Abstract
Imaging features of fat necrosis vary depending on its stage of evolution and can mimic malignancy in late stages. Imaging may suffice to differentiate fat necrosis in the early stages from malignancy and thus avoid unnecessary biopsy. In this pictorial essay, we present combination of benign features in mammography and/or ultrasonography (USG) that can lead to imaging diagnosis of fat necrosis. The follow-up imaging features of fat necrosis which mirror its pathophysiological evolution have also been demonstrated. To summarize, in the appropriate clinical setting, no mammographic features suspicious for malignancy should be present. When the typical mammographic features are not present, USG can aid with the diagnosis and follow up USG can confirm it.
Keywords: Breast lump, complex nodule, fat necrosis, imaging, solid nodule
Introduction
Fat necrosis is a benign non-suppurative inflammatory process of adipose tissue. The incidence of the disease is estimated to be 0.6%, accounting for 2.75% of all benign breast lesions.[1] Majority present with a palpable lump, typically periareolar,[2] that can clinically mimic malignancy and pose a diagnostic challenge.
If a patient presents for evaluation in the early stages of fat necrosis, a systematic approach using American College of Radiology (ACR) guidelines[3] can help to avoid misdiagnosis and ensure confident exclution of malignancy. In this essay, we highlight the various combinations of imaging features of fat necrosis that suggest benignity in appropriate clinical setting.
Clinical Features
The presentation can vary from being clinically occult to a hard lump with skin changes highly suspicious for malignancy. A history of accidental trauma raises the suspicion of fat necrosis in a breast lump. The other common predisposing causes include surgery and radiation.[2] The possibility of malignancy should not be overlooked in these cases. The absence of history of trauma does not exclude fat necrosis.[4]
Evolution of Fat Necrosis and Corresponding Imaging Findings
Imaging appearances of fat necrosis depend on its stage of evolution.[1,2] In the early phase, when there is hemorrhage in the fat, initiating edema of the breast trabeculae, it may be seen as an area of hyperreflectivity on USG.
Those which do not resolve, progress to cystic degeneration within weeks to months and are seen as oil-containing cavities on gross pathology[2] If large, the corresponding imaging findings at this stage would include oil cyst on mammogram, and if small, these would be anechoic areas within the hyperreflective area on USG. Calcification and fibrotic reaction occur late over months or years and imaging appearances at this stage can mimic malignancy[2] unless the characteristic benign lucent-centered or coarse rim calcifications are seen.
The diagnosis can be confirmed on the basis of either serial imaging studies that show chronological changes compatible with the evolution of fat necrosis or improvement in clinical symptoms (lump no longer detectable on palpation). Histological confirmation may be reserved for indeterminate or suspicious imaging features.
Mammographic Appearances
Mammography is the most important diagnostic tool in early fat necrosis.[2] However a normal mammogram does not always exclude underlying pathology[5] and the same holds true for fat necrosis.
A radiolucent well-defined cyst [Figure 1] is one of the pathognomonic mammographic features seen in early fat necrosis when there is little associated fibrosis. Fat-fluid level when observed [Figure 2] is due to oil and serosanguinous fluid layering.[6] Benign lucent-centered calcification, a characteristic late stage feature[2] [Figure 3], also requires no additional workup.[4,6]
Figure 1.
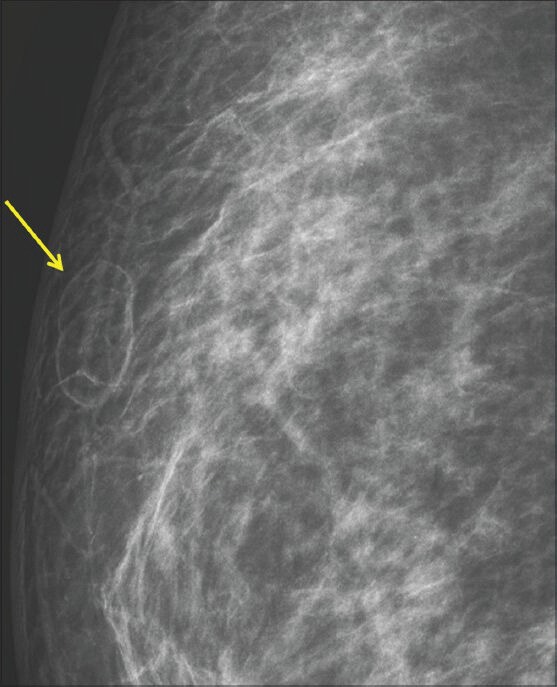
A 32-year-old female referred for imaging with bimanual fine needle aspiration results suspicious for malignancy. There was a history of prior insignificant trauma. Right mediolateral oblique (MLO) zoomed view shows fat density lesion (arrow) in the upper outer quadrant of the breast (BIRADS 2)
Figure 2.
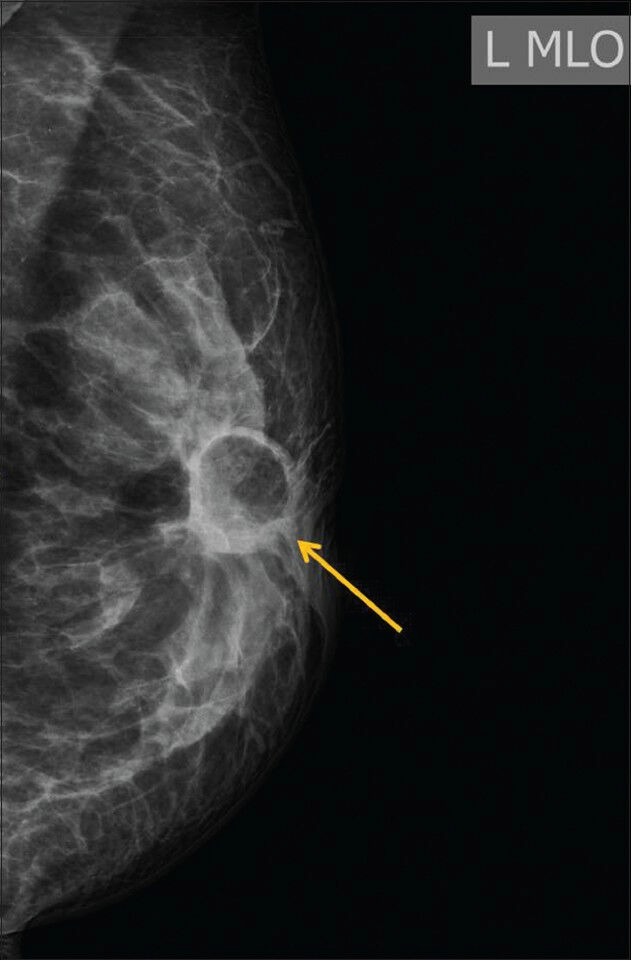
A 38-year-old female presented with hard lump at the site of lumpectomy performed 9 months prior to presentation. Mammogram shows a fat-containing lesion with a small fat-fluid level at the scar site (BIRADS 2)
Figure 3.
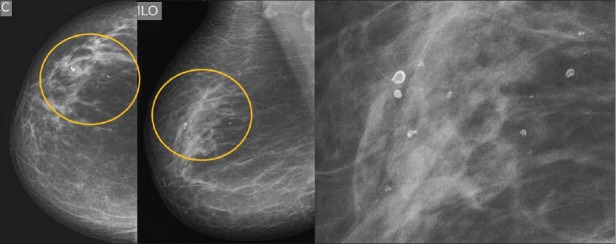
Screening mammogram in a 58-year-old female with history of trauma 20 years ago is showing benign lucent-centered calcification in the upper outer quadrant (regional distribution) of the right breast (BIRADS 2). Zoomed view of the calcification (extreme right)
If a soft tissue density mass with partial halo [Figure 4] or focal asymmetric density without architectural distortion [Figure 5] is seen on mammogram at site of palpable abnormality, US can confirm diagnosis of fat necrosis.
Figure 4.
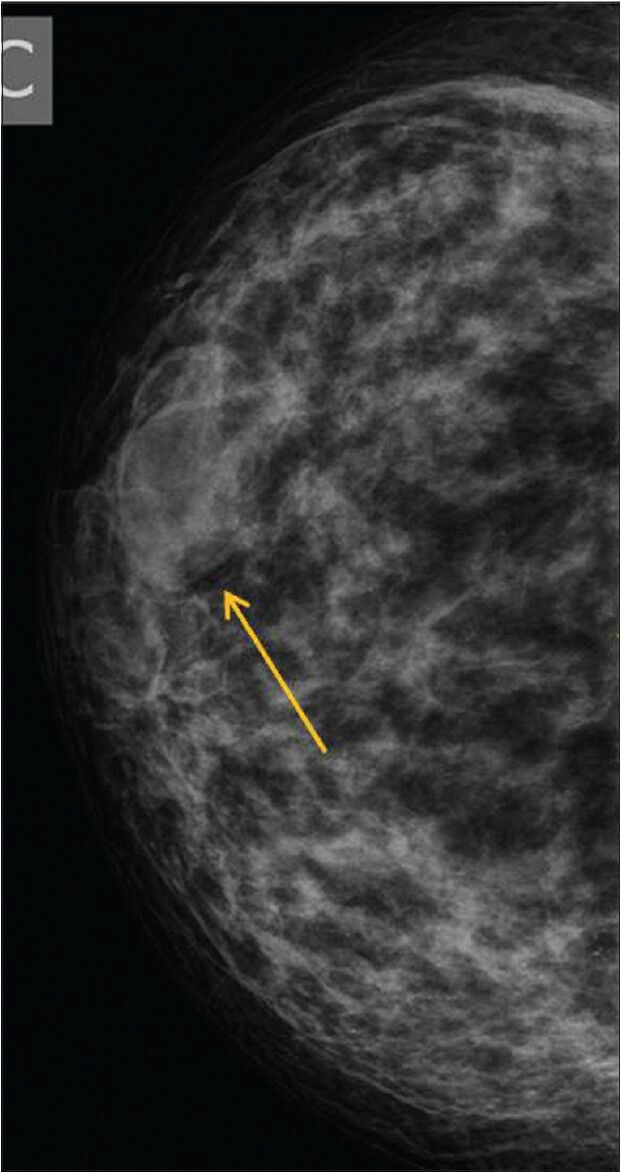
A 35-year-old female with pain and swelling of 1 year duration. There was no history of trauma. Right MLO view showing a soft density with peripheral halo suggesting benign lesion (BIRADS 3)
Figure 5.
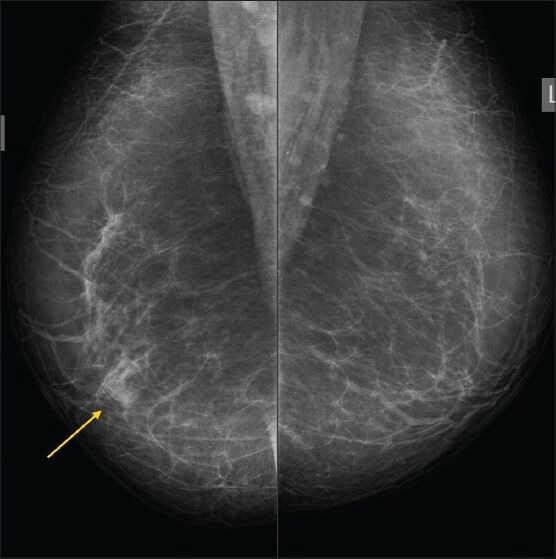
A 54-year-old female with painful lump of short duration. There was no history of trauma. Focal asymmetric density (arrow) is seen in the region of the palpable lump on the right MLO view (BIRADS 3)
When any suspicious features for malignancy such as dense/irregular/spiculated mass, architectural distortion, or suspicious calcification are found on mammogram, the categorization would be BIRADS 4 or 5 (suspicious for malignancy) and biopsy should be considered according to ACR recommendation. It is important to categorize the BIRADS for mammography first before performing the USG to minimize the chances of overlooking malignancy, as USG is less specific than mammography. Hence, even if the USG features appear probably benign, combined BIRADS 4a categorization with biopsy would be considered more appropriate than BIRADS 3 [Figure 6].[3,7]
Figure 6 (A, B).
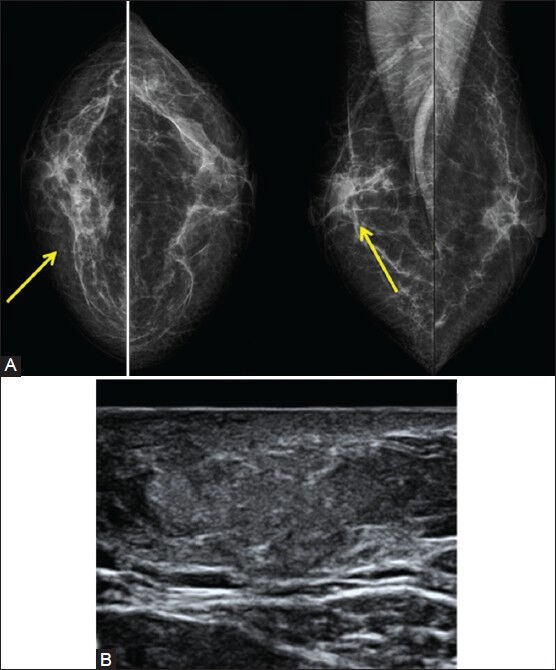
(A) A 50-year-old female presented with hard tender lump. Bilateral mammogram shows an area of architectural distortion (arrows) at the symptomatic site, BIRADS 4. (B) USG image was less suspicious with a homogenous ill-defined hyperreflective lesion. A combined BIRADS 4a was assigned and USG-guided biopsy confirmed fat necrosis. The history of trauma was elicited on direct questioning in retrospect
Sonographic Appearances
USG plays an important role in ruling out malignancy and suggesting fat necrosis as the diagnosis. The USG examination is abnormal in almost all the cases including those cases with normal mammogram.[8]
An echogenic band within an oil cyst that shifts in orientation with changes in patient position [Figure 7] is the most specific feature of fat necrosis[8]
Figure 7.
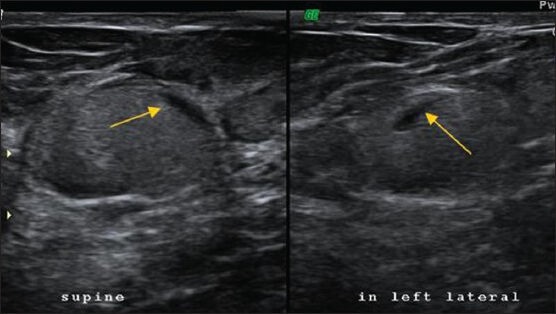
USG image of an ovoid lesion with homogenous mobile echoes tender on probe pressure. The fluid-fluid level (arrow) was mobile with patient movement. BIRADS 2. Note that the plane of the lesion is in the deeper fibroglandular plane and not the typical superficial fat plane
Hyperechogenicity in the subcutaneous tissue [Figure 8], which is a reliable predictor of benignity, is the most common presentation of fat necrosis on USG and often seen in all cases with history of trauma.[4] In the anterior superficial plane of the breast, there is subcutaneous fat with connective tissue, but in the deeper parenchymal layer, fat is interspersed in between the fibroglandular tissue.[9] Hence, one needs to be cautious about hyperechoic nodule in the deeper tissue planes, and findings like “taller-than-wide” morphology, irregular shape, posterior acoustic shadowing need to be given due importance when considering the nature of the lesion.[10] The plane of location [Figure 7] is not particularly important when an oil cyst can be identified as it is a very specific sign for fat necrosis.
Figure 8.
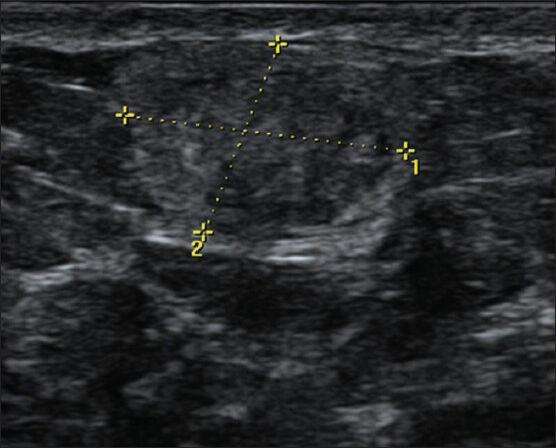
USG image showing a partly ill-defined isoechoic parallel lesion in a superficial location. BIRADS 3
Parallel orientation, an USG feature supportive of benign etiology, is very important to note in cases with solid abnormality [Figure 9]. Lack of flow on Doppler [Figure 10] is a supportive feature of fat necrosis,[2] but literature suggests that it is not a reliable discriminator between benign and malignant nodules.[11]
Figure 9.
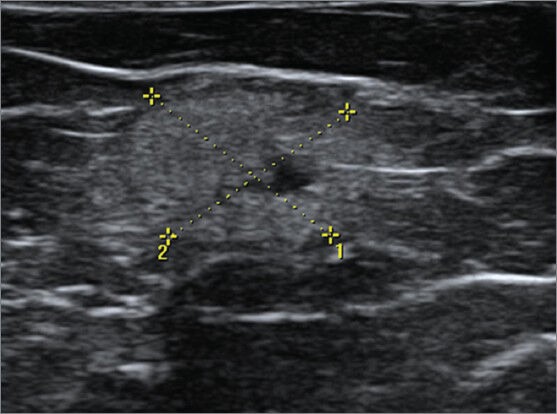
A 56-year-old female with history of trauma and lump. USG shows an ill-defined hyperechoic mass with anechoic component at the site of the palpable lump quite superficial in location. Note the displacement of the tissue planes and the parallel orientation of the lesion suggesting benignity. BIRADS 3
Figure 10.
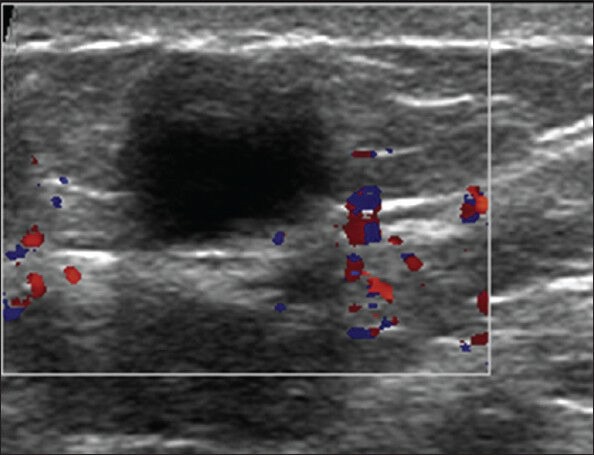
USG image of an anechoic lesion in superficial location showing no vascularity within the color box
The criteria for probably benign nodule are solid mass with oval shape, circumscribed margins, and parallel orientation as per the BIRADS lexicon.[3] However, in the study by Soo et al.,[8] the BIRADS criteria pertaining to shape and margins were not fulfilled by the solid nodules. Some malignant and indeterminate features on USG which may be observed include noncircumscribed (indistinct) border with anechoic areas within [Figure 11] and posterior acoustic shadowing [Figure 12].
Figure 11.
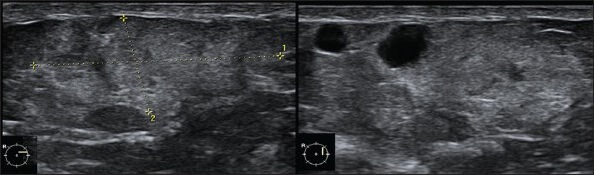
A 34-year-old female with breast lump and history of trauma. USG image of the palpable lump shows an ill-defined iso- to hyperechoic area in the superficial tissues with anechoic areas within. No obvious architectural distortion (BIRADS 3). The lump resolved in 12 months time
Figure 12.
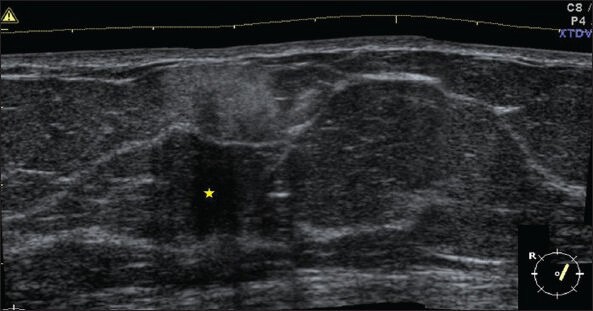
USG image of cytologically proven fat necrosis shows superficially located ill-defined hyperreflective lesion with marked posterior acoustic shadowing (*)
Follow-up Imaging
The recommended follow-up of probably benign USG lesions includes repeat imaging at 6-monthly interval for a year and annually for either 1or 2 years with a diagnostic mammogram and/or USG.[11] The common findings in follow-up USGs include normalization of the subcutaneous reflectivity, development of anechoic areas [Figures 8 and 13], and solid and complex lesions becoming more cystic. The lesion size either remains stable or decreases.[4] The lesions can become more solid or increase in size.[8] In cases of uncertainty, especially with a complex nodule at initial presentation, fluid aspiration is recommended.[12] If the aspirate is oily material, the differential diagnosis of malignant complex mass is excluded and fat necrosis is confirmed. Biopsy should be reserved for cases with bloody fluid aspirate.[12] Other findings include flattening of the lesion [Figure 14] and resorption of the more pure oil fat and increase in the serosanguinous fluid [Figure 15].
Figure 13.
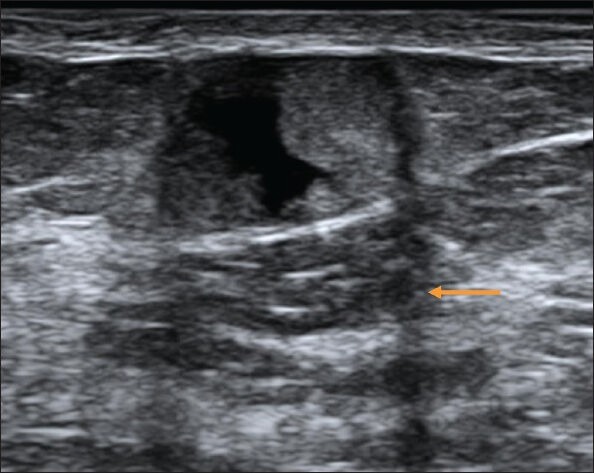
Follow-up USG image of the lesion shown in Figure 8 at 3 months, which shows anechoic component within the lesion. Edge shadowing seen here (arrow) is neither a benign nor a malignant feature
Figure 14.
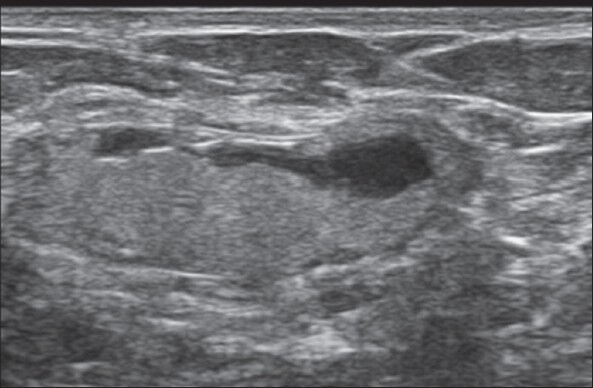
Follow-up image at 4 months of the lesion shown in Figure 7, which shows flattening of the ovoid lesion. Excision biopsy for signs of infection at 10 months showed no evidence of malignancy
Figure 15.
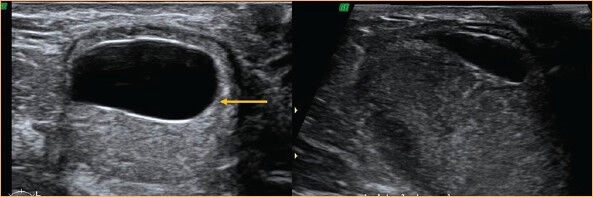
USG image of an oil cyst with echogenic band (arrow) is shown on the left. Follow-up scan at 3 months (right) shows resorption of much of the fluid component
To conclude, when clinically fat necrosis is suspected, the mammogram is the most important diagnostic tool. If there are no suspicious features of malignancy on the mammogram, then USG appearances can be relied upon to give a diagnosis of early fat necrosis. Oil cyst is a pathognomonic USG finding. Other features include a complex nodule in superficial location or solid hyperreflective focal abnormality with parallel orientation. USG follow-up can be sufficient to confirm diagnosis. However, in case of suspicious features on mammogram, biopsy is recommended, despite apparently benign sonographic appearances. Figure 16 summarizes the approach to diagnose fat necrosis.
Figure 16.
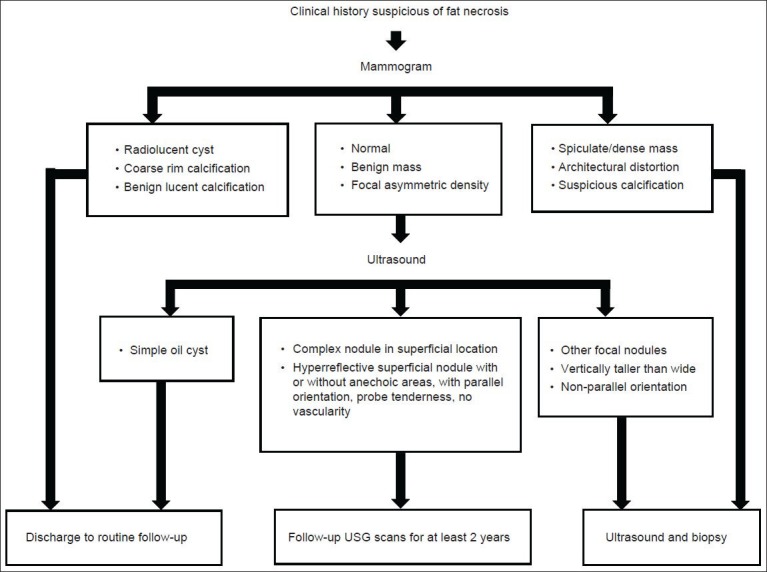
A suggested approach for diagnosis of fat necrosis
Acknowledgement
We thank Anupama Bhat (Strand Life Sciences) for helpful edits.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Tan PH, Lai LM, Carrington EV, Opaluwa AS, Ravikumar KH, Chetty N, et al. Fat necrosis of the breast-a review. Breast. 2006;15:313–8. doi: 10.1016/j.breast.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Taboada JL, Stephens TW, Krishnamurthy S, Brandt KR, Whitman GJ. The many faces of fat necrosis in the breast. AJR Am J Roentgenol. 2009;192:815–25. doi: 10.2214/AJR.08.1250. [DOI] [PubMed] [Google Scholar]
- 3.ACR BI-RADS Breast imaging reporting and data system: Breast imaging: Atlas. 1st ed, 4th ed. Reston, Virginia: American College of Radiology; 2003. BI-RADS®–Ultrasound. American College of Radiology (ACR) [Google Scholar]
- 4.Bilgen IG, Ustun EE, Memis A. Fat necrosis of the breast: Clinical, mammographic and sonographic features. Eur J Radiol. 2001;39:92–9. doi: 10.1016/s0720-048x(00)00303-x. [DOI] [PubMed] [Google Scholar]
- 5.Moy L, Slanetz PJ, Moore R, Satija S, Yeh ED, McCarthy KA, et al. Specificity of mammography and US in the evaluation of a palpable abnormality: Retrospective review. Radiology. 2002;225:176–81. doi: 10.1148/radiol.2251010999. [DOI] [PubMed] [Google Scholar]
- 6.Evers K, Troupin RH. Lipid cyst: Classic and atypical appearances. AJR Am J Roentgenol. 1991;157:271–3. doi: 10.2214/ajr.157.2.1853804. [DOI] [PubMed] [Google Scholar]
- 7.Harvey JA, Nicholson BT, Cohen MA. Finding early invasive breast cancers: A Practical Approach. Radiology. 2008;248:61–76. doi: 10.1148/radiol.2481060339. [DOI] [PubMed] [Google Scholar]
- 8.Soo MS, Kornguth PJ, Hertzberg BS. Fat necrosis in the breast: Sonographic features. Radiology. 1998;206:261–9. doi: 10.1148/radiology.206.1.9423681. [DOI] [PubMed] [Google Scholar]
- 9.Dixon AM. How, Why and When. Amsterdam: Elsevier; 2008. Breast ultrasound; pp. 71–3. [Google Scholar]
- 10.Linda A, Zuiani C, Lorenzon M, Furlan A, Londero V, Machin P, et al. The wide spectrum of hyperechoic lesions of the breast. Clin Radiol. 2011;66:559–65. doi: 10.1016/j.crad.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Venkataraman S, Slanetz PJ, editors. Breast imaging: Mammography and ultrasonography. Uptodateonline. [Last updated on 2012 Mar]. Topic 7561 Version 15.0. Available from: http://www.uptodate.com .
- 12.Doshi DJ, March DE, Crisi GM, Coughlin BF. Complex cystic breast masses: Diagnostic approach and imaging-pathologic correlation. Radiographics. 2007;27:S53–64. doi: 10.1148/rg.27si075508. [DOI] [PubMed] [Google Scholar]


