgenic mice by 7,12-dimethylbenz[a]anthracene. Cancer Lett. 2001;167:125–133. doi: 10.1016/s0304-3835(01)00478-5. [DOI] [PubMed] [Google Scholar]
- 40.Umar A, Kang H, Timmermans AM, Look MP, Meijer-van Gelder ME, et al. Identification of a putative protein profile associated with tamoxifen therapy resistance in breast cancer. Mol Cell Proteomics. 2009;8:1278–9124. doi: 10.1074/mcp.M800493-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–290. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 42.Ewald D, Li M, Efrat S, Wall RJ, Furth PA, et al. Time-sensitive reversal of hyperplasia in transgenic mice expressing SV40 T antigen. Science. 1996;273:1384–1386. doi: 10.1126/science.273.5280.1384. [DOI] [PubMed] [Google Scholar]
- 43.Tilli MT, Hudgins SL, Frech MS, Halama ED, Renou J-P, et al. Loss of PP2A Expression Correlates with Phosphorylation of DP-1 and Reversal of Dysplasia through Differentiation in a Conditional Mouse Model of Cancer Progression. Cancer Res. 2003;63:7668–7673. [PubMed] [Google Scholar]
- 44.Higgins MJ, Stearns V. Understanding resistance to tamoxifen in hormone receptor-positive breast cancer. Clin Chem. 2009;55:1453–1455. doi: 10.1373/clinchem.2009.125377. [DOI] [PubMed] [Google Scholar]
- 45.Watts CK, Sweeney KJ, Warlters A, Musgrove EA, Sutherland RL. Antiestrogen regulation of cell cycle progression and cyclin D1 gene expression in MCF-7 human breast cancer cells. Breast Cancer Res Treat. 1994;31:95–105. doi: 10.1007/BF00689680. [DOI] [PubMed] [Google Scholar]
- 46.Maas RA, Bruning PF, Top B, Breedijk AJ, Peterse HL. Growth arrest associated changes of mRNA levels in breast cancer cells measured by semi-quantitative RT-PCR: potential early indicators of treatment response. Cancer Lett. 1995;97:107–116. doi: 10.1016/0304-3835(95)03959-z. [DOI] [PubMed] [Google Scholar]
- 47.Erbas B, Provenzano E, Armes J, Gertig D. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat. 2006;97:135–144. doi: 10.1007/s10549-005-9101-z. [DOI] [PubMed] [Google Scholar]
- 48.Ashbeck EL, Rosenberg RD, Stauber PM, Key CR. Benign breast biopsy diagnosis and subsequent risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:467–472. doi: 10.1158/1055-9965.EPI-06-0394. [DOI] [PubMed] [Google Scholar]
- 49.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]