Abstract
Tumors of the pleura are not uncommon and diagnosis is clinched by combined imaging and clinical correlation. Malignant tumors are more common than benign tumors. Initial imaging modalities are chest radiography and Computed Tomography (CT). Further characterization may be required using Ultrasoundgraphy (USG), Magnetic resonance Imaging (MRI) and PET-CT. Biopsy remains gold standard. This article highlights various common and uncommon tumors of pleura and characteristic imaging findings.
Keywords: Fibroma, mesothelioma, nodular pleural thickening, pleura
Introduction
Radiological assessment of pleural tumors requires complete knowledge of pleural anatomy. Various benign, malignant and tumor-like conditions can involve the pleura. Malignant pleural neoplasms are more common. The most common tumor-like condition involving the pleura is pleural thickening. Radiological features of pleural disease can have varied spectrum including pleural effusion, pleural plaques, and nodular pleural thickening. Differentiation of pleural neoplasms from pulmonary and extrapleural neoplasms is crucial in making appropriate diagnosis.
Anatomy
Pleura is a serous membrane composed of mesothelial cells and loose connective tissue. It is divided into parietal pleura and visceral pleura. Parietal pleura is again divided into costal, diaphragmatic, mediastinal, and cervical pleura [Figure 1]. Up to 5 ml of fluid may be present in normal individuals within the two layers of pleura. Normal thickness of pleura including the pleural space is 0.2-0.44 mm. Normally pleura is not separately seen unless outlined by fluid, air, fat, or fascia. Usually it is seen as major, minor, and accessory fissures (two invaginated sheets of visceral pleura) or as junctional lines (comprising four sheets of pleura) [Figure 2]. Azygous fissure consists of four layers of pleura. There is no communication between the right and left pleural cavities. Parietal pleura is innervated by sensory nerves. Parietal pleura has systemic supply, whereas visceral pleura is supplied by pulmonary and bronchial arteries.
Figure 1.
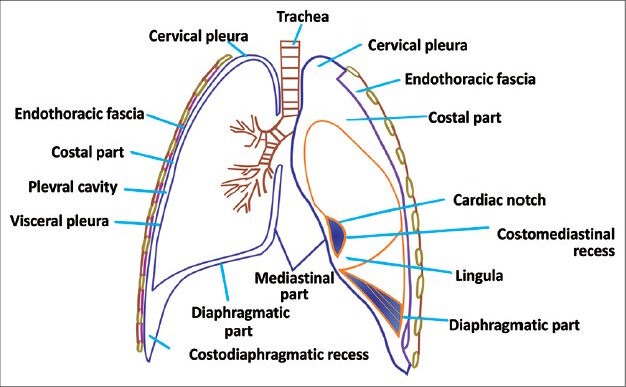
Line diagram of pleura showing different components of pleura
Figure 2.
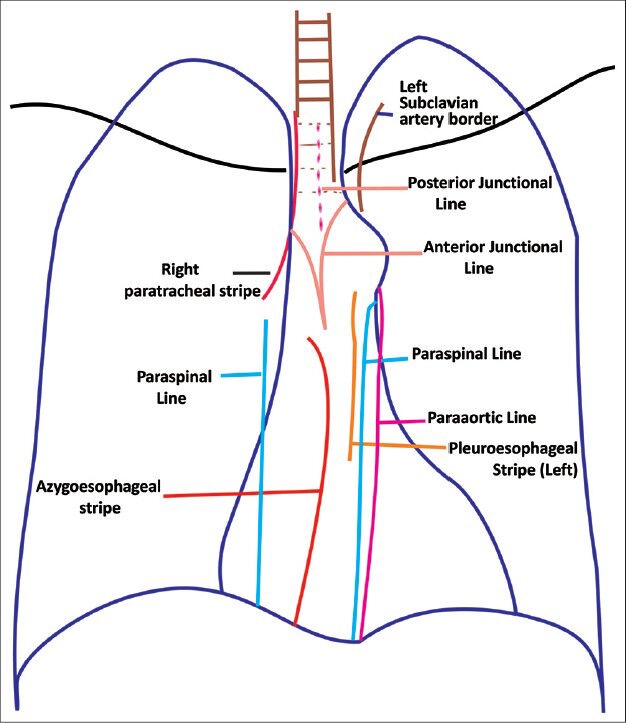
Line diagram showing junctional lines formed by pleural invaginations
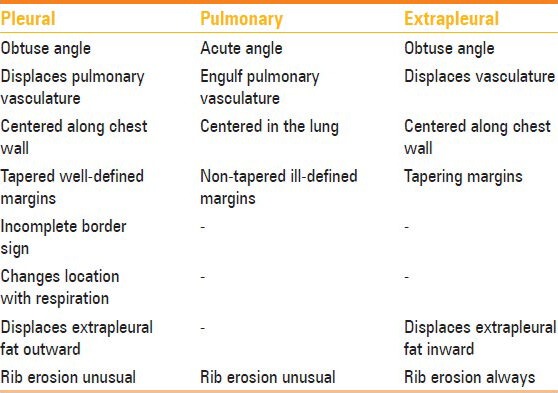
Differentiating pleural, pulmonary, and extrapleural neoplasms
Pulmonary neoplasms usually have acute angles with the chest wall, are centered in the lung, and engulf the pulmonary vasculature. A pleural neoplasm shows obtuse angles with the lateral chest wall with tapered margins [Figures 3 and 4], displaces the pulmonary vasculature, changes its location on respiration, and may show incomplete border sign on chest radiograph – i.e., only a portion of the margin of mass is depicted on chest radiograph. Next step lies in differentiation of pleural from extrapleural origin of the mass. Extrapleural neoplasms may arise from extrapleural fat, ribs, intercostal muscles, and neurovascular bundle; typical pleural neoplasms do not cause erosion of ribs and displace the extrapleural fat outward, while extrapleural neoplasms displace the extrapleural fat inward [Table 1].
Figure 3 (A, B).
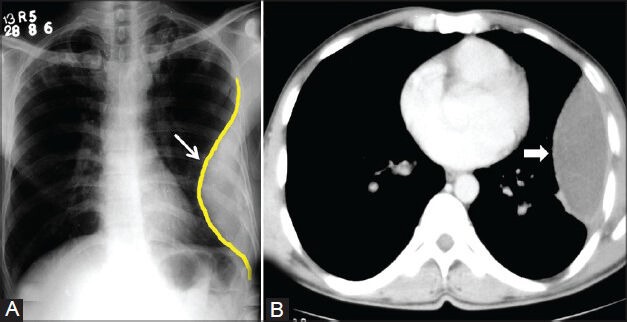
Loculated empyema: (A) Chest radiograph showing pleural-based opacity (arrow) with tapering obtuse margins in left hemithorax; (B) axial contrast-enhanced CT scan showing loculated collection (arrowhead) with peripherally enhancing thick walls
Figure 4 (A, B).
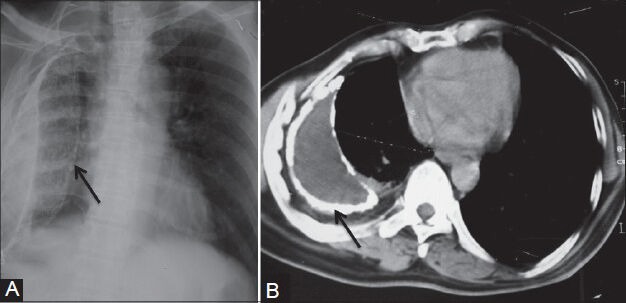
Calcified empyema: (A) Chest radiograph showing volume loss right hemithorax with veil-like calcified (arrow) pleural opacity; (B) axial contrast-enhanced CT scan showing evidence of calcified chronic empyema (arrow) with proliferation of extrapleural fat and crowding of ribs suggestive of volume loss in right hemithorax
Table 1.
Differentiating pleural, pulmonary, and extrapleural neoplasms
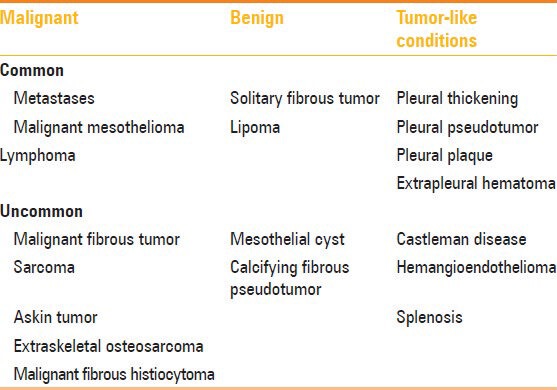
Tumors and tumor-like conditions involving the pleura
Various benign, malignant, and tumor-like conditions can involve the pleura [Table 2]. Malignant neoplasms are more common than benign neoplasms. Pleural tumors can have a varied imaging spectrum – may be unilateral or bilateral, calcified, or noncalcified, and focal or diffuse.
Table 2.
Benign and malignant pathologies of pleura
Pleural thickening
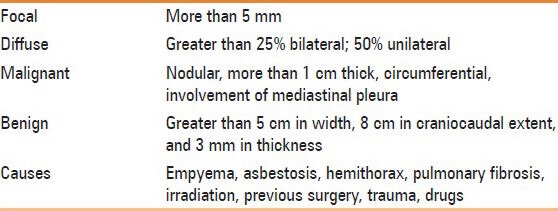
Pleural thickening may be focal or diffuse. Diffuse pleural thickening is defined as thickening of pleura (more than 5 mm) with combined area of involvement more than 25% of chest wall if bilateral and 50% involvement if unilateral.[1] Apical pleural thickening is a normal aging process, but if the thickening is more than 2 cm, it requires further work-up [Figure 5]. On Computed Tomography (CT) scan, malignant pleural thickening is nodular (>1 cm), shows circumferential involvement, and involves the mediastinal pleura. On imaging, benign pleural thickening appears as a diffuse involvement of pleura. Pleural thickening greater than 5 cm in width, 8 cm in craniocaudal extent, and 3 mm in thickness usually suggests a benign etiology [Table 3].[2] Causes of diffuse pleural thickening are empyema, asbestosis, hemothorax, pulmonary fibrosis, irradiation, previous surgery, trauma, and drugs. In developing countries, tuberculosis is an important cause of pleural thickening. Pleural involvement in tuberculosis is either due to rupture of subpleural caseous focus within the lung, hematogenous dissemination, or involvement from an adjacent lymph node. Tubercular pleural involvement may be in the form of pleural effusion, pleural thickening, empyema, bronchopleural or pleurocutaneous fistula, or calcifications. On imaging, volume loss, calcifications, and proliferation of extrapleural fat are suggestive of diffuse benign pleural thickening. Fluorine-18 fluorodeoxyglucose positron emission Computed Tomography (18F-FDG PET CT) cannot reliably differentiate benign and malignant pleural thickening. However, a standardized uptake value (SUVmax) greater than 2 requires further evaluation with clinical correlation or image-guided biopsy.[3,4] Pleural plaques are deposits of hyalinized collagen fibers in the parietal pleura. Pleural plaques may be calcified or noncalcified. On imaging, pleural plaques are seen as focal pleural thickening.
Figure 5.
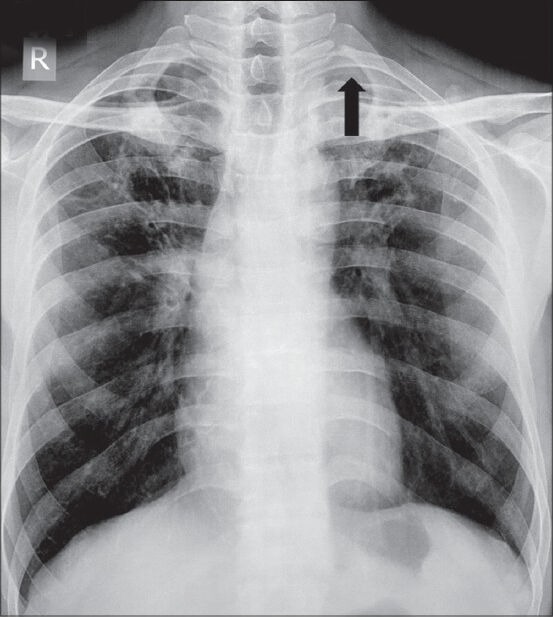
Apical pleural thickening: Chest radiograph showing apical pleural thickening (arrowhead) in left apical region
Table 3.
Pleural thickening
Solitary fibrous tumor
Solitary fibrous tumor of pleura (SFTP) is also known as localized fibrous tumor or localized pleural mesothelioma.[5] It is usually seen in the age group of 45-60 years. Most of these tumors are benign, but in 20% cases, they can be malignant. The tumor usually arises from the visceral pleura in 80% of cases. On imaging, SFTP appears as a soft tissue pleural-based neoplasm with areas of necrosis, hemorrhage, and cystic changes [Figures 6 and 7]. Calcification may be seen in up to 26% of cases. Heterogeneous enhancement is seen post-contrast. On magnetic resonance imaging (MRI), hypointense solid mass is seen on T1- and T2-weighted images. Necrosis and cystic degeneration changes show high T2 signal intensity. Differentiation of benign and malignant fibrous tumors is difficult on imaging. Features suggestive of malignant fibrous tumors are presence of calcification, effusion, atelectasis, mediastinal shift, and chest wall invasion [Figures 8 and 9].[6,7] Presence of stalk also suggests benign nature. On CT, the stalk is identified as a linear soft tissue extending into the pleura/interlobar fissure/hilum. Presence of stalk is also confirmed by change in its location on respiration. Associations of SFTP are clubbing, hypertrophic osteoarthropathy (Pierre–Marie–Bamberger syndrome), and hypoglycemia (Doege–Potter syndrome). Hypoglycemia occurs as a result of the production of insulin-like growth factor II (IGF-II) by these tumors.[8] Hypertrophic osteoarthropathy occurs as a result of production of ectopic growth hormone-like substance and is more common with tumors greater than 7 cm. Histologically, morphology is similar to that of a low-grade spindle cell neoplasm.
Figure 6 (A, B).
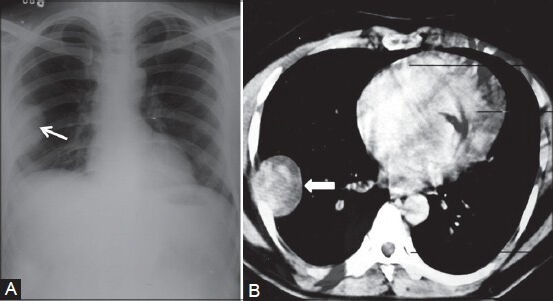
Benign solitary fibrous tumor: (A) Chest radiograph showing pleural-based opacity (arrow) in right hemithorax with peripheral obtuse margins; (B) axial contrast-enhanced CT scan showing heterogeneously enhancing pleural-based mass (arrowhead) proved to be benign fibrous pleural tumor
Figure 7 (A, B).
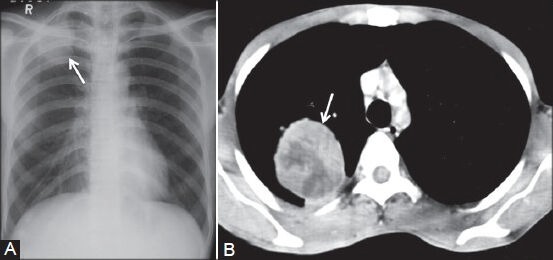
Pleural fibroma: (A) Chest radiograph showing lobulated pleural-based opacity (arrow) in right apical region; (B) axial contrast-enhanced CT scan showing heterogeneously enhancing peripheral mass lesion (arrow) in a biopsy-proven case of benign pleural fibroma
Figure 8.
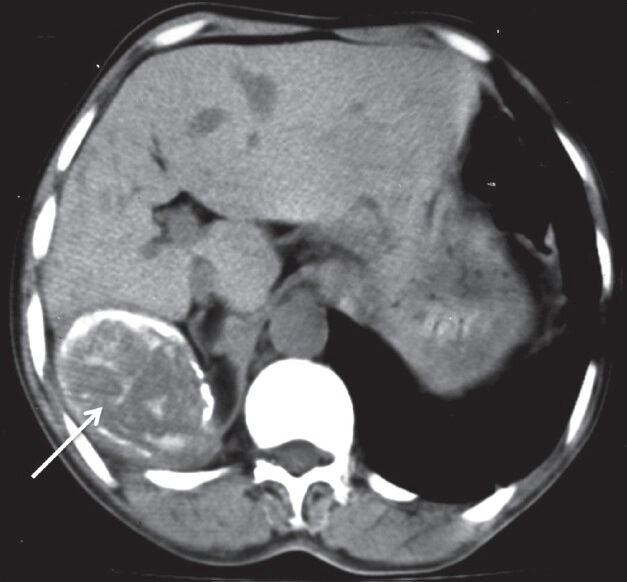
Malignant solitary fibrous tumor of pleura: Plain axial CT scan showing pleural-based soft tissue lesion with peripheral as well as internal calcification (arrow) abutting the liver
Figure 9.
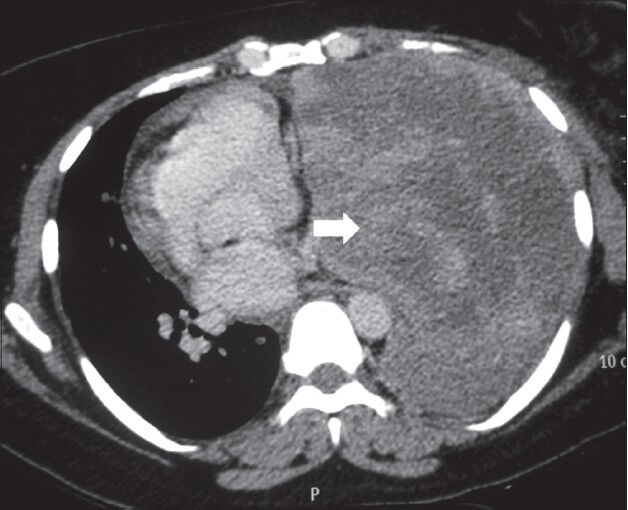
Malignant fibrous tumor of pleura: Axial contrast-enhanced CT scan showing heterogeneously enhancing mass lesion left hemithorax (arrowhead) causing mediastinal displacement to the right
Malignant mesothelioma
Mesothelioma is a highly malignant and locally aggressive tumor seen in the sixth or seventh decade of life. It is associated with asbestos exposure, with an average latency of 35-40 years for its development. Hypertrophic osteoarthropathy and intermittent hypoglycemia are less common than SFTP. Most carcinogenic form of asbestos is crocidolite. Insulation workers, shipyard workers, construction workers, workers in heating trades, and asbestos miners are at greatest risk. Other factors which predispose to development of mesothelioma are radiation therapy, tuberculosis, and chronic empyema. On imaging, diffuse nodular pleural thickening, pleural plaques, and pleural effusion are usually seen [Figures 10 and 11]. The latent period for pleural plaque formation is usually 20 years and presence of pleural plaques is a strong indicator of asbestos exposure. Typically, pleural plaque is seen adjacent to ribs, involving sixth to ninth ribs. Pleurae along the intercostal spaces, costophrenic angles, and lung apices are less frequently involved. Large pleural effusion without mediastinal shift may also be seen [Figures 12 and 13]. Calcifications are seen involving the diaphragmatic parietal pleura [Figure 14].[9,10] On MRI, the lesions show low to intermediate signal intensity on T1-W images and high signal intensity on T2-W images with post-contrast enhancement. Differentiation from metastatic carcinoma is difficult; however, unilateral involvement and volume loss of affected hemithorax favors mesothelioma. Imaging criteria for unresectability includes encasement of diaphragm and involvement of extrapleural fat, ribs, or other mediastinal structures.[11]
Figure 10.
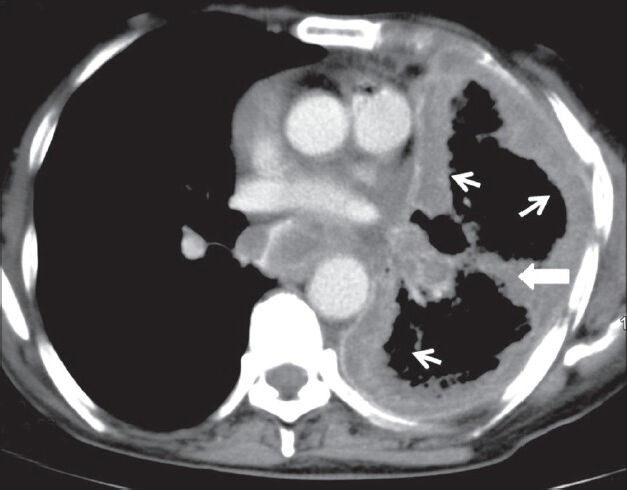
Malignant mesothelioma: Axial contrast-enhanced CT scan showing enhancing nodular pleural thickening (arrows) involving the costal and mediastinal pleura, extending into the major fissure (arrowhead) with crowding of ribs suggestive of volume loss changes in left hemithorax
Figure 11.
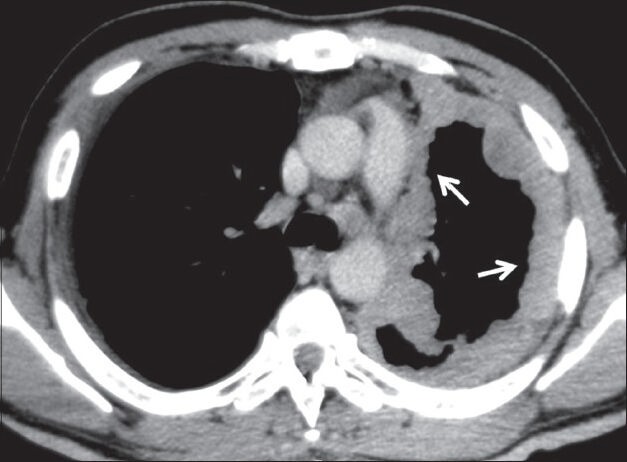
Malignant mesothelioma: Axial contrast-enhanced CT scan showing homogeneously enhancing nodular pleural thickening (arrows) involving the mediastinal and costal pleura with volume loss changes in left hemithorax
Figure 12.
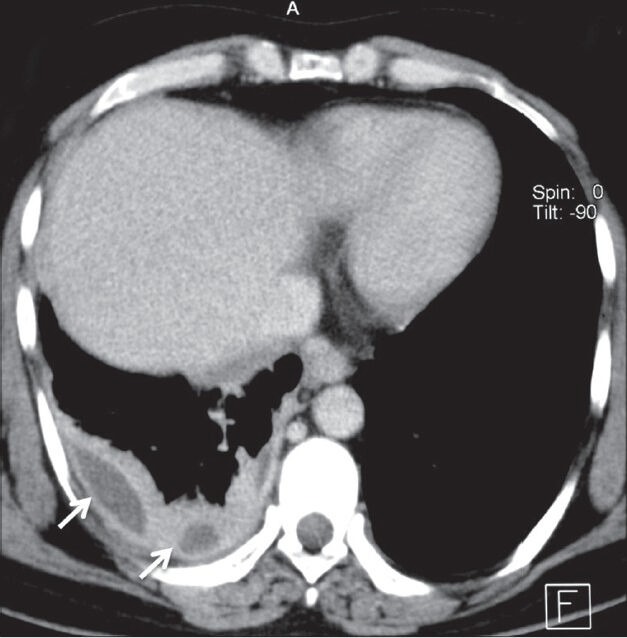
Mesothelioma presenting as pleural collections: Axial contrast-enhanced CT scan showing nodular thickening of pleura involving right hemithorax with small pleural collections (arrows)
Figure 13.
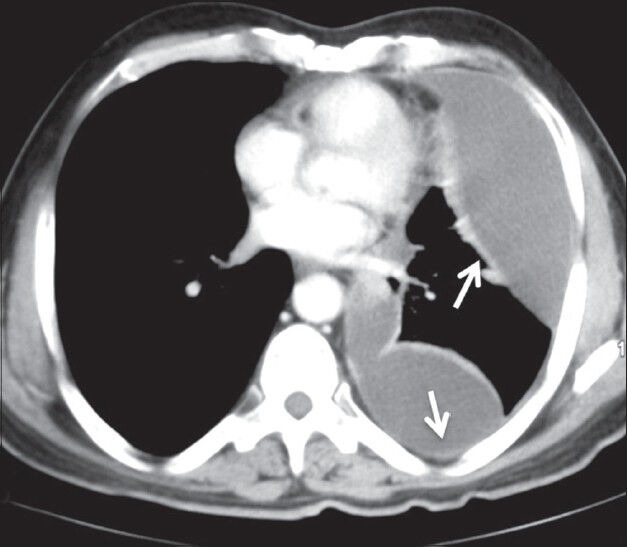
Mesothelioma presenting as a pleural effusion: Axial contrastenhanced CT scan showing moderate left pleural effusion as loculated collection with thickening of pleura (arrows) in a case of mesothelioma
Figure 14 (A, B).
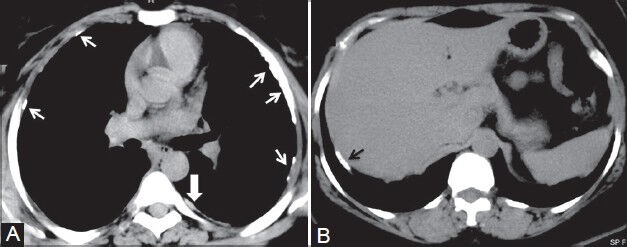
Mesothelioma and pleural plaques: (A) Axial plain CT scan showing calcified (arrows) and noncalcified (arrowhead) pleural plaques; (B) axial plain CT scan image showing calcified plaque (black arrow) classically involving the diaphragmatic parietal pleura in a construction worker
Lymphoma
Both Hodgkin's and non-Hodgkin's lymphoma can involve the pleura. On imaging, effusion, pleural nodules, focal or diffuse pleural thickening may be seen, which show homogeneous contrast enhancement. Associated mediastinal and hilar lymphadenopathy is also seen [Figures 15 and 16]. Cystic/necrotic changes and calcification are seen post-chemotherapy. Circumferential pleural involvement is less common in lymphoma.
Figure 15.
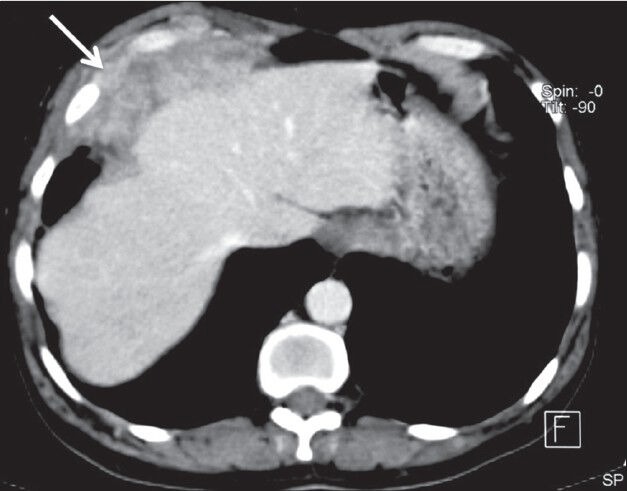
Pleural lymphoma: Axial contrast-enhanced CT scan showing heterogeneously enhancing lobulated mass lesion involving the diaphragmatic pleura (arrow) and invading the chest wall in a case of high-grade lymphoma
Figure 16.
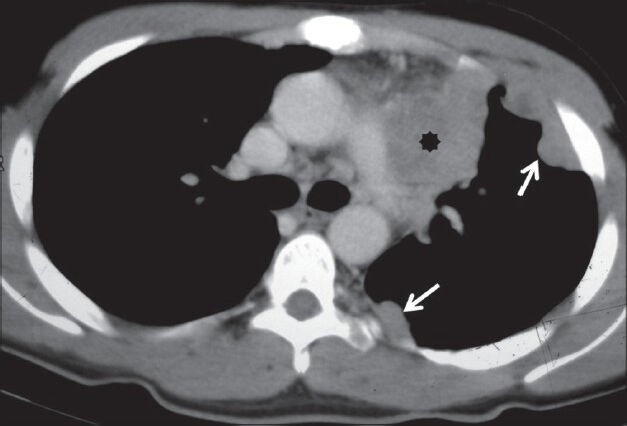
Pleural lymphoma: Axial contrast-enhanced CT scan showing homogeneously enhancing nodular pleural thickening (arrows) involving the costal pleura with mediastinal lymphadenopathy (asterisk)
Calcifying fibrous pseudotumor
The term calcifying fibrous pseudotumor was coined by Fetsch et al. in 1993.[12] Previously, these tumors were termed as “childhood fibrous tumor with psammoma bodies.” These neoplasms occur in children and young adults. History of previous inflammation is a prerequisite for the diagnosis. On imaging, extensive solitary or multifocal masses with calcifications are seen [Figures 17 and 18].[13,14]
Figure 17 (A, B).
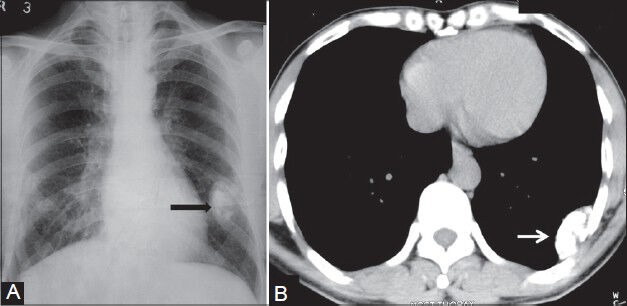
Calcifying fibrous pseudotumor: (A) Chest radiograph showing pleural-based calcified opacity (arrowhead) left hemithorax with incomplete border sign; (B) plain axial CT scan image showing pleural-based calcified lesion (arrow) with no destruction of underlying ribs
Figure 18.
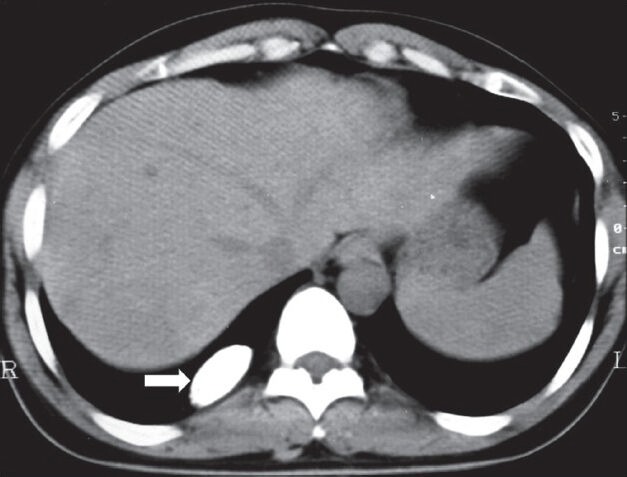
Calcifying fibrous pseudotumor: Plain axial CT scan showing calcified pleural-based opacity in right hemithorax (arrowhead)
Pleural metastases
Adenocarcinomas are known to cause pleural metastasis more frequently than other histological types of cancers. Common primary sites are from lung, breast, lymphoma, and ovary [Figures 19–21]. Invasive thymoma can also involve the pleura [Figure 22]. On imaging, pleural effusion is the most common finding. Diffuse or focal nodular pleural thickening may be seen. Increased 18F-FDG uptake on PET-CT is seen in malignant pleural thickening and effusion.[2]
Figure 19.
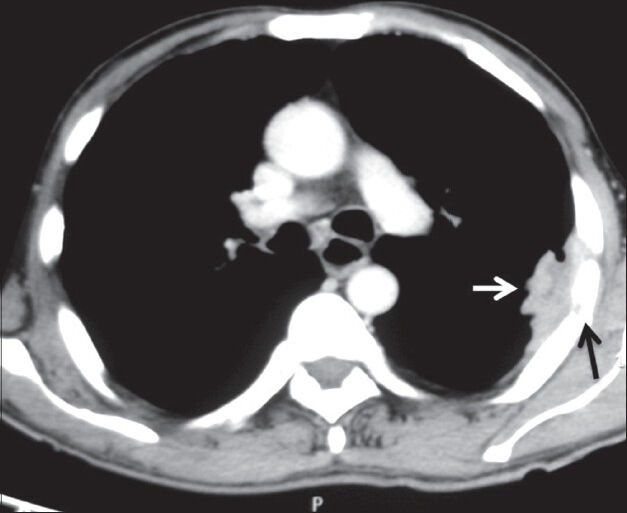
Pleural metastases: Axial contrast-enhanced CT scan showing heterogeneously enhancing pleural-based soft tissue (white arrow) with rib destruction (black arrow) in a case of pleural metastases from renal cell carcinoma
Figure 21.
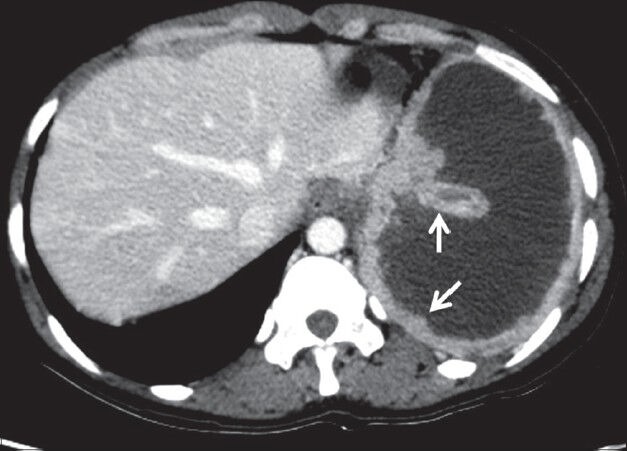
Pleural metastases: Axial contrast-enhanced CT scan showing nodular pleural thickening (arrows) involving the costal and mediastinal pleura with malignant pleural effusion in a case of metastatic ovarian adenocarcinoma
Figure 22.
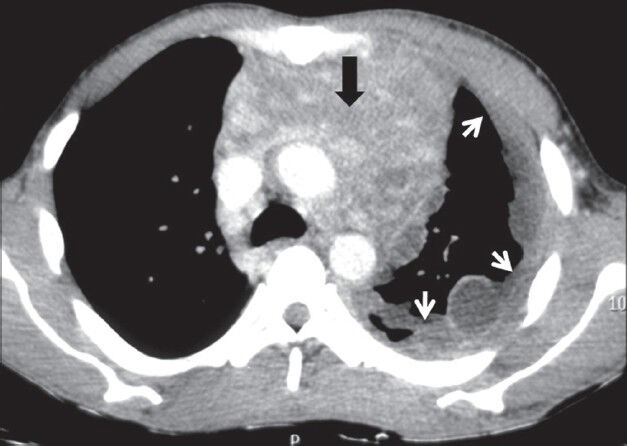
Pleural drop metastases in invasive thymoma: Axial contrast-enhanced CT image showing heterogeneously enhancing anterior mediastinal mass (arrowhead) with mild left pleural effusion and ipsilateral pleural implants (arrows)
Figure 20.
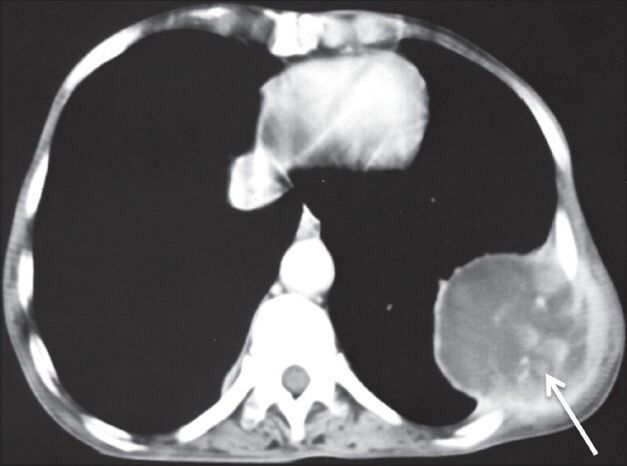
Pleural metastases: Axial contrast-enhanced CT scan showing heterogeneously enhancing pleural-based mass lesion (arrow) in left hemithorax with extrathoracic extension in a case of metastatic adenocarcinoma
Askin tumor
Askin tumor is an aggressive malignant tumor of primitive neuroectodermal origin belonging to the Ewing tumor family. Most of these tumors arise from the soft tissues of the chest wall or lung periphery. It is usually seen in children and adolescents. On histopathology, malignant, small round cells with Homer–Wright rosettes are seen. Balanced reciprocal chromosomal translocation between chromosomes 11 and 22 is diagnostic. On imaging, unilateral involvement is generally seen in the form of nodular pleural thickening. Infiltration into the chest wall, mediastinum, and sympathetic chain is pathognomonic. Pleural effusion and rib destruction may or may not be seen [Figure 23].
Figure 23 (A, B).
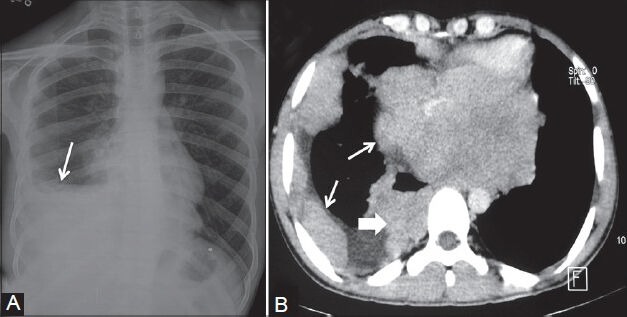
Askin tumor: (A) Chest radiograph showing inhomogeneous opacity (arrow) right hemithorax obscuring right hemidiaphragm without mediastinal shift; (B) axial contrast-enhanced CT scan showing heterogeneously enhancing nodular pleural-based lesions (arrows) involving the costal and mediastinal pleura with characteristic involvement of the sympathetic chain (arrowhead) in right paraspinal region
Rare pathologies of pleura
Pleural lipoma
Pleural lipoma is often an incidental finding. It is one of the most common benign tumors of pleura. On CT, lipoma shows fat density and no contrast enhancement. Presence of enhancing septa within the mass suggests liposarcoma.
Pleural splenosis
Pleural splenosis results from displaced splenic tissue into the thorax following trauma on the left side. On imaging, multiple soft tissue lesions of variable sizes are seen implanted on pleura, with enhancement similar to splenic tissue. Gold standard for diagnosis is scintigraphy with 99mTc heat-damaged tagged erythrocytes.
Other rare pathologies of pleura are mesothelial cysts, epithelioid hemangioendothelioma, Castleman disease, sarcomas [Figure 24], malignant fibrous histiocytoma, leukemic infiltration, Erdheim–Chester disease, and extraskeletal osteosarcoma. Extraskeletal osteosarcoma is a rare malignant neoplasm and constitutes 1.2% of all soft tissue sarcomas. It should be considered in the differential diagnosis for a rapidly growing calcified pleural mass in an elderly. Other causes of malignant pleural calcification are metastasis from osteosarcoma, chondrosarcoma, parosteal osteosarcoma, and mesothelioma.[15]
Figure 24 (A, B).
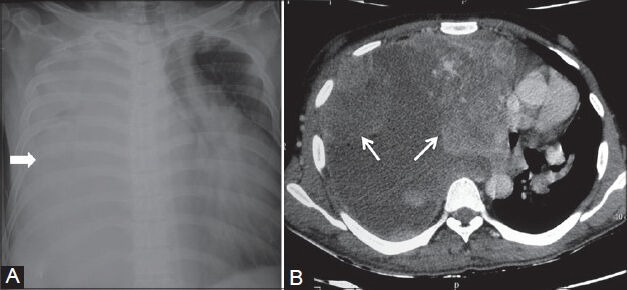
Spindle cell sarcoma of pleura: (A) Chest radiograph showing complete opacification of right hemithorax (arrowhead) with mediastinal shift to the left; (B) axial contrast-enhanced CT scan showing heterogeneously enhancing nodular pleural-based lesions with pleural effusion displacing the heart to the left
Pleural pseudotumor[16] is fluid collection within a lung fissure. Most common site for pseudotumor is minor fissure. Common causes of pleural pseudotumor are congestive heart failure, cirrhosis, and renal insufficiency. On chest radiographs, classical lenticular or biconvex opacity is seen in the fissure. It usually resolves after therapy with diuretic agents.
Conclusion
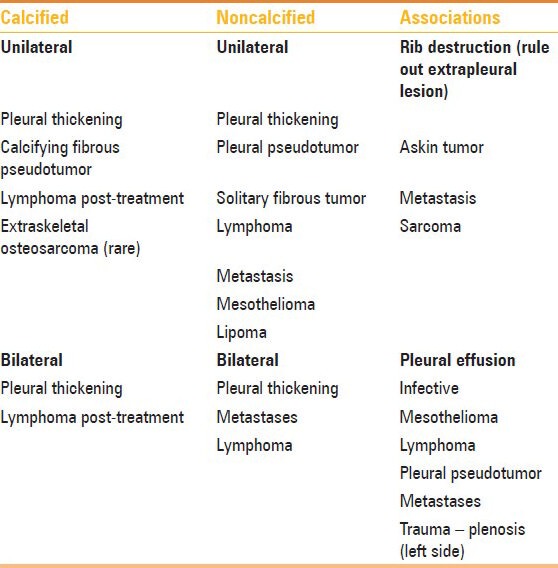
An approach to correct diagnosis of pleural tumors depends on the pattern of involvement – focal or diffuse, unilateral or bilateral, and calcified or noncalcified [Table 4]. The role if imaging is to identify pleural thickening, differentiate benign and malignant pleural thickening, and identify the cause if possible. An appropriate clinical history, imaging findings and, if required, image-guided biopsy may be used to clinch the diagnosis.
Table 4.
Approach to diagnosis of pleural pathologies
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Downer NJ, Ali NJ, Au-Yong IT. Investigating pleural thickening. BMJ. 2013;346:e8376. doi: 10.1136/bmj.e8376. [DOI] [PubMed] [Google Scholar]
- 2.Jelen TH, Bankier AA, Eisenberg RL. Solid Pleural Lesions. Am J Roentgenol. 2012;198:W512–20. doi: 10.2214/AJR.11.7626. [DOI] [PubMed] [Google Scholar]
- 3.Alavi A, Gupta N, Alberini JL, Hickeson M, Adam LE, Bhargava P, et al. Positron emission tomography imaging in nonmalignant thoracic disorders. Semin Nucl Med. 2002;32:293–321. doi: 10.1053/snuc.2002.127291. [DOI] [PubMed] [Google Scholar]
- 4.Makis W, Ciarallo A, Hickeson M, Rush C, Novales-Diaz JA, Derbekyan V, et al. Spectrum of malignant pleural and pericardial disease on FDG PET/CT. AJR Am J Roentgenol. 2012;198:678–85. doi: 10.2214/AJR.11.7076. [DOI] [PubMed] [Google Scholar]
- 5.Cardillo G, Facciolo F, Cavazzana AO, Capece G, Gasparri R, Martelli M. Localized (solitary) fibrous tumors of the pleura: An analysis of 55 patients. Ann Thorac Surg. 2000;70:1808–12. doi: 10.1016/s0003-4975(00)01908-1. [DOI] [PubMed] [Google Scholar]
- 6.Fraser MD, Müller NL, Colman N, Paré PD. Pleural neoplasms. In: Fraser MD, Paré PD, editors. Diagnosis of diseases of the chest. 4th ed. Philadelphia: WB Saunders Company; 1999. pp. 2807–47. [Google Scholar]
- 7.Rosado-de-Christenson ML, Abbott GF, McAdams HP, Franks TJ, Galvin JR. Localized fibrous tumors of the pleura. Radiographics. 2003;23:759–83. doi: 10.1148/rg.233025165. [DOI] [PubMed] [Google Scholar]
- 8.Sun ZG, Wang Z, Zhang M. A 70-year-old man with hypoglycemia, clubbing of fingers and toes, and a large mass of the right hemithorax. Chest. 2011;139:1528–31. doi: 10.1378/chest.10-2463. [DOI] [PubMed] [Google Scholar]
- 9.Larson TC, Meyer CA, Kapil V, Gurney JW, Tarver RD, Black CB, et al. Workers with Libby amphibole exposure: Retrospective identification and progression of radiographic changes. Radiology. 2010;255:924–33. doi: 10.1148/radiol.10091447. [DOI] [PubMed] [Google Scholar]
- 10.Pairon JC, Laurent F, Rinaldo M, Clin B, Andujar P, Ameille J, et al. Pleural plaques and the risk of pleural mesothelioma. J Natl Cancer Inst. 2013;105:293–301. doi: 10.1093/jnci/djs513. [DOI] [PubMed] [Google Scholar]
- 11.Weyant MJ, Flores RM. Imaging of pleural and chest wall tumors. Thorac Surg Clin. 2004;14:15–23. doi: 10.1016/S1547-4127(04)00033-7. [DOI] [PubMed] [Google Scholar]
- 12.Fetsch JF, Montgomery EA, Meis JM. Calcifying fibrous pseudotumor. Am J Surg Pathol. 1993;17:502–8. doi: 10.1097/00000478-199305000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Erasmus JJ, McAdams HP, Patz EF, Jr, Murray JG, Pinkard NB. Calcifying fibrous pseudotumor of pleura: Radiologic features in three cases. J Comput Assist Tomogr. 1996;20:763–5. doi: 10.1097/00004728-199609000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Suh JH, Shin OR, Kim YH. Multiple calcifying fibrous pseudotumor of the pleura. J Thorac Oncol. 2008;3:1356–8. doi: 10.1097/JTO.0b013e318186a87a. [DOI] [PubMed] [Google Scholar]
- 15.Sabloff B, Munden RF, Melhem AI, El-Naggar AK, Putnam JB. Extraskeletal Osteosarcoma of the Pleura. Am J Roentgenol. 2003;180:972. doi: 10.2214/ajr.180.4.1800972. [DOI] [PubMed] [Google Scholar]
- 16.Walker CM, Takasugi JE, Chung JH, Reddy GP, Done SL, Pipavath SN, et al. Tumorlike conditions of the pleura. Radiographics. 2012;32:971–85. doi: 10.1148/rg.324115184. [DOI] [PubMed] [Google Scholar]


