Abstract
Background:
Chemotherapy induced nausea and vomiting (CINV) is one of the most disturbing side-effects in children receiving highly emetogenic chemotherapy. We aimed to assess whether the addition of an antiemetic cocktail containing midazolam and diphenhydramine to granisetron plus dexamethasone combination could ameliorate CINV in this study.
Patients and Methods:
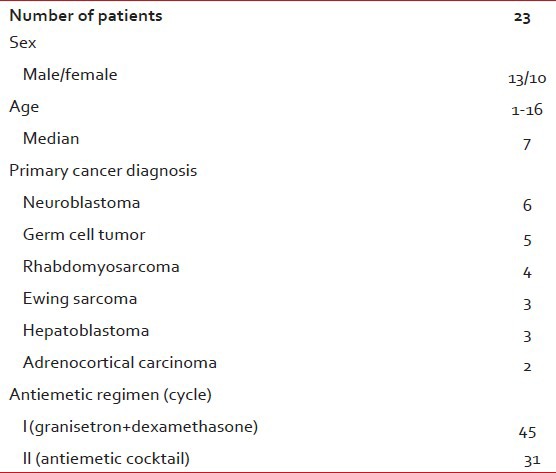
A total of 23 children aged between 1 and 16 years to receive cisplatin containing chemotherapy in our clinic were included in this study from April 2007 to April 2008. 76 cycles in 23 patients were randomly assigned to receive either antiemetic regimen 1 or antiemetic regimen 2. Antiemetic regimen 1 containing granisetron 0, 04 mg/kg plus dexamethasone 0, 2 mg/kg were given in 45 chemotherapy cycles. In 31 cycles, an antiemetic cocktail containing midazolam 0, 04 mg/kg, diphenhyramine 2, 5 mg/-kg in addition to granisetron plus dexamethasone was given. Number of vomiting, severity of nausea, the use of rescue therapy and adverse events were assessed between day 1 and day 5.
Results:
Complete response for the acute phase was observed 38/45 (84, 4%) cycles in regimen 1 as compared with 28/31 (90, 3%) in regimen 2, antiemetic cocktail regimen (P > 0.05). Complete response for delayed emesis after 24 h of the beginning of chemotherapy was observed in 29/45 (64, 4 %) in regimen 1 and 16/31 (51, 6%) in regimen 2. Antiemetic cocktail was not superior to the granisetron plus dexamethasone combination in controlling emesis in acute and delayed phase. Furthermore, patients receiving antiemetic regimen 2 were noted significantly more side effects.
Conclusion:
Our data showed that antiemetic cocktail containing midazolam and diphenhydramine was not better in controlling acute and delayed emesis. A slightly more toxicity with additional drugs was also observed.
Keywords: Antiemetics, cancer, chemotherapy, children, nausea, vomiting
INTRODUCTION
Chemotherapy induced nausea and vomiting (CINV) are major adverse effects of chemotherapy in children with cancer. CINV can lead to serious medical problems such as dehydration and electrolyte imbalances, increased duration of hospital stay and impaired quality-of-life for children and their parents.[1,2] Current standard recommendation in children with cancer is the use of a 5-hydroxytryptamine 3 receptor antagonist plus a corticosteroid to prevent emesis. Despite this treatment, more than 40% of patients still vomit in response to highly emetogenic chemotherapy.[3,4] New antiemetic agents such as neurokinin 1 receptor inhibitor, aprepitant, have been reported to improve control of emesis in adults. But, it is not yet established for pediatric use.[5,6]
To improve our prophylactic approach, we have conducted a study to compare the antiemetic activity of granisetron plus dexamethasone with those of an antiemetic cocktail containing midazolam and diphenhydramine in children receiving highly emetogenic chemotherapy.
PATIENTS AND METHODS
From April 2007 to April 2008, all consecutive pediatric patients to receive cisplatin containing regimen were included in our study. Criteria for exclusion were the presence of nausea and vomiting or the use of anti-emetics in the 24 h before chemotherapy, those with brain tumors and other causes of vomiting such as gastrointestinal obstruction, infection. The institutional review board of our hospital approved this study. All parents gave written informed consent.
A total of 23 patients were randomly assigned to receive either antiemetic regimen 1 or antiemetic regimen 2 at alternating cycles until the end of the study period. In this way, the patients acted as their own controls. Each patient received both antiemetic regimens in different cycles. The primary cancer diagnosis was neuroblastoma (6 patients), germ cell tumor (5 patients) rhabdomyosarcoma (4 patients), ewing sarcoma (3 patients), hepatoblastoma (3 patients) and adrenocortical carcinoma (2 patients). Seventy-six cycles containing cisplatin have been evaluated. Antiemetic regimen 1 containing granisetron 0, 04 mg/kg plus dexamethasone 0, 2 mg/kg were given in 45 chemotherapy cycles. In 31 cycles, an antiemetic cocktail containing midazolam 0, 04 mg/kg, diphenhydramine 2, 5 mg/kg in addition to granisetron plus dexamethasone were given. Antiemetic combination were diluted in 100 ml of 5% dextrose and given as 1 h infusion before chemotherapy. A diary form was completed on each day of chemotherapy cycle in every patient by parents and nurses. Number of vomiting, severity of nausea, the use of rescue therapy and adverse events were assessed between day 1 and day 5. Complete response was defined as no nausea and vomiting, partial response as one or two vomiting, but no rescue therapy, no response as more than three emetic episodes or rescue therapy.
Statistical analysis
The data analysis was performed using SPSS 15.0 software products, (SPSS inc. 233 South Wacker Drive, 11th Floor, Chicago, IL) for windows program. Descriptive statistics were shown as % of the number of observations. Analyses of nausea and vomiting were done separately for day 1 and days 2-5 (delayed emesis). The χ2 test and Fischer's exact test were used to compare the difference in efficacy of the two antiemetic treatments. P < 0.05 was considered to be statistically significant.
RESULTS
Demographic and clinical characteristics of the patients are outlined in Table 1. All chemotherapy regimens containing cisplatin were classified as highly emetogenic. Response rates were evaluated in the first 24 h (acute phase) and in between the day 2 day 5 (delayed phase). All 23 patients received regimen 1 twice, whereas 9 patients received regimen 2 twice.”
Table 1.
Patient characteristics
Complete response was observed 38/45 (84, 4%) cycles in regimen 1 as compared to 28/31 (90, 3%) in regimen 2, antiemetic cocktail regimen (95% confidence interval 0, 78-1, 96, P = 0.37). Antiemetic cocktail regimen was slightly superior to regimen 1 in the percentage of patients with a major control of emesis in the acute phase. However, there was no statistically significant difference between the groups. Partial response rates were 7/45 (15, 5%) in regimen1 and 3/31 (9, 6%) in antiemetic cocktail regimen. There were no patients treated with rescue antiemetic therapy in the first 24 h after chemotherapy.
Complete response for delayed emesis after 24 h of the beginning of chemotherapy was observed in 29/45 (64, 4 %) in regimen 1 and 16/31 (51, 6%) in regimen 2. Partial response rates were 14/45 (31, 1%) and 13/31 (41, 9%) in regimen1 and 2, respectively [Table 2]. The rates of treatment failure were 2/45 (4, 4%) and 2/31 (6, 45%) in regimen 1 and regimen 2. Antiemetic cocktail was not superior to the granisetron plus dexamethasone combination in controlling emesis in delayed phase. In terms of side effects, no severe or unexpected adverse events were observed in patients receiving antiemetic regimen 1. Adverse events were significantly more common among those receiving antiemetic cocktail treatment [Table 3]. Hypotension was observed during the antiemetic infusion in 2 patients. A marked sedation developed in 4 patients.
Table 2.
Antiemetic efficacy of different regimens
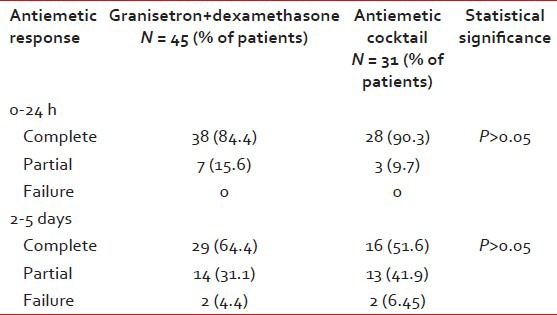
Table 3.
Clinical adverse events

DISCUSSION
The incidence of CINV is estimated at about 70% of children receiving highly emetogenic chemotherapy.[1,2,3] CINV is a very disturbing issue for children with cancer. They are more prone to vomiting than adults. These symptoms may compromise their quality-of-life and compliance with chemotherapy schedule. However, the knowledge of the most effective antiemetic to prevent CINV is not fully adequate in children, the combination of a 5HT-3 receptor antagonist with a corticosteroid is the recommended standard therapy for chemotherapy induced emesis in children with cancer.[2] Antiemetic agents that are recommended for use in adult patients with cancer are not included in the present pediatric guidelines. Aprepitant has been shown to provide superior protection against CINV in adult patients.[5] However, pediatric data about its efficacy and side effects is limited, yet.[6] There is no data to recommend the selection of alternative antiemetic drugs for pediatric cancer patients who do not respond sufficiently to the standard antiemetic therapy.
Some reports suggest that sedating a patient may be of value in cases of refractory emesis. Midazolam, short acting benzodiazepine, has been demonstrated to improve antiemetic effect for prolonged post-operative emesis.[7] Diphenhydramine, an antihistaminic, has a role in the treatment of nausea. It is thought to be mediated by the vestibular system. It has as well as sedative and antiemetic effects.[8]
Various types of antiemetic drugs can be combined with the aim of increasing antiemetic efficacy.[9,10] The drugs used in this cocktail were selected for their different mechanisms of action against emesis. We also aimed to provide a broad scope of antiemetic action. The primary goal of our study was to determine whether the addition of midazolam plus diphenhydramine to standard antiemetic therapy was superior in control of chemotherapy induced emesis. Granisetron plus dexamethasone combination was compared in a randomized trial with an antiemetic cocktail consisting of midazolam, diphenhydramine, granisetron and dexamethasone in our study. To the best of our knowledge, this is the first study in which antiemetic cocktail addition to standard therapy has been evaluated.
Our study showed no significant benefit for the addition of midazolam and diphenhydramine to antiemetic regimen. We also observed more serious side-effects during antiemetic cocktail infusion. We had to stop the infusion in 2 patients because of hypotension. We also observed excess sedation in 4 patients. Excess sedation may be dangerous in children receiving chemotherapy if vomiting occurs in some cases. Therefore, the addition of midazolam and diphenhydramine may be considered in only selected patients whose vomiting are due to psychological or emotional factors. If anticipatory emesis is considered, this antiemetic cocktail can be useful by reducing anxiety and causing sedation. There were important limitations in our study. The study group was too small to allow definitive statistical conclusions. Our study showed no expressive benefit and slightly more toxicity for the antiemetic cocktail. However, further studies with larger number of patients are necessary to prove this opinion.
In conclusion, since no statistically significant difference in antiemetic efficacy could be detected, our data seem to suggest that midazolam plus diphenhydramine may not useful in addition to standard antiemetic drugs in most patients. However, it should be taken into consideration that our study has some major limitations such as inadequate sample size, unequal randomization. The efficacy of antiemetic cocktail in children should be explored in further larger studies.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Dewan P, Singhal S, Harit D. Management of chemotherapy-induced nausea and vomiting. Indian Pediatr. 2010;47:149–55. doi: 10.1007/s13312-010-0023-4. [DOI] [PubMed] [Google Scholar]
- 2.Jordan K, Roila F, Molassiotis A, Maranzano E, Clark-Snow RA, Feyer P, et al. Antiemetics in children receiving chemotherapy. MASCC/ESMO guideline update 2009. Support Care Cancer. 2011;19(Suppl 1):S37–42. doi: 10.1007/s00520-010-0994-7. [DOI] [PubMed] [Google Scholar]
- 3.Phillips RS, Gopaul S, Gibson F, Houghton E, Craig JV, Light K, et al. Antiemetic medication for prevention and treatment of chemotherapy induced nausea and vomiting in childhood. Cochrane Database Syst Rev. 2010 Sep 8;9:CD007786. doi: 10.1002/14651858.CD007786.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Berrak SG, Ozdemir N, Bakirci N, Turkkan E, Canpolat C, Beker B, et al. A double-blind, crossover, randomized dose-comparison trial of granisetron for the prevention of acute and delayed nausea and emesis in children receiving moderately emetogenic carboplatin-based chemotherapy. Support Care Cancer. 2007;15:1163–8. doi: 10.1007/s00520-007-0242-y. [DOI] [PubMed] [Google Scholar]
- 5.Schmoll HJ, Aapro MS, Poli-Bigelli S, Kim HK, Park K, Jordan K, et al. Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol. 2006;17:1000–6. doi: 10.1093/annonc/mdl019. [DOI] [PubMed] [Google Scholar]
- 6.Gore L, Chawla S, Petrilli A, Hemenway M, Schissel D, Chua V, et al. Aprepitant in adolescent patients for prevention of chemotherapy-induced nausea and vomiting: A randomized, double-blind, placebo-controlled study of efficacy and tolerability. Pediatr Blood Cancer. 2009;52:242–7. doi: 10.1002/pbc.21811. [DOI] [PubMed] [Google Scholar]
- 7.Mandalà M, Cremonesi M, Rocca A, Cazzaniga M, Ferretti G, Di Cosimo S, et al. Midazolam for acute emesis refractory to dexamethasone and granisetron after highly emetogenic chemotherapy: A phase II study. Support Care Cancer. 2005;13:375–80. doi: 10.1007/s00520-004-0741-z. [DOI] [PubMed] [Google Scholar]
- 8.Jordan K, Schmoll HJ, Aapro MS. Comparative activity of antiemetic drugs. Crit Rev Oncol Hematol. 2007;61:162–75. doi: 10.1016/j.critrevonc.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Grunberg SM. Antiemetic activity of corticosteroids in patients receiving cancer chemotherapy: Dosing, efficacy, and tolerability analysis. Ann Oncol. 2007;18:233–40. doi: 10.1093/annonc/mdl347. [DOI] [PubMed] [Google Scholar]
- 10.Dix S, Cord M, Howard S, Coon J, Belt R, Geller R. Safety and efficacy of a continuous infusion, patient controlled anti-emetic pump to facilitate outpatient administration of high-dose chemotherapy. Bone Marrow Transplant. 1999;24:561–6. doi: 10.1038/sj.bmt.1701909. [DOI] [PubMed] [Google Scholar]


