Abstract
In the fifty years since the organizational hypothesis was proposed, many sex differences have been found in behavior as well as structure of the brain that depend on the organizational effects of gonadal hormones early in development. Remarkably, in most cases we do not understand how the two are related. This paper makes the case that overstating the magnitude or constancy of sex differences in behavior and too narrowly interpreting the functional consequences of structural differences are significant roadblocks in resolving this issue.
Keywords: Sex differences, sex similarities, organizational effects, sexual behavior, dual function hypothesis
W.C. Young and his colleagues outlined the principles of gonadal steroid actions on sexual differentiation of the brain (reviewed by Young, 1961 and 1969; Young et al., 1964). They used the term activational for the reversible effects by which gonadal hormones stimulate sexual behaviors in adult animals, and organizational for the permanent effects that testosterone (T) exerts in perinatal life in decreasing adult sensitivity to ovarian hormones (Phoenix et al., 1959) and increasing adult sensitivity to T (Grady et al., 1965). Young and colleagues recognized that the concepts of activation and organization were implicitly, if not explicitly, stated in the work by Dantchakoff (1938) (Young et al., 1964). Their expectation was that the morphological changes in genital anatomy induced by early androgen treatment would not be equaled by “visible” structural changes in the brain (Phoenix et al., 1959). Despite presenting compelling evidence that prenatal androgen treatment causes behavioral “´differentiation´ in the direction of masculinization”, and assuming “…that T or some metabolite acts on those central nervous tissues in which patterns of sexual behavior are organized,” Phoenix et al. (1959) were not “… prepared to suggest whether the site of action is general or localized”.
Beach (1971), in a charismatic and skeptical essay went even further and questioned the concepts introduced by Young: “The sex hormones are best regarded not as organizing agents but as chemical sensitizers, which alter the stimulability of critical mechanisms within the central nervous system”. Beach held the alternative view that sexual differentiation of the periphery has the more important role for sex differences in adult behavior (Forger, 2009). Currently, however, thousands of studies have documented sex differences in the brain in practically any parameter imaginable (see for example, Morris et al., 2004; McCarthy et al., 2009), and in at least some cases there is evidence that gonadal hormones directly influence the development of these differences (McCarthy et al., 2009). Remarkably, in most cases we do not understand how, or even whether, these sex differences contribute to sex differences in behavior.
Three reasons may contribute to our ignorance. Firstly, it is difficult to unravel the function of neural circuitry in general. Just finding a sex difference in the brain does not speak to function. Secondly, behavioral sex differences are often exaggerated, sometimes even portrayed as absolute. This makes it tricky to link relatively consistent sex differences in brain structure to more fickle sex differences in brain function. Thirdly, we are drawn to the idea that sex differences in brain structure generate sex differences in behavior. In fact, Phoenix et al. (1959) pointed out: “An assumption seldom made explicit is that modification of behavior follows an alteration in structure or function of the neural correlates of behavior.” Note, however, that this does not imply that a change in structure always results in a change in behavior, or conversely, that in the absence of modification of behavior the underlying neural substrate is similar. This review will elaborate on each of these three reasons.
1. The Structure – Function Relationship
The prediction in the Phoenix et al., 1959, paper that early androgen exposure causes “a more subtle change reflected in function rather than in visible structure” proved to be too cautious after the first reports on sex differences in the brain started to trickle in. For example, in 1960 Kato detected higher serotonin levels in female than in male rat brains. Not much later Pfaff (1966) showed that neonatal castration of rats permanently changed the size of nucleoli in the hypothalamus. In 1970 McEwen and his colleagues showed that neonatal steroid treatment changed T and estradiol (E) uptake in rat brains (McEwen and Pfaff, 1970; McEwen et al., 1970). One year later Raisman and Field (1971) reported that male rats have more synapses of non-strial origin on dendritic shafts and fewer on dendritic spines on neurons in the preoptic area (POA) than do females, a difference that could be eliminated by treating females neonatally with T (Raisman and Field, 1973). Although this sex difference’s function remains unclear, it was consistent with the organizational hypothesis.
The preoptic area and male sexual behavior: an impasse?
The first clearly visible differences concerned song control nuclei in zebra finches and canaries, which are much larger in males than in females (Nottebohm and Arnold, 1976). This difference elegantly mirrored sex differences in bird song: males sing, females typically don’t. Just two years later, Gorski’s group discovered a nucleus in the POA / anterior hypothalamic area (AH) that is five times larger in male rats than in females, the sexually dimorphic nucleus of the POA (SDN) (Gorski et al., 1978). Similar sex differences have since been found in the POA/AH in non-mammalian and mammalian vertebrates, including humans (Swaab and Fliers, 1885; Allen et al., 1989). Besides being a hotbed for sex differences in the brain, the POA also illustrates how difficult it is to relate structure to function.
Because the medial POA (MPOA) has long been known to be essential for male sexual behavior (e.g., Hillarp et al., 1954; Larsson and Heimer, 1964; Davidson, 1966; Hansen et al., 1982), it has been attractive to link sex differences in MPOA morphology to differences in male sexual behavior. However, lesions centered in the SDN or in similarly dimorphic areas in the MPOA of ferrets produced little to no decrements in male sexual behavior (Arendash and Gorski, 1983; Turkenburg et al., 1988; de Jonge et al., 1989; Cherry and Baum, 1990). Discrepancies in the effects of perinatal endocrine manipulations on SDN morphology and male sexual behavior further weakened the link. For example, prenatal T treatment increased SDN volume in female rats but not their propensity to show masculine behavior as adults (Ito et al., 1986). Likewise, treating males prenatally with an aromatase inhibitor reduced SDN volume (Houtsmuller et al., 1994) but had little or no effect on male sexual behavior (Brand et al., 1991). Inhibiting steroid receptor coactivator SRC-1 expression reduced SDN volume as well but not male sexual behavior even though it blocked the defeminizing action of T on female sexual behavior (Auger et al., 2000). Vice versa, inhibiting prostaglandin-E2 action, a mediator of the organizing effects of gonadal steroids on male sexual behavior (McCarthy, 2008), blocked masculinization of male sexual behavior but did not reduce the size of the SDN nor did it block defeminization of female sexual behavior (Todd et al., 2005). These last two experiments point at a possible link between the SDN and female sexual behavior. Lesions of the MPOA that include the SDN-POA can, in fact, disinhibit female sexual behavior, in females (Powers and Valenstein, 1972) as well as in males (Hennesey et al. 1986). Perhaps a larger SDN translates in stronger inhibition of female sexual behavior. It is unknown, however, whether lesions of merely the SDN-POA still affect female sexual behavior.
Inconsistencies between sex differences in male sexual behavior and sexual dimorphism are also reported for the ‘male nucleus’ of the POA in ferrets (MN). This sex difference is absolute (females lack an MN) and depends on the presence of T before birth (Cherry et al., 1990). Neonatal T treatment of female ferrets, however, masculinizes sexual behavior without inducing an MN (Cherry et al., 1991). Interestingly, however, the MN may be important for partner preference as lesions of the MPOA centered around the MN cause male ferrets to prefer male over female conspecifics (Paredes and Baum, 1995; Kindon et al., 1996), or male over female body odors (Alekseyenko et al., 2007). The same lesions also changed the pattern of Fos activation in response to male odors from a male to a female-typical pattern (Alekseyenko et al., 2007).
It may be that homologous sexually dimorphic structures play a similar role in other mammals. Lesions of the SDN disrupt partner preference in rats as it does in ferrets (Paredes et al., 1998). Natural variation in male versus female preference also correlates with the size of sexually dimorphic nuclei in the MPOA. For example, about 8% of rams prefer mounting male rather than female sheep. These male-oriented rams have an ‘ovine SDN’ (oSDN) only half the size of that of female-oriented rams (Roselli et al., 2004). This difference cannot be ascribed to adult T levels (Roselli et al., 2008), but may be related to differences in prenatal levels as prenatal exposure to T masculinizes the oSDN in females (Roselli et al., 2007). It is not known, however, whether such masculinized females show similar variability in oSDN size and sexual orientation as do rams. Interestingly, humans have an area in the same region, the third interstitial nucleus of the AH (INAH3), which is larger in heterosexual males than in females (Allen et al., 1989). The INAH3 of homosexual males, however, is intermediate to that of heterosexual male and females (LeVay, 1991). A more recent report failed to replicate this finding but showed a trend in the same direction (Byne et al., 2001). If these sex differences in the size of subnuclei in the MPOA/AH are indeed important for sexual preference, it is still puzzling how, for example, mice can show partner preference while lacking similar sex differences in MPOA/AH structure.
Even if a structural sex difference in the brain correlates well with a sex difference in behavior, such as appears to be the case with sexual preference, there is still the question of what, for example, a difference in cell number buys one sex over the other in terms of function. For this, one has to understand the connections and functions of the components that make up a sexually dimorphic structure. In the spinal cord, this is relatively easy (Forger, 2009); in the brain it is much harder because connections are far more numerous. More consistent sex differences may be found in the wiring of the MPOA. For example, the density of dendritic spine synapses on MPOA neurons correlates well with measures of male sexual behavior (Wright et al., 2008), but to understand the functional significance of this correlation, one has to identify the source and nature of the neurons that synapse onto these MPOA neurons. It will also be useful to study neurochemical markers in sexually dimorphic systems. Focusing on neurotransmitter systems, for example, helps trace the anatomical connections of subsets of cells within sexually dimorphic areas (De Vries, 1990). It also allows more specific manipulations than lesioning entire cell groups or transsecting projections; for example, injecting receptor agonists and antagonists or making conditional knock-outs or knock-ins have proven useful in delineating systems engaged in food intake and energy balance (e.g., Elmquist et al., 2005).
Kisspeptin: a sunshine story?
Kisspeptin neurons in the anteroventral periventricular nucleus (AVPV) of mice and rats provide an example of a sex difference in neurochemistry. The AVPV is larger and contains more neurons in females than in males (Bleier et al., 1982; Arai et al., 1994; Forger et al., 2004), sex differences that obey the organizational hypothesis (Arai et al., 1993; Simerly et al., 1985). A subset of the AVPV neurons express Kiss1-mRNA and its gene product, kisspeptin (Dungan et al., 2006). Female mice have ten times more AVPV kisspeptin neurons than males (Clarkson and Herbison, 2006); in rats, males express almost no kisspeptin, making the sex difference nearly absolute (Kauffman et al., 2007; Gonzalez-Martinez et al., 2008). As kisspeptin-containing projections appear to contact GnRH neurons (Clarkson and Herbison, 2006), and kisspeptin triggers a surge of luteinizing hormone (LH) by stimulating these neurons (Gottsch et al., 2004; Irwig et al., 2004), the higher number of kisspeptin neurons in the female AVPV may explain the sex difference in the induction of an LH surge, as males do not show this neuroendocrine response. Kisspeptin is probably not the only factor, however. For example, compared to male rats, female rats have twice as many AVPV neurons that express markers of both glutamatergic and GABAergic signaling (Ottem et al., 2004). These ‘dual phenotype’ neurons may synapse on GnRH neurons to modulate the effect of E on LH secretion.
The key to the success of the kisspeptin story may be similar to that of the sexually dimorphic nuclei in the spinal cord (Morris et al., 2004; Forger, 2009); kisspeptin neurons form part of a final common pathway, in this case, of the sexually dimorphic control of the LH surge. The search for the function of sexually dimorphic neural systems with no clear links to peripheral structures has turned out to be much more difficult. And even with the niftiest techniques available, there is little chance of answering the structure-function question if one overestimates functional sex differences or interprets potential functions of sex differences in the brain too narrowly as we will now illustrate.
2. Sex Similarities
A recent study suggested that a single gene deletion can wipe out sex differences in male sexual behavior. Female mice that are deficient in Trpc2, an ion channel that is expressed in the epithelium of the vomeronasal organ (VNO) and probably functions in pheromone detection (Liman et al., 1999), showed high levels of male sexual behavior (Kimchi et al., 2007). The data suggest that VNO-mediated input represses male and activates female behavior and that functional neuronal circuits underlying male-specific behavior co-exist in normal female mouse brains. The report caused quite a stir but perhaps for the wrong reason, as the idea that males and females have similar behavioral capacities is not at all new. In fact, an important reason why brain dimorphism cannot readily be related to sex differences in behavior is probably that such differences have been overestimated.
Female sexual behavior
Beach (1971) suggested that, in rodents, perinatal T-stimulation defeminizes and, at the same time masculinizes the animal. In the rat, defeminization is traditionally measured as a reduced display of female sexual behavior (lordosis to mount ratio = lordosis quotient, LQ) after gonadectomy and exogenous treatment with E benzoate (EB) and progesterone (P); male rats are thought to be less sensitive to EB and insensitive to P (McEwen, 1981; Rainbow et al., 1982). Indeed at a given dose, males showed lower LQs than females (Södersten, 1976). However, merely increasing the dose of EB (100 µg) and P (2 mg) induced not only high LQs in castrated males but also ear wiggling and hopping behavior, i.e., higher levels of what Beach (1976) has termed proceptive behavior. Clearly, males possess the neural circuitry of all aspects of female sexual behavior.
The same study showed that females given T neonatally did not differ from control females (Södersten, 1976), suggesting that the reduced LQs of males cannot be due only to neonatal androgen secretions. A subsequent experiment showed that the retained E-sensitivity of the androgenized females was due to the presence of the ovaries until adulthood (Södersten, 1976). As these rats were ovariectomized no sooner than four weeks after puberty, post-pubertal ovarian secretions may have boosted female sexual behavior in the androgenized females. Moreover, Gerall and colleagues (Gerall et al., 1973) had already demonstrated that the presence of normal cyclic ovaries boosts behavioral sensitivity to E in rats. This raised the possibility that the sex difference in female sexual behavior could be eliminated if normal males were treated with E in adulthood in a manner that replicates the episodic release of E during the estrous cycle in rats. Indeed, adult gonadectomized males and females showed equally high LQs in response to a wide range of low doses of E, administered in two pulses and followed by P. In contrast, if the rats were given constant-release E-filled implants, clear cut sex differences emerged (Södersten et al., 1983a). Similar finding have been reported for guinea pigs (Olster and Blaustein, 1992). Obviously, male rats exposed to testicular secretions in early life are capable of showing the same behavioral responses to physiological doses of E and P as females. This challenges the notion that perinatal T stimulation irreversibly changes adult behavioral sensitivity to ovarian hormones and suggests that many of the reported sex differences in female sexual behavior may be at best context-specific and, at worst, artifacts of pharmacological methods of hormone treatment (Södersten et al., 1983a). Most hormones are secreted episodically (Maywood et al., 2007); physiology demands that to test their effect, hormones should be administered in a manner that replicates their inherent pulsatile pattern of release.
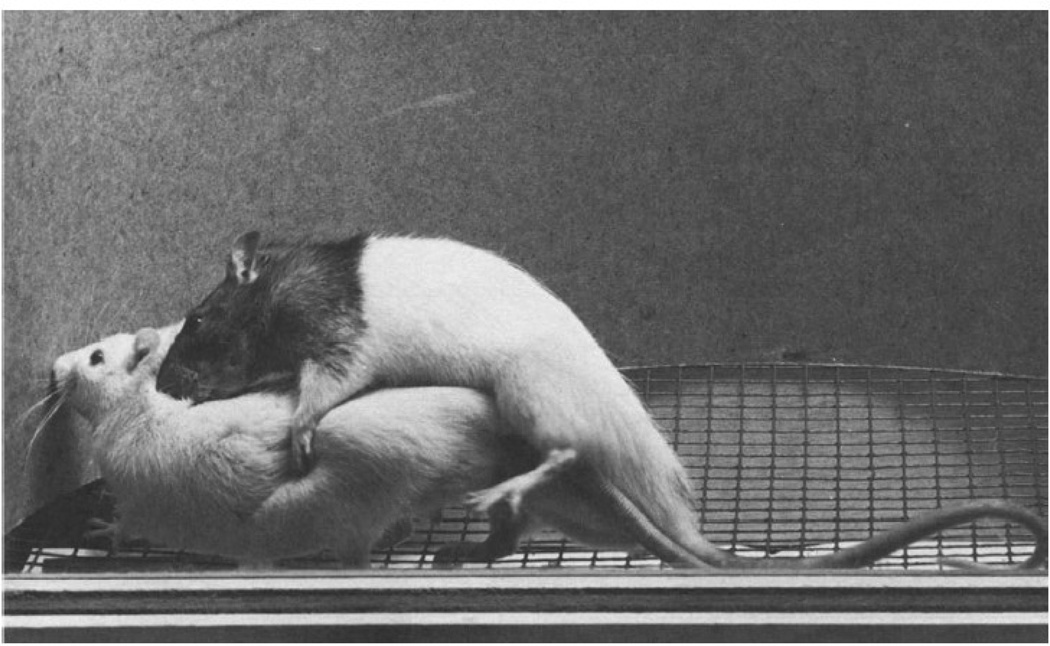
In some strains of rats up to 50% of intact males show the behavior of the opposite sex without hormone treatment (Fig. 1; Södersten and Larsson 1974; Södersten et al., 1974). While this occurs at reduced levels--males and females certainly behave differently--this is not a reflection of a difference in capacity between the sexes.
Fig. 1.
Lordosis response displayed by an intact untreated male rat in response to a mount by another male. Photo Courtesy: Dr. M.J. Baum. Reprinted from Södersten et al., 1974, with permission.
Sex differences in LQ may depend on the time of day. Females rats show a rhythm with an LQ of 100% during the dark phase of the light:darkness (LD) cycle but only around 30% during the light phase. Males treated with E, however, show about a 75% response rate at any time of the day (Hansen et al., 1979). Thus, whereas behavioral E-sensitivity is retained in male rats, the temporal organization of behavioral responses has changed. Interestingly, in females intracerebral injection of vasopressin (VP), one of the output peptides of the brain´s rhythm generator, the suprachiasmatic nucleus (SCN), inhibits the lordosis response during the dark phase but not during the light phase. Conversely, a VP antagonist facilitates the behavior during the light phase but not during the dark phase; moreover, VP levels in the cerebrospinal fluid vary inversely with the display of lordosis in female rats (Södersten et al., 1983b).
Sunshine, rhythms, and ovulation
It has long been known that, in rats, there is a 24-hour periodicity in the "LH-release apparatus" (Everett and Sawyer, 1950) that is controlled by the circadian oscillator and entrained by the LD cycle (Alleva et al. (1971) and can be generated by E in females (Legan et al. (1975), but not in males (Henderson et al., 1977). Thus, surge secretion of LH displays a sexually dimorphic rhythm that is controlled by light via partially known neural pathways (Maywood et al., 2007).
Rusak and Zucker (1975) extended this hypothesis by suggesting that neonatal androgen stimulation uncouples the SCN rhythm generator from the substrates of sexual behavior. We verified this hypothesis by demonstrating that this is true for the sexually dimorphic rhythm in lordosis in rats (Hansen et al., 1979); the rhythm generator can be coupled to the external photoperiod in males by blocking T action during development (Södersten and Eneroth, 1980) and subsequently uncoupled by lesioning the SCN (Södersten et al., 1981). These maneuvers produced male rats with normal endogenous levels of T that showed all aspects of male and female sexual behavior at all times of day without hormone treatment (Södersten et al., 1981). Thus, while the neural tissues controlling male and female mating behavior can develop side by side, photoperiod influences sexual behavior in female but not male rats (Södersten, 1984). These results were elegantly extended to choice of sexual partner; male rats treated neonatally with an aromatize inhibitor to block the conversion of T into E, showed a LD-dependent rhythm in partner preference similar to female rats (Bakker et al., 1993; Brand et al., 1991). Kisspeptin may spread more light on this story. Thus, it has been suggested that the SCN induces activity both in the kisspeptin and GnRH neurons (Tsukahara, 2006) and that AVPV kisspeptin neurons mediate the well-known influence of photoperiod on timing of ovulation (Morgan and Hazlerigg, 2008; Simonneaux et al., 2009).
Male sexual behavior
If Young’s group had worked with mice rather than with guinea pigs a different picture might have emerged, as mice do not show consistent sex differences in male sexual behavior. For example, several papers report that female mice treated with T or E show mounting and thrusting at levels that are similar to or even higher than those of males (Wersinger et al., 1997; Jiyotika et al., 2006). Others report a clear sex difference in, or an organizational effect on, male sexual behavior (Vale et al., 1973; Bakker et al., 2006). In rats, a sex difference is generally found, but not an absolute one. Most intact untreated female rats show male sexual behavior (Södersten, 1972); even the pattern displayed by an ejaculating male can be activated pharmacologically in normal female rats (Emery and Sachs, 1975; Södersten et al., 1976). As with female behavior in males, male behavior in females occurs at reduced levels, but again, this does not reflect a difference in capacity. For these reasons, a researcher of sex similarities pointed out long ago that: “The search for morphological sex differences in adult rat brains that are caused by the 'organizing effect of perinatal androgen' and that can be related to sex differences in behavior has not been fruitful and may continue unrewarded” (Södersten, 1987).
Full-blown male mating behaviors in Martians
The recent report of male behavior in Trpc2-/- female mice made the same point (Kimchi et al., 2007). This study, however, tested mice for only 15 minutes, about a third of the time needed for male mice to ejaculate and much shorter than other studies of male sexual behavior in mice (e.g., Vale et al., 1973; Mosig and Dewsbury, 1976; Wersinger et al., 1997; Bakker et al., 2006; Jiyotika et al., 2006). Based on similarities in mount latency and an increase in time spent mounting male mice or “intruder females”, neither of which showed sexual receptivity in supplementary videos, the authors concluded that “a functional neuronal network mediating male sexual behavior develops and persists in females”. This conclusion over-interprets the data and merely iterates the conclusions from earlier research. Nevertheless, few people in our field would have predicted that Trpc2-/- mice would show increased mounting and a reversal of a sex difference in vocalizations. Careful testing of these mice may reveal whether these animals show indeed “full blown male mating behaviors” or are truly “mice from Mars” (Shah and Breedlove, 2007).
Testing conditions
Testing conditions likely play a role in the variability in behavioral sex differences. For example, sex differences in response to sleep deprivation depends on stress level, with stressed animals showing bigger sex differences in sleep recovery than unstressed animals (Koehl et al., 2006). Developmental history may also contribute to variability. While male prairie voles are spontaneously parental, most virgin females avoid or attack pups (Lonstein and De Vries, 1999a). This sex difference, however, depends on rearing conditions; females raised in the presence of their parents are parental as adults (Lonstein and De Vries, 2001). Even apparently very subtle changes in developmental history can make a big difference: prairie voles show sex differences in pair bond formation depending on whether they were transported by a gloved hand or in a plastic cup during routine cage changing in the first three weeks of life (Bales et al., 2007). If transported by hand, females form partner preference within six hours but males don’t, if transported by cup, neither sex forms partner preference (Bales et al., 2007).
Testing conditions also affect sex differences in human behavior. For example, the Mental Rotations Test, in which subjects have to mentally rotate a block figure to match it with a congruent object in a line-up of similarly shaped but not identical objects, shows one of the most consistent cognitive sex differences with males outperforming females in a wide range of studies. This difference shrinks considerably if instead of interconnected cubes, the figures take on a human shape (Alexander and Evardone, 2008). Similarly, the male advantage in certain math tests are eliminated or reduced if female subjects are told in advance that females do as well as males or better, but sex differences are exacerbated if females are told the opposite (Spelke, 2005). In all of these cases, context, whether defined as the conditions before or during the test, clearly influences the outcome. To what extent these different behavioral outcomes are reflected in context-dependent differences in brain structure is unknown.
The structural basis of sex differences in behavior
Clearly males as well as females have the capacity to generate behaviors considered to be male-or female-typical even though the levels to activate these behaviors may differ. Two different, not mutually exclusive scenarios may explain this. The first is that males and females share most of the circuitry needed to generate these behaviors, but a limited set of factors determine whether the circuitry is repressed or activated. This may be true for courtship song in fruit flies, which is produced only by males by rapidly oscillating the wing closest to the courted female (Rideout et al., 2007). In an elegant set of studies, Dylan et al., (2008), show that females have the neural circuitry needed to generate this behavior, but it lies dormant and when activated sings ‘out of tune.’ A combination of different inputs and sex-specific expression of a sex-determination gene, fruitless, within the circuitry may account for the sex difference in song (Dylan et al., 2008). Given the availability of a powerful arsenal of genetic and physiological methods available to study the fly nervous system, there is good hope that, in this case, the question as to how differences in neural structure contribute to sex differences in this behavior may be answered satisfactorily (Dornan and Goodwin, 2008).
An alternative scenario is that males and females can both generate male- and female-typical behaviors, but their brains use different strategies to do so. Functional imaging studies have shown this to be true for a variety of tasks on which men and women perform similarly (e.g., Shaywitz et al., 1995; Grabowski et al., 2003; Piefke et al., 2005). In the following section we will give an example of such a scenario for parental behavior in bi-parental species.
3. Sex Differences in Brain Structure May Cause as well as Prevent Sex Differences in Behavior
We now know that organizational effects impinge on neural structures and behaviors beyond those related to reproduction. Intuitively, we associate structural differences with differences in physiological and behavioral endpoints. If the latter do not differ, we typically do not search for differences in mechanisms. However, sex differences at one or another level in the mechanisms underlying any physiological or behavioral endpoint, sexually dimorphic or not, may be the norm, not the exception.
For mammals, this is certainly true at the molecular level. In females, practically all cells, including those of the brain, show X inactivation, the silencing of genes on one of the two X chromosomes; in males, which typically have only one X chromosome, this does not happen (Lyon, 1999). X inactivation is most likely a compensatory mechanism to ensure that X chromosomal genes, many of which serve basic cell maintenance functions, are expressed at roughly the same rate in males as in females (Lyon, 1999). The energy spent on X inactivation is, presumably, the price we pay for the evolution of two sexes. Clearly, differential expression of genes on the XX and XY chromosomes is necessary to generate the male and female phenotype. But for all we know, much of this differential expression is restricted to only a few tissues during short, critical periods of life. In mice, for example, the Sry gene has to be expressed for only half a day in only one cell-type, Sertoli cells, to trigger the development of the male phenotype (Burgoyne et al., 1988; Lovell-Badge and Hacker, 1995). In contrast, in females, X inactivation must take place throughout life, in all cells of any tissue, sexually dimorphic or not (Lyon, 1999). Compensatory processes to avoid undesired side effects of sexual differentiation, may take place again and again, in developing as well as adult animals, from the molecular to the macroscopic level. Brains may be the perfect place to detect such compensation, especially since neural circuits often serve more than one function, some dimorphic, others not.
Vasopressin dimorphism: the cause of sex differences
A good example is the VP-innervation of the brain, which shows perhaps the most consistent neural sex difference among vertebrates (De Vries and Panzica, 2006) with males having more VP neurons in the bed nucleus of the stria terminalis (BNST) and medial amygdaloid nucleus (MeA) and denser projections from these areas than do females across many mammalian species (De Vries and Panzica, 2006). Non-mammalian vertebrates show similar sex differences in homologous vasotocin (VT) projections. This sex difference, which depends on organizational effects of gonadal steroids, has been particularly well studied in rodents (De Vries and Panzica, 2006).
When we found this difference, we suggested that it was implicated in sexually dimorphic behaviors, such as female sexual behavior (e.g., De Vries, 1990). Lesion and stimulation studies suggested that areas innervated by the sexually dimorphic VP projections from the BNST and MeA, such as the lateral septum, influence female sexual behavior, in this case inhibiting it (Nance et al., 1974; Zasorin et al., 1975). Moreover, as mentioned before, intraventricular injections of VP inhibited female sexual behavior whereas similar injections of an antagonist facilitated it (Södersten et al., 1983b). These injections, however, may have interacted with any VP system in the brain. In fact, the circadian nature both of the effects of VP and its antagonist on lordosis behavior and of VP levels in the brain suggest that VP projections from the SCN formed the substrate for VP’s effects on female sexual behavior (Södersten et al., 1985). A direct link between the VP innervation of the lateral septum and female sexual behavior has never been tested. Nevertheless, if septal VP inhibits female sexual behavior, then the higher levels of VP in males versus females correlate well with males being less likely to show lordosis behavior.
Sex differences in VP also appear to match sex differences in social behaviors that are modulated by VP, for example, aggressive behavior. Just as the VP content of the BNST and MeA projections declines gradually after castration, so does intermale aggression (DeBold and Miczek, 1984), and injections of VP into the lateral septum or the MeA reverse the decline (Koolhaas et al., 1990, 1991). In fact, in all vertebrate classes, VP and its non-mammalian homologue VT have been linked to territorial aggression (Goodson and Bass, 2001; Ferris, 2005). As with female sexual behavior, the higher levels of VP / VT correlate well with the higher levels of resident-intruder aggression displayed by males. Interestingly, in hyenas, in which females are more aggressive and dominant than males (Matthews, 1939; Hamilton, 1986), males have either the same or lower VP fiber densities in the septum and in other sites that receive projections from the BST and MeA (Rosen et al., 2007). Interestingly, in Syrian hamsters, which lack the VP projections of the BNST and MeA altogether (Albers et al., 1991), females are as aggressive as males (Payne and Swanson, 1970; Huhman et al., 2003). All these examples are compatible with the idea that sex differences in VP innervation beget sex differences in function. They also invoke the idea that size and direction of sex differences in VP innervation correlate directly with size and direction of sex differences in behavior. Research in prairie voles, however, paints a different picture.
Vasopressin dimorphism: the cause of sex similarities
Unlike most other mammals, prairie voles show almost no sex differences in aggressive behavior (Villalba et al., 1997). Yet, sex differences in VP expression are larger in this species than in any other mammal (Bamshad et al., 1993; 1994). In males, mating increases VP mRNA expression in the BNST while reducing VP immunostaining in BNST terminals, suggesting increased VP release (Bamshad et al., 1994; Wang et al., 1994b). These changes, which do not occur in females, may precipitate mating-induced changes in aggression and social behavior, which indeed can be blocked with a VP antagonist (Winslow et al., 1993). They may also underlie the change in parental behavior in males after mating (Bamshad et al., 1994), it is blocked by VP antagonists as well (Wang et al., 1994a). The sex difference in VP- innervation may therefore be key to the similarities in parental behavior observed in parental voles (Lonstein and De Vries, 1999a). In female prairie voles, pregnancy-associated hormonal changes and parturition appear necessary to trigger parental behavior (Lonstein and De Vries, 1999b, Hayes and De Vries, 2007), very similar to what is found in other rodents (Bridges, 1990). As male prairie voles do not get pregnant, let alone give birth, they must use a different strategy to boost parental responsiveness. Part of this strategy may involve engaging the higher density of VP-innervation.
The dual function hypothesis
In prairie voles, the presence of a sex difference in the brain clearly does not correlate with the size of a sex difference in behavior. The species with the largest sex difference in VP innervation reported to date shows some of the least conspicuous sex differences in social behavior. Apparently, the sex difference in VP can cause as well as prevent sex difference in social behavior. Because there is no reason that this dual function is VP’s prerogative, we suggested that in general sex differences in brain structure may cause as well as prevent sex differences in specific behaviors or centrally regulated functions (De Vries and Boyle, 1998; De Vries, 2004). This dual function hypothesis is perfectly testable. To take the VP system as an example, one would predict in the former case, that blocking VP neurotransmission would blunt or eliminate sex differences and in the latter case that the same treatment would cause a sex difference that wasn´t there. In fact, such tests have already been done; treatment with a VP antagonist blocked social recognition memory in male but not in female rats, thereby creating a new sex difference (Bluthe and Dantzer, 1990). Related to this, the V1a receptor knock-out mouse has a behavioral phenotype in males but not in females (Bielsky et al., 2005), exactly what one would predict for a system that is more important for a function in one sex than in the other.
Epilogue
While the search for the “Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior…” (Phoenix et al., 1959) remains elusive, selective genetic elimination or re-introduction of elements of sexually dimorphic neural systems may change the scene. To ensure success, however, we have to realize that sex differences in function and behavior are context-dependent; context can alter gene expression in the brain, and genes, of course, “… do not specify behavior directly but rather encode molecular products that build and govern the functioning of the brain through which behavior is expressed” (Robinson et al., 2008). Overlooking these issues makes forging links between structure and function akin to building a house of cards. The possibility that sex differences in brain structure can cause as well as prevent sex differences in function should also be considered; the VP innervation may merely be one of many dual-function neural networks. Hundreds of sex differences have been found in the central nervous system, but only a handful can be clearly linked to sex differences in behavior, the best one is in the spinal cord (Morris et al., 2004; Forger, 2009); we do not know the functional consequences of most of the others, for example, the ones in the SDN-POA. As biologists, we should consider any biological phenomenon as a possible adaptation to the circumstances. The evolutionary biologist, Dobzhanski (1973) stated that ‘nothing in biology makes sense except in the light of evolution.’ For us, this means that male neural systems have evolved to control behavior most optimally in a male body and likewise for females. The dual-function hypothesis, therefore, may assist us in taking a step ahead in answering the questions regarding the site and specificity as well as the nature of the effect of neonatal androgen on adult behavior.
Acknowledgments
Recent research by GJD was funded by NIH grants MH47538 and MH01497. We thank Elsevier for permission to reproduce Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers HE, Rowland CM, Ferris CF. Arginine-vasopressin immunoreactivity is not altered by photoperiod or gonadal hormones in the Syrian hamster (Mesocricetus auratus) Brain Res. 1991;539:137–142. doi: 10.1016/0006-8993(91)90696-s. [DOI] [PubMed] [Google Scholar]
- Alekseyenko OV, Waters P, Zhou H, Baum MJ. Bilateral damage to the sexually dimorphic medial preoptic area/anterior hypothalamus of male ferrets causes a female-typical preference for and a hypothalamic Fos response to male body odors. Physiol Behav. 2007;90:438–449. doi: 10.1016/j.physbeh.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Evardone M. Blocks and bodies: sex differences in a novel version of the Mental Rotations Test. Horm. Behav. 2008;53:177–184. doi: 10.1016/j.yhbeh.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LS, Hines M, Shryne JE, Gorski RA. Two sexually dimorphic cell groups in the human brain. J. Neurosci. 1989;9:497–506. doi: 10.1523/JNEUROSCI.09-02-00497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LS, Hines M, Shryne JE, Gorski RA. Two sexually dimorphic cell groups in the human brain. J. Neurosci. 1989;9:497–506. doi: 10.1523/JNEUROSCI.09-02-00497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleva JJ, Waleski MV, Alleva FR. A biological clock controlling the estrous cycle of the hamster. Endocrinology. 1971;88:1368–1379. doi: 10.1210/endo-88-6-1368. [DOI] [PubMed] [Google Scholar]
- Arai Y, Nishizuka M, Murakami S, Miyakawa M, Machida M, Takeuchi H, Sumida H. Morphological correlates of neuronal plasticity to gonadal steroids: Sexual differentiation of the preoptic area. In: Haug M, Whalen RE, Aron C, Olsen KL, editors. The Development of Sex Differences and Similarities in Behavior. Dordrecht: Kluwer Academic Publishers; 1993. pp. 311–323. [Google Scholar]
- Arendash GW, Gorski RA. Effects of discrete lesions of the sexually dimorphic nucleus of the preoptic area or other medial preoptic regions on the sexual behavior of male rats. Brain Res. Bull. 1983;10:147–154. doi: 10.1016/0361-9230(83)90086-2. [DOI] [PubMed] [Google Scholar]
- Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 (SRC-1) mediates the development of sex- specific brain morphology and behavior. Proc. Natl. Acad. Sci. U. S. A. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat. Neurosci. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- Bakker J, van Ophemert J, Slob AK. Organization of partner preference and sexual behavior and its nocturnal rhythmicity in male rats. Behav. Neurosci. 1993;107:1049–1058. doi: 10.1037//0735-7044.107.6.1049. [DOI] [PubMed] [Google Scholar]
- Bales KL, Lewis-Reese AD, Pfeifer LA, Kramer KM, Carter CS. Early experience affects the traits of monogamy in a sexually dimorphic manner. Dev Psychobiol. 2007;49:335–342. doi: 10.1002/dev.20216. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, De Vries GJ. Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles, Microtus ochrogaster, and meadow voles, Microtus pennsylvanicus. J. Neuroendocrinol. 1993;5:247–255. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, De Vries GJ. Cohabitation alters vasopressin innervation and paternal behavior in Prairie voles, Microtus ochrogaster. Physiol. Behav. 1994;56:751–758. doi: 10.1016/0031-9384(94)90238-0. [DOI] [PubMed] [Google Scholar]
- Beach FA. Hormonal factors controlling the differentiation. development, and display ofcopulatory behavior in the ramstergig and related species. In: Tobach E, Aronson LR, Shaw E, editors. The Biopsychology of Development. New York: Academic Press; 1971. pp. 249–296. [Google Scholar]
- Beach FA. Bisexual mating behavior in the male rat: Effects of castration and hormone administration. Physiol. Zool. 1945;18:390–402. [Google Scholar]
- Beach FA. Sexual attractivity, proceptivity'. and receptivity in female mammals. Hormone Behar. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Young LJ. Sexual dimorphism in the vasopressin system: lack of an altered behavioral phenotype in female V1a receptor knockout mice. Behav. Brain Res. 2005;164:132–136. doi: 10.1016/j.bbr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Bleier R, Byne W, Siggelkow I. Cytoarchitectonic sexual dimorphisms of the medial preoptic and anterior hypothalamic areas in guinea pig, rat, hamster, and mouse. J. Comp. Neurol. 1982;212:118–130. doi: 10.1002/cne.902120203. [DOI] [PubMed] [Google Scholar]
- Bluthe R-M, Dantzer R. Social recognition does not involve vasopressinergic neurotransmission in female rats. Brain Res. 1990;535:301–304. doi: 10.1016/0006-8993(90)91613-l. [DOI] [PubMed] [Google Scholar]
- Brand T, Kroonen J, Mos J, Slob AK. Adult partner preference and sexual behavior of male rats affected by perinatal endocrine manipulations. Horm. Behav. 1991;25:323–341. doi: 10.1016/0018-506x(91)90005-3. [DOI] [PubMed] [Google Scholar]
- Bridges RS. Endocrine regulation of parental behavior in rodents. In: Krasgenor NA, Bridges RS, editors. Mammalian Parenting. Biochemical Neurobiological, and Behavioral Determinants. New York: Oxford University Press; 1990. pp. 93–117. [Google Scholar]
- Browne P, Place NJ, Vidal JD, Moore IT, Cunha GR, Glickman SE, Conley AJ. Endocrine differentiation of fetal ovaries and testes of the spotted hyena (Crocuta crocuta): timing of androgen-independent versus androgen-driven genital development. Reproduction. 2006;132:649–659. doi: 10.1530/rep.1.01120. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Buehr M, Koopman P, Rossant J, McLaren A. Cell-autonomous action of the testis-determining gene: Sertoli cells are exclusively XY in XX-XY chimaeric mouse testes. Development. 1988;102:443–450. doi: 10.1242/dev.102.2.443. [DOI] [PubMed] [Google Scholar]
- Byne W, Tobet S, Mattiace LA, Lasco MS, Kemether E, Edgar MA, Morgello S, Buchsbaum MS, Jones LB. The interstitial nuclei of the human anterior hypothalamus: an investigation of variation with sex, sexual orientation, and HIV status. Horm. Behav. 2001;40:86–92. doi: 10.1006/hbeh.2001.1680. [DOI] [PubMed] [Google Scholar]
- Cherry JA, Basham ME, Baum MJ. Neonatal testosterone masculinizes sexual behavior without affecting the morphology of the dorsal preoptic/anterior hypothalamic area of female ferrets. Brain Res. 1991;546:321–328. doi: 10.1016/0006-8993(91)91496-n. [DOI] [PubMed] [Google Scholar]
- Cherry JA, Basham ME, Weaver CE, Krohmer RW, Baum MJ. Ontogeny of the sexually dimorphic male nucleus in the preoptic/anterior hypothalamus of ferrets and its manipulation by gonadal steroids. J. Neurobiol. 1990;21:844–857. doi: 10.1002/neu.480210603. [DOI] [PubMed] [Google Scholar]
- Cherry JA, Baum MJ. Effects of lesions of a sexually dimorphic nucleus in the preoptic/anterior hypothalamic area on the expression of androgen- and estrogen-dependent sexual behaviors in male ferrets. Brain Res. 1990;522:191–203. doi: 10.1016/0006-8993(90)91461-o. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantchakoff V. Rôle des hormones dans la manifestation des instincts sexuels. Compt. rend. Acad. sci. 1938;206:945–948. 1938. [Google Scholar]
- Davidson JM. Activation of the male rat’s sexual behavior by intracerebral implantation of androgen. Endocrinology. 1966;79:783–794. doi: 10.1210/endo-79-4-783. [DOI] [PubMed] [Google Scholar]
- de Jonge FH, Louwerse AL, Ooms MP, Evers P, Endert E, Van de Poll NE. Lesions of the SDN-POA inhibit sexual behavior of male Wistar rats. Brain Res. Bull. 1989;23:483–492. doi: 10.1016/0361-9230(89)90194-9. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Sex differences in neurotransmitter systems. J. Neuroend. 1990;2:1–13. doi: 10.1111/j.1365-2826.1990.tb00385.x. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Sex differences in adult and developing brains; compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Boyle PA. Double duty for sex differences in the brain. Behav. Brain Res. 1998;92:205–213. doi: 10.1016/s0166-4328(97)00192-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBold JE, Miczek KA. Aggression persist after gonadectomy in female rats. Horm. Behav. 1984;18:177–190. doi: 10.1016/0018-506x(84)90041-2. [DOI] [PubMed] [Google Scholar]
- Dornan AJ, Goodwin SF. Fly courtship song: triggering the light fantastic. Cell. 2008;133:210–212. doi: 10.1016/j.cell.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA. Kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- Dylan Clyne J, Miesenböck G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J. Comp. Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- Emery DE, Sachs BD. Ejaculatory pattern in female rats without androgen treatment. Science. 190:484–486. doi: 10.1126/science.1174387. [DOI] [PubMed] [Google Scholar]
- Everett JW, Sawyer CH. A 24 hour periodicity in the’LH’release apparatus’ disclosed by barbitu rate sedation. Endocrinology. 1950;47:198–218. doi: 10.1210/endo-47-3-198. [DOI] [PubMed] [Google Scholar]
- Ferris CF. Vasopressin/oxytocin and aggression. Novartis Found. Symp. 2005;268:190–198. [PubMed] [Google Scholar]
- Forger NG. The organizational hypothesis and final common pathways: sexual differentiation of the spinal cord and peripheral nervous system. Horm. Behav. 2009 doi: 10.1016/j.yhbeh.2009.03.008. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, De Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerall AA, Dunlap JL, Hendricks SE. Effect of ovarian secretions on female behavioral potentiality in the rat. J. Comp. Physiol. Psychol. 1973;82:449–465. doi: 10.1037/h0034113. [DOI] [PubMed] [Google Scholar]
- González-Martínez D, De Mees C, Douhard Q, Szpirer C, Bakker J. Absence of gonadotropin-releasing hormone 1 and Kiss1 activation in alpha-fetoprotein knockout mice: prenatal estrogens defeminize the potential to show preovulatory luteinizing hormone surges. Endocrinology. 2008;149:2333–2340. doi: 10.1210/en.2007-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res. Brain Res. Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Grady KL, Phoenix CH, Young WC. Role of the developing rat testis in the differentiation of the neural tissues mediating mating behavior. J. Comp. Physiol. Psvchol. 1965;59:176–182. doi: 10.1037/h0021824. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Eichhorn GR, Tranel D. Effects of gender on blood flow correlates of naming concrete entities. Neuroimage. 2003;20:940–954. doi: 10.1016/S1053-8119(03)00284-2. [DOI] [PubMed] [Google Scholar]
- Hamilton WJ, Tilson RL, Frank LG. Sexual monomorphism in spotted hyenas, Crocuta crocuta. Ethology. 1986;71:63–73. [Google Scholar]
- Hansen S, Köhler C, Goldstein M, Steinbusch HVM. Effects of ibotenic acid-induced neuronal degeneration in the medial preoptic area and the lateral hypothalamic area on sexual behavior in the male rat. Brain Res. 1982;239:213–232. doi: 10.1016/0006-8993(82)90843-5. [DOI] [PubMed] [Google Scholar]
- Hansen S, Södersten P, Eneroth P, Srebro B, Hole K. A sexually dimorphic rhythm in oestradiol activated lordosis behaviour in the rat. J. Endocrinol. 1979;33:267–274. doi: 10.1677/joe.0.0830267. [DOI] [PubMed] [Google Scholar]
- Hayes UL, De Vries GJ. Role of pregnancy and parturition in induction of maternal behavior in prairie voles (Microtus ochrogaster) Horm. Behav. 2007;51:265–272. doi: 10.1016/j.yhbeh.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey AC, Wallen K, Edwards DA. Preoptic lesions increase the display of lordosis by male rats. Brain Res. 1986;370:21–28. doi: 10.1016/0006-8993(86)91100-5. [DOI] [PubMed] [Google Scholar]
- Hillarp NA, Olivecrona H, Silfverskiöld W. Evidence for the participation of the preoptic area in male mating behaviour. Experientia. 1954;10:224–225. doi: 10.1007/BF02159285. [DOI] [PubMed] [Google Scholar]
- Houtsmuller EJ, Brand T, de Jonge FH, Joosten RN, Van de Poll NE, Slob AK. SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol Behav. 1994;56:535–541. doi: 10.1016/0031-9384(94)90298-4. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm. Behav. 2003;44:293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Ito S, Murakami S, Yamanouchi K, Arai Y. Prenatal androgen exposure, preoptic area and reproductive functions in the female rat. Brain Dev. 1986;8:463–468. doi: 10.1016/s0387-7604(86)80070-5. [DOI] [PubMed] [Google Scholar]
- J. Comp. Psychol. 115:53–61. [Google Scholar]
- Jyotika J, McCutcheon J, Laroche J, Blaustein JD, Forger NG. Deletion of the Bax gene disrupts sexual behavior and modestly impairs motor function in mice. Dev. Neurobiol. 2007;67:1511–1519. doi: 10.1002/dneu.20525. [DOI] [PubMed] [Google Scholar]
- Kato R. Serotonin content of rat brain in relation to sex and age. J. Neurochem. 1960;5:202. doi: 10.1111/j.1471-4159.1960.tb13355.x. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Kindon HA, Baum MJ, Paredes RJ. Medial preoptic/anterior hypothalamic lesions induce a female-typical profile of sexual partner preference in male ferrets. Horm. Behav. 1996;30:514–527. doi: 10.1006/hbeh.1996.0055. [DOI] [PubMed] [Google Scholar]
- Koehl M, Battle S, Meerlo P. Sex differences in sleep: the response to sleep deprivation and restraint stress in mice. Sleep. 2006;29:1224–1231. doi: 10.1093/sleep/29.9.1224. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Moor E, Hiemstra Y, Bohus B. The testosterone-dependent vasopressinergic neurons in the medial amygdala and lateral septum: involvement in social behaviour of male rats. In: Jard S, Jamison R, editors. Vasopressin. Colloque INSERM. Vol. 208. Montrouge: John Libbey Eurotext Ltd; 1991. pp. 213–219. [Google Scholar]
- Koolhaas JM, Van den Brink THC, Roozendaal B, Boorsma F. Medial amygdala and aggressive behavior: interaction between testosterone and vasopressin. Aggressive Behav. 1990;16:223–229. [Google Scholar]
- Larsson K, Heimer L. Mating behaviour of male rats after lesions in the preoptic area. Nature. 1964;202:413–414. doi: 10.1038/202413a0. [DOI] [PubMed] [Google Scholar]
- Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of the daily LH surges in the ovariectomized rat. Endocrinology. 1975;96:50–56. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- LeVay S. A difference in hypothalamic structure between heterosexual and homosexual men. Science. 1991;253:1034–1037. doi: 10.1126/science.1887219. [DOI] [PubMed] [Google Scholar]
- Liman ER, Corey DP, Dulac C. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc. Natl. Acad. Sci. U. S. A. 1999;96:5791–5796. doi: 10.1073/pnas.96.10.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Sex differences in the parental behaviour of adult virgin prairie voles: independence from gonadal hormones and vasopressin. J. Neuroendocrinol. 1999b;11:441–449. doi: 10.1046/j.1365-2826.1999.00361.x. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Comparison of the parental behavior of pair-bonded female and male prairie voles (Microtus ochrogaster) Physiol. Behav. 1999a;66:33–40. doi: 10.1016/s0031-9384(98)00270-4. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Social influences on parental and nonparental responses toward pups in virgin female prairie voles (Microtus ochrogaster) 2001 doi: 10.1037/0735-7036.115.1.53. [DOI] [PubMed] [Google Scholar]
- Lovell-Badge R, Hacker A. The molecular genetics of Sry and its role in mammalian sex determination. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1995;350:205–214. doi: 10.1098/rstb.1995.0153. [DOI] [PubMed] [Google Scholar]
- Lyon MF. X-chromosome inactivation. Curr. Biol. 1999;9:R235–R237. doi: 10.1016/s0960-9822(99)80151-1. [DOI] [PubMed] [Google Scholar]
- Matthews LH. Reproduction in the spotted hyena (Crocuta crocuta, Erxleben) Philos. Trans. R. Soc. London., Ser. B. 1939;230:1–78. [Google Scholar]
- Maywood ES, O’Neill JS, Chesham JE, Hastings MH. The circadian clockwork of the suprachiasmatic nuclei--analysis of a cellular oscillator that drives endocrine rhythms. Endocrinology. 2007;148:5624–5634. doi: 10.1210/en.2007-0660. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol. Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, De Vries GJ, Forger NG. Sexual differentiation of the brain: mode, mechanisms, and meaning. In: Pfaff DW, et al., editors. Hormones, Brain, and Behavior. Elsevier; 2009. In Press. [Google Scholar]
- McEwen BS, Pfaff DW. Factors influencing sex hormone uptake by rat brain regions. I. Effects of neonatal treatment, hypophysectomy, and competing steroid on estradiol uptake. Brain Res. 1970;21:1–16. doi: 10.1016/0006-8993(70)90016-8. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Pfaff DW, Zigmond RE. Factors influencing sex hormone uptake by rat brain regions. II. Effects of neonatal treatment and hypophysectomy on testosterone uptake. Brain Res. 1970;21:17–28. doi: 10.1016/0006-8993(70)90017-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Neural gonadal steroid actions. Science. 1981;2ll:1303–l3ll. doi: 10.1126/science.6259728. [DOI] [PubMed] [Google Scholar]
- Moralí G, Asunción Pía Soto M, Luis Contreras J, Arteaga M, González-Vidal MD, Beyer C. Detailed analysis of the male copulatory motor pattern in mammals: hormonal bases. Scand. J. Psychol. 2003;44:279–288. doi: 10.1111/1467-9450.00346. [DOI] [PubMed] [Google Scholar]
- Morgan PJ, Hazlerigg DG. Photoperiodic signalling through the melatonin receptor turns full circle. J Neuroendocrinol. 2008;20:820–826. doi: 10.1111/j.1365-2826.2008.01724.x. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Mosig DW, Dewsbury DA. Studies of the copulatory behavior of house mice (Mus musculus) Behav. Biol. 1976;16:463–473. doi: 10.1016/s0091-6773(76)91635-7. [DOI] [PubMed] [Google Scholar]
- Nance DM, Shryne J, Gorski RA. Septal lesions: effects on lordosis behavior and pattern of gonadotropin release. Horm. Behav. 1974;5:73–81. doi: 10.1016/0018-506x(74)90008-7. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Olster DH, Blaustein JD. Progesterone facilitation of lordosis in male and female Sprague-Dawley rats following priming with estradiol pulses. Horm. Behav. 1988;22:294–304. doi: 10.1016/0018-506x(88)90002-5. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J. Neurosci. 2004;24:8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RG, Baum MJ. Altered sexual partner preference in male ferrets given excitotoxic lesions of the preoptic area/anterior hypothalamus. J. Neurosci. 1995;15:6619–6630. doi: 10.1523/JNEUROSCI.15-10-06619.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RG, Lopez ME, Baum MJ. Testosterone augments neuronal Fos responses to estrous odors throughout the vomeronasal projection pathway of gonadectomized male and female rats. Horm. Behav. 1998;33:48–57. doi: 10.1006/hbeh.1998.1435. [DOI] [PubMed] [Google Scholar]
- Payne AP, Swanson HH. Agonistic behaviour between pairs of hamsters of the same and opposite sex in a neutral observation area. Behaviour. 1970;36:260–269. [PubMed] [Google Scholar]
- Pfaff DW. Morphological changes in the brains of adult male rats after neonatal castration. J. Endocrinol. 1966;36:415–416. doi: 10.1677/joe.0.0360415. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Markowitsch HJ, Fink GR. Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Hum. Brain Mapp. 2005;24:313–324. doi: 10.1002/hbm.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers B, Valenstein ES. Sexual receptivity: facilitation by medial preoptic lesions in female rats. Science. 1972;175:1003–1005. doi: 10.1126/science.175.4025.1003. [DOI] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, McEwen BS. Sex differences in rat brain oestrogen and progestin receptors. Nature. 1982;300:648–649. doi: 10.1038/300648a0. [DOI] [PubMed] [Google Scholar]
- Raisman G, Field PM. Sexual dimorphism in the preoptic area of the rat. Science. 1971;173:731–733. doi: 10.1126/science.173.3998.731. [DOI] [PubMed] [Google Scholar]
- Raisman G, Field PM. Sexual dimorphism in the neuropil of the preoptic area of the rat and its dependence on neonatal androgen. Brain Res. 1973;54:1–29. doi: 10.1016/0006-8993(73)90030-9. [DOI] [PubMed] [Google Scholar]
- Rideout EJ, Billeter JC, Goodwin SF. The sex-determination genes fruitless and doublesex specify a neural substrate required for courtship song. Curr. Biol. 2007;17:1473–1478. doi: 10.1016/j.cub.2007.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GE, Fernald RD, Clayto DF. Genes and social behavior. Science. 2008;322:896–900. doi: 10.1126/science.1159277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Estill CT, Stadelman HL, Stormshak F. The volume of the ovine sexually dimorphic nucleus of the preoptic area is independent of adult testosterone concentrations. Brain Res. 2008 doi: 10.1016/j.brainres.2008.10.047. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology. 2004;145:478–483. doi: 10.1210/en.2003-1098. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Stadelman H, Reeve R, Bishop CV, Stormshak F. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology. 2007;148:4450–4457. doi: 10.1210/en.2007-0454. [DOI] [PubMed] [Google Scholar]
- Rosen GJ, De Vries GJ, Villalba C, Weldele ML, Place NJ, Coscia EM, Glickman SE, Forger NG. The distribution of vasopressin in the forebrain of spotted hyenas. J. Comp. Neurol. 2006;498:80–92. doi: 10.1002/cne.21032. [DOI] [PubMed] [Google Scholar]
- Rusak B, Zucker I. Biological rhythms and animal behavior. Annu. Rev. Psychol. 1975;26:131–171. doi: 10.1146/annurev.ps.26.020175.001033. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweiler DP, Katz L, Gore JC. Sex differences in the functional organization of the brain for language. Nature. 1995;373:607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- Shah NM, Breedlove SM. Behavioural neurobiology: females can also be from Mars. Nature. 2007;448:999–1000. doi: 10.1038/nature05892. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Handa RJ, Gorski RA. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology. 1985;40:501–510. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- Simonneaux V, Ansel L, Revel FG, Klosen P, Pévet P, Mikkelsen JD. Kisspeptin and the seasonal control of reproduction in hamsters. Peptides. 2009;30:146–153. doi: 10.1016/j.peptides.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Södersten P. Mounting behavior in female rat during estrous-cycle, after ovariectomy, and after estrogen or testosterone administration. Horm. Behav. 1972;3:307–320. [Google Scholar]
- Södersten P. How different are male and female brains. TINS. 1987;10:197–198. [Google Scholar]
- Södersten P, de Jong FH, Vreeburg JT, Baum MJ. Lordosis behavior in intact male rats: absence of correlation with mounting behavior or testicular secretion of estradiol-17 beta and testosterone. Physiol. Behav. 1974;13:803–808. doi: 10.1016/0031-9384(74)90265-0. [DOI] [PubMed] [Google Scholar]
- Södersten P, De Vries GJ, Buijs RM, Melin P. A daily rhythm in behavioural vasopressin sensitivity and brain vasopressin concentrations. Neurosci. Lett. 1985;58:37–41. doi: 10.1016/0304-3940(85)90325-8. [DOI] [PubMed] [Google Scholar]
- Södersten P, Eneroth P. Neonatal treatment with anti oestrogen increases the diurnal rhythmicity in the sexual behavior of adult male rats. J. Endocrinol. 1980;85:331–339. doi: 10.1677/joe.0.0850331. [DOI] [PubMed] [Google Scholar]
- Södersten P, Hansen S, Srebro B. Suprachiasmatic lesions disrupt the daily rhythmicity in the sexual behaviour of normal male rats and of male rats treated neonatally with antioestrogen. J. Endocrinol. 1981b;88:125–130. doi: 10.1677/joe.0.0880125. [DOI] [PubMed] [Google Scholar]
- Södersten P, Henning M, Melin P, Lundin S. Vasopressin alters female sexual behaviour by acting on the brain independently of alterations in blood pressure. Nature. 1983b;301:608–610. doi: 10.1038/301608a0. [DOI] [PubMed] [Google Scholar]
- Södersten P, Larsson K. Lordosis behavior in castrated male rats treated with estradiol benzoate or testosterone propionate in combination with an estrogen antagonist, MER-25, and in intact male rats. Horm. Behav. 1974;5:13–18. doi: 10.1016/0018-506x(74)90003-8. [DOI] [PubMed] [Google Scholar]
- Södersten P, Larsson K. Sexual behavior in castrated male rats treated with monoamine synthesis inhibitors and testosterone. Pharmacol. Biochem. Behav. 1976;5:319–327. doi: 10.1016/0091-3057(76)90084-8. [DOI] [PubMed] [Google Scholar]
- Södersten P, Pettersson A, Eneroth P. Pulse administration of estradiol-17 beta cancels sex difference in behavioral estrogen sensitivity. Endocrinology. 1983a;112:1883–1885. doi: 10.1210/endo-112-5-1883. [DOI] [PubMed] [Google Scholar]
- Södersten P. Lordosis behaviour in male. female and androgenized female rats. J. Endocrinol. 1976;l0:409–420. doi: 10.1677/joe.0.0700409. [DOI] [PubMed] [Google Scholar]
- Spelke ES. Sex differences in intrinsic aptitude for mathematics and science?: a critical review. Am. Psychol. 2005;60:950–958. doi: 10.1037/0003-066X.60.9.950. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E. A sexually dimorphic nucleus in the human brain. Science. 1985;228:1112–1115. doi: 10.1126/science.3992248. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Schwarz JM, McCarthy MM. Prostaglandin-E2: a point of divergence in estradiol-mediated sexual differentiation. Horm. Behav. 2005;48:512–521. doi: 10.1016/j.yhbeh.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Tsukahara S. Increased Fos immunoreactivity in suprachiasmatic nucleus before luteinizing hormone surge in estrogen-treated ovariectomized female rats. Neuroendocrinology. 2006;83:303–212. doi: 10.1159/000095341. [DOI] [PubMed] [Google Scholar]
- Turkenburg JL, Swaab DF, Endert E, Louwerse AL, Van de Poll NE. Effects of lesions of the sexually dimorphic nucleus on sexual behavior of testosterone-treated female Wistar rats. Brain Res. Bull. 1988;21:215–224. doi: 10.1016/0361-9230(88)90234-1. [DOI] [PubMed] [Google Scholar]
- Vale JR, Ray D, Vale CA. The interaction of genotype and exogenous neonatal androgen and estrogen: Sex behavior in female mice. Dev. Psychobiol. 1973;6:319–327. doi: 10.1002/dev.420060405. [DOI] [PubMed] [Google Scholar]
- Villalba C, Boyle PA, De Vries GJ. Effects of the selective serotonin reuptake inhibitor, fluoxetine, on social behaviors in male and female prairie voles (Microtus ochrogaster) Horm. Behav. 1997;32:184–191. doi: 10.1006/hbeh.1997.1420. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Ferris CF, De Vries GJ. The role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc. Natl. Acad. Sci. U.S.A. 1994a;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, Smith W, Major DE, De Vries GJ. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain Res. 1994b;650:212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Horm. Behav. 1997;32:176–183. doi: 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- Wright CL, Burks SR, McCarthy MM. Identification of prostaglandin E2 receptors mediating perinatal masculinization of adult sex behavior and neuroanatomical correlates. Dev. Neurobiol. 2008;68:1406–1419. doi: 10.1002/dneu.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WC. The hormones and mating behavior. In: Young WC, editor. Sex and lnternal Secretions. Baltimore. MD: Williams and Wilkins; 1961. pp. 1l73–1239. [Google Scholar]
- Young WC, Goy RW, Phoenix CH. Hormones and sexual behavior. Science. 1964;143:212–218. doi: 10.1126/science.143.3603.212. [DOI] [PubMed] [Google Scholar]
- Young WC. Psychobiology of sexual behavior in the guinea pig. In: Lehrman DS, Hinde RA, Shaw E, editors. Advances in the Study of Behavior. Vol.2. New York: Academic Press; 1969. pp. 1–1l0. [Google Scholar]
- Zasorin NL, Malsbury CW, Pfaff DW. Suppression of lordosis in the hormone-primed female hamster by electrical stimulation of the septal area. Physiol. Behav. 1975;14:595–599. doi: 10.1016/0031-9384(75)90187-0. [DOI] [PubMed] [Google Scholar]