Abstract
BACKGROUND
Type 2 diabetes (T2D) is a metabolic disease with significant medical complications. Roux-en-Y gastric bypass (RYGB) surgery is one of the few interventions that remit T2D in ~60% of patients. However, there is no accurate method for predicting preoperatively the probability for T2D remission.
METHODS
A retrospective cohort of 2,300 RYGB patients at Geisinger Clinic was used to identify 690 patients with T2D and complete electronic data. Two additional T2D cohorts (N=276, and N=113) were used for replication at 14 months following RYGB. Kaplan-Meier analysis was used in the primary cohort to create survival curves until remission. A Cox proportional hazards model was used to estimate the hazard ratios on T2D remission.
FINDINGS
Using 259 preoperative clinical variables, four (use of insulin, age, HbA1c, and type of antidiabetic medication) were sufficient to develop an algorithm that produces a type 2 diabetes remission (DiaRem) score over five years. The DiaRem score spans from 0 to 22 and was divided into five groups corresponding to five probability-ranges for T2D remission: 0–2 (88%–99%), 3–7 (64%–88%), 8–12 (23%–49%), 13–17 (11%–33%), 18–22 (2%–16%). The DiaRem scores in the replication cohorts, as well as under various definitions of diabetes remission, conformed to the DiaRem score of the primary cohort.
INTERPRETATION
The DiaRem score is a novel preoperative method for predicting the probability (from 2% to 99%) for T2D remission following RYGB surgery.
FUNDING
This research was supported by the Geisinger Health System and the National Institutes of Health.
INTRODUCTION
Type 2 diabetes (T2D) is a chronic metabolic disease with potentially severe medical and socioeconomic effects.1 Roux-en-Y gastric bypass (RYGB) surgery is a particularly effective intervention in humans that remits T2D2–4, with ~60% of patients achieving T2D remission.5, 6 RYGB has also been proposed as a therapy for T2D resolution in cases where weight loss may not be the primary objective7, 8 including cases with low body mass index (BMI) ranging from 25 to 35 Kg/m2.9, 10 It would therefore benefit patients and clinicians to have a means for predicting the probability of T2D remission by RYGB, using preoperative criteria.
Various mechanisms have been proposed for predicting T2D remission after RYGB surgery. Durable T2D remission has been associated with early diabetes stage11 and significant percent excess body weight loss (% EWL)12, while, failure to achieve long-term remission has been associated with inadequate weight loss.13 Young age and low BMI (25–35 Kg/m2) are also predictors of long-term T2D remission9, 10, while, use of insulin, high percent glycated hemoglobin A1c (HbA1c), and low %EWL are predictors of decreased rates of remission after RYGB surgery.14 Glycemic response to gastric bypass has also been correlated with BMI, duration of diabetes, fasting C-peptide, and weight loss.15 A few reports using algorithmic prediction models have shown that preoperative BMI, HbA1c, plasma glucose, hypertension, and better control of diabetes can predict diabetes remission after RYGB.16, 17
Our goal was to develop a simple and effective method based on preoperative clinical criteria for predicting diabetes remission by RYGB. After screening 259 variables, four of them formed an algorithmic model and a scoring system that predicts probabilities ranging from 2% to 99% for diabetes remission after RYGB.
RESEARCH DESIGN AND METHODS
Study design and participants
A retrospective cohort of 2,300 patients that underwent Roux-en-Y gastric bypass at Geisinger Clinic between 1/1/2004 and 2/15/201118 was used to identify 690 T2D patients with available electronic medical records (EMR). The cohort consisted predominantly of severely obese (mean BMI 49.2 kg/m2) White Caucasians (97%) from central Pennsylvania who had voluntarily enrolled into our RYGB surgery program.18, 19 The mean age of the primary cohort was 51.2 years and the female/male ratio of 73/27% (Tables 1, S1). These 690 T2D cases were divided into T2D patients not using insulin preoperatively (the “T2D” group) and T2D patients using insulin preoperative (the “T2D+I” group) (Table S1). Each category was further divided into patients with early or late T2D remission (details in sections below). A flow chart of patient groups and samples sizes is provided in Figure 1. These studies were approved by the Geisinger Clinic Institutional Review Board (IRB). All participants provided informed written consent.
Table 1. Demographics and basic characteristics of the primary and replication cohorts.
Primary: the primary cohort from central Pennsylvania that was used to develop the DiaRem score prediction tool. AZ: the first replication cohort from the Scottsdale area in Arizona. PA: the second replication cohort from central Pennsylvania. The data shown are at 14 months following RYGB surgery, in all three cases. ISA: insulin sensitizing agent. (Age: years; HbA1c: percent; Serum insulin: μU/mL).
| Primary cohort | AZ cohort | PA cohort | P-value | |
|---|---|---|---|---|
|
| ||||
| Sample size | 690 | 276 | 113 | - |
|
| ||||
| Geographic location | Central PA | Scottsdale, AZ | Central PA | - |
|
| ||||
| % Female | 73% | 68% | 74% | 0·272 |
|
| ||||
| % Caucasian | 97% | 89% | 96% | <0·0001 |
|
| ||||
| Age (years): <40 (%) | 14% | 13% | 15% | |
| 40–49 | 30% | 34% | 32% | 0·119 |
| 50–59 | 37% | 41% | 36% | |
| 60+ | 19% | 11% | 17% | |
|
| ||||
| Pre-surgery BMI (kg/m2), mean (SD) | 49·4 (8·3) | 48·4 (8·3) | 49·5 (9·3) | 0·200 |
|
| ||||
| HbA1c (%): <6·5 | 38% | 28% | 43% | |
| 6·5–6·9 | 16% | 18% | 14% | 0·056 |
| 7·0–8·9 | 30% | 39% | 34% | |
| 9·0+ | 15% | 15% | 9% | |
|
| ||||
| Metformin use | 78% | 74% | 83% | 0·103 |
|
| ||||
| Sulfonylureas use | 31% | 33% | 32% | 0·851 |
|
| ||||
| Other ISA | 32% | 41% | 20% | 0·0004 |
|
| ||||
| Insulin use | 36% | 28% | 38% | 0·032 |
|
| ||||
| Serum Insulin (μU/mL): >=30 | 72% | 71% | 71% | 0·868 |
|
| ||||
| DiaRem score: 0–2 | 27% | 18% | 27% | |
| 3–7 | 30% | 43% | 32% | |
| 8–12 | 10% | 14% | 11% | 0·0031 |
| 13–17 | 24% | 17% | 22% | |
| 18–22 | 8% | 7% | 8% | |
Figure 1. Flow chart describing the patient selection strategy for the Primary cohort.
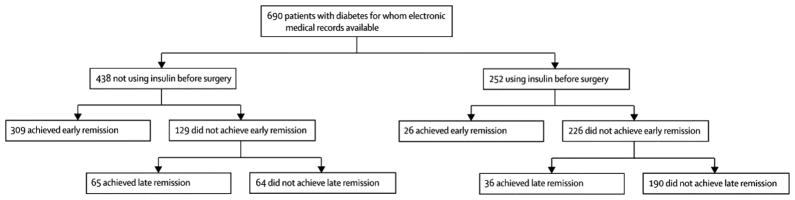
The indicated sample sizes (N) were used for the corresponding type of analysis, before and after stratification by insulin use [i.e., overall remission (partial + complete), predictors of early or late (partial + complete) remission].
Definition of type 2 diabetes and remission of type 2 diabetes
The definition of type 2 diabetes was according to the American Diabetes Association (ADA) guidelines.20 T2D was defined by fasting glucose > 126 mg/dL or HbA1c > 6·5%. Additional confirmation was obtained by examining the EMR for the ICD9 code for T2D diabetes. Preoperative medication use included biguanides (metformin), insulin sensitizer, sulfonylureas, insulin, or combinations of these before surgery.
Remission of T2D was defined according to ADA criteria.21 Specifically, “partial” remission of T2D was defined by HbA1c < 6·5%, fasting blood glucose levels < 125 mg/dL and no use of anti-diabetic medications, for a minimum of 12 months. “Complete” remission was defined by HbA1c < 6.0%, fasting glucose < 100 mg/dL, and no use of antidiabetic medication for least 12 months. The DiaRem score was developed by using patients in “partial” and “complete” remission combined.
Early and late remission of diabetes
Early T2D remission was defined as the period of remission commencing within the first two months after surgery and lasting for at least an additional 12 months. The period of two months after surgery was required to ensure that patients in this classification had their glucose and HbA1c normalized before they were taken off any anti-diabetic medications. Late T2D remission was defined as the remission commencing more than two months after surgery and lasting at least for another 12 months.
Development of the algorithm, weighting of scores, and statistical analyses
A total of 259 clinical variables (including 51 co-morbidities, 93 medications, 78 laboratories, 19 survey scores, and 18 other miscellaneous factors (age, gender, smoking, alcohol, use, etc.) were considered as we have previously described.18 Multiple logistic regression models were used to identify independent predictors of early diabetes remission. To evaluate predictors of late diabetes remission, the patients with early remission were excluded, and multivariate Cox regression models were used to identify time until late remission. When building the multivariate models, each of the clinical variables listed above were evaluated in univariate models, and those with p-values < 0.10 were considered for inclusion in the multivariate model. Continuous covariates were checked for non-linearity by categorizing the data into groups (for example, using quartiles of the distribution or scientifically valid cutoff values). For each model, the subset was entered into the relevant multiple regression model with the goal of identifying a set of clinical variables that independently predict remission. Model results were evaluated to identify a consistent subset of variables that predict T2D remission for each regression model. A final Cox regression model for time until T2D remission using this consistent subset of variables was evaluated. The resulting hazard ratios were used to guide the creation of a weighting system (Table S5). We evaluated interactions between all items in the DiaRem score and with baseline BMI but none were significant. All tests were two-sided and p < 0·05 was considered significant. SAS version 9·2 was used for statistical analyses.
Means (standard deviation) and percentages were used to describe the demographics, BMI, and diabetes/lipid laboratory measurements. Kaplan-Meier (K-M) analysis was used to create K-M survival curves of time until remission. Patients that never reached remission were censored. The K-M curves were stratified by pre-operative insulin use and were compared using a Log-rank test. A Cox proportional hazards model was used to estimate the hazard ratio for insulin use on T2D remission.
Other cohorts used in replication analysis of the DiaRem score
To evaluate the validity of the DiaRem score, two independent cohorts were used:
Scottsdale, Arizona: (Scottsdale Healthcare Bariatric Center, AZ made kindly available by Dr. Robin Blackstone). The remission algorithm was based on the initial 14 months post-surgery as defined in a recent manuscript.5 The diabetes remission criteria were for partial remission under ADA-recommendations, as follows: diabetes-free > 14 months after RYGB, no antidiabetic medications, HbA1c < 6·0%, and glucose < 125 mg/dL.
Danville, Pennsylvania, 2nd Geisinger Clinic’s cohort: The DiaRem score was analyzed using a second sample from Geisinger Clinic’s RYGB program who had surgery between 2/16/2011 and 12/31/2011. None of these patients had been used in the original analysis. The diabetes remission criteria were for partial remission under ADA-recommendations, as follows: diabetes-free >14 months after RYGB, no antidiabetic medications, HbA1c < 6·5, and glucose < 125 mg/dL. Basic characteristics of this cohort (termed “PA”) (N = 113), and the Arizona cohort (termed “AZ”) (N = 276) are presented in Table 1.
For patients within each cohort, the DiaRem score was calculated, the patients were categorized into the DiaRem groups defined in the primary analysis (0–2, 3–7, 8–12, 13–17, 18–22), and the percent remission within each group was calculated. Cochran-Armitage trend tests were used to confirm that lower DiaRem scores were associated with higher chance of remission.
Role of the funding source
The sponsors had no role in the study design, data interpretation, or the writing of the manuscript. The corresponding author had full access to all the data and had final responsibility for the decision to submit the manuscript.
RESULTS
Overall, 436 patients (63%) out of a total of 690 had partial or complete diabetes remission. Based on Kaplan-Meier analysis, the overall remission at 14-months, 2-years, 3-years, 4-years, and 5-years was 49%, 58%, 65%, 66%, and 68%, respectively.
Preoperative use of insulin predisposes to low rates of T2D remission (partial + complete)
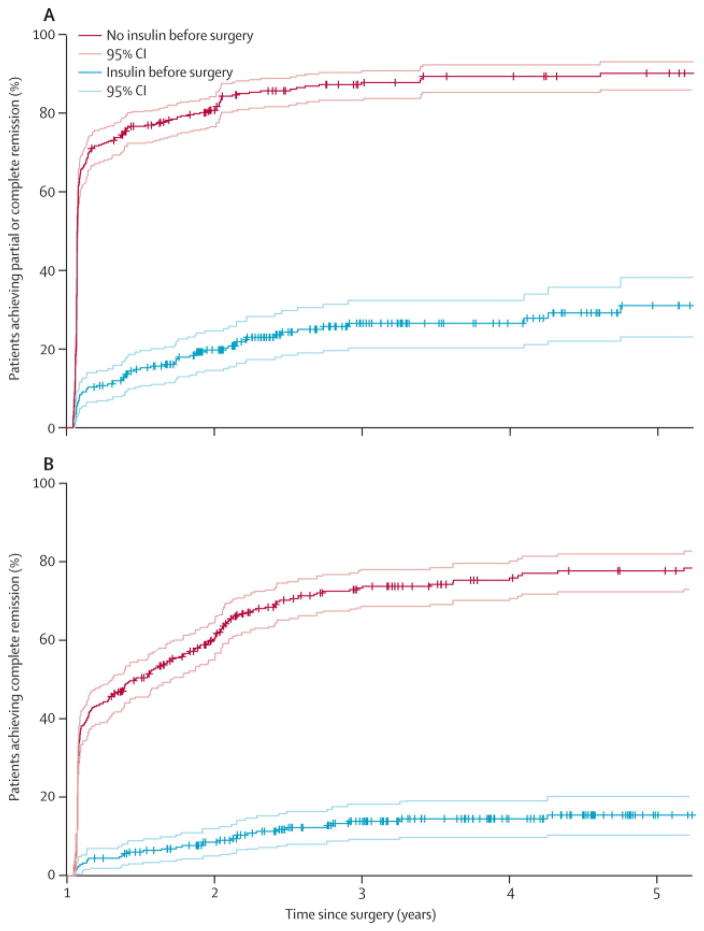
Kaplan-Meier survival estimates using the definition of “partial + complete” remission of diabetes according to ADA recommendations, showed that 70·6% T2D patients (i.e., T2D patients not using insulin) had early T2D remission up to 14 months after RYGB, whereas 10·3% of T2D+I patients (i.e., insulin users) had early T2D remission (Tables S1 & S2, Figure 2A). In Cox regression, the hazard ratio comparing T2D+I to T2D was 7·25 (95% CI=[5·52, 9·52], P-value < 0·0001), meaning that T2D+I patients were 7·25 times less likely to have diabetes remission. By the fifth year after surgery, 90·1% T2D patients had T2D remission while 31·1% T2D+I had T2D remission (Figure 2A). Further Kaplan-Meier analysis using the definition of “complete” diabetes remission according to ADA recommendations (Table S3) predicted lower probabilities of remission for patients not taking insulin (42·4%–77·7%, Figure 2B) as well for patients taking insulin (4·4%–15·4%, Figure 2B), compared to the “partial + complete” definition of diabetes remission.
Figure 2. Kaplan-Meier survival estimates with 95% confidence intervals over five years showing the percent (%) number of patients with diabetes remission after RYGB surgery, stratified by pre-operative use of insulin.
(A) Percent remission according to the definition of “partial + complete” remission of T2D. Patients that were not using insulin preoperatively had a probability range of 70·6%-90·1% for achieving remission of T2D (early or late). T2D patients using insulin preoperatively (T2D+I), on the other hand, had a probability range of 10·3%–31·1% for achieving remission of T2D (early or rate). (B) Percent remission according to the definition of “complete” remission of T2D. Patients that were not using insulin preoperatively had a probability range of 42·4%–77·7% for achieving remission of T2D (early or late). T2D patients using insulin preoperatively (T2D+I), on the other hand, had a probability range of 4·4%–15·4% for achieving remission of T2D (early or rate). More cohort information is provided in the Supplemental information section (Tables S1–S3).
Preoperative prediction of early and late remission in T2D and T2D+I patients
The two subsets of T2D and T2D+I patients were further divided into two groups of early and late remission in order to dissociate in the first group the effects of weight loss. In the T2D group of patients, early remission was correlated with younger age (for each 10 year decrease), low HbA1c (< 6.5%), high insulin levels (> 30 μU/mL), and to a lesser degree with LDL (≥ 125 mg/dL) (Tables 2 and S4). Factors associated with a decreased chance of early remission included combined use of other insulin sensitizing agents (non-metformin) with sulfonylureas and the use of Leukotriene modifiers. Late T2D remission in the T2D group was also correlated with younger age, lower HbA1c, higher insulin levels, and higher % EWL after surgery (for every 10% increase) (Tables 2 and S4). Decreased chance of late remission was associated with combined use of other insulin sensitizing agents (non-metformin) with sulfonylureas. In the T2D+I group of patients, early remission was correlated with younger age, lower HbA1c, the use of an incretin mimetic (defined as use or no use of the incretin), and to a lesser degree to hypertension (Tables 3 and S5). Late remission, in the T2D+I group, was correlated with higher % EWL, younger age, and lower HbA1c (Tables 3 and S5).
Table 2. Predictors for early and late T2D remission after RYGB surgery for patients not using insulin (T2D) preoperatively.
Early remission (partial + complete) was defined by reaching diabetes-free status during the first two months after surgery. Late remission (partial + complete) was defined by reaching diabetes-free status more than two months after surgery. In univariate analysis for early T2D remission, there were 59 variables with p-value < 0·10 (35 with p-values < 0·05). In univariate analysis for late T2D remission, there were 54 variables with p-value < 0·10 (37 with p-values < 0·05). In both events, individual variables were added until the models below were developed. (% EWL: percent excess body weight loss).
| Early T2D remission
| ||||
|---|---|---|---|---|
| Odds ratio | 95% Confidence interval | p-value | ||
|
| ||||
| Age (years) | Each 10 year decrease | 1·41 | [1·10, 1·80] | 0·0071 |
|
| ||||
| Pre-operative HbA1c (%) | <6·5 | 11·53 | [4·32, 30·79] | <0·0001 |
| 6·5–6·9 | 6·27 | [2·21, 17·77] | 0·0006 | |
| 7·0–8·9 | 1·83 | [0·70, 4·83] | 0·220 | |
| 9·0+ | Reference | - | - | |
|
| ||||
| Pre-operative diabetes medications | Other* | 3·13 | [1·70, 5·75] | 0·0003 |
| ISA+Sulf‡ | Reference | - | - | |
|
| ||||
| Serum Insulin (μU/mL) | <17 | Reference | - | - |
| 17–30 | 1·13 | [0·64, 2·02] | 0·668 | |
| 30+ | 2·75 | [1·43, 5·28] | 0·0024 | |
|
| ||||
| Pre-operative use of leukotriene modifiers | Yes | 0·28 | [0·11, 0·70] | 0·0062 |
| No | Reference | - | - | |
|
| ||||
| Pre-operative LDL (mg/dL) | <125 | Reference | - | - |
| ≥125 | 2·26 | [1·17, 4·38] | 0·016 | |
|
| ||||
|
Late T2D remission
| ||||
| Hazard ratio | 95% Confidence interval | p-value | ||
|
| ||||
| Post-op % EWL | Each 10% increase | 1·31 | [1·18, 1·45] | <0·0001 |
|
| ||||
| Age (years) | Each 10 year decrease | 1·45 | [1·10, 1·92] | 0·0085 |
|
| ||||
| Pre-operative HbA1c (%) | <6·5 | 4·73 | [1·78, 12·59] | 0·0019 |
| 6·5–6·9 | 1·65 | [0·57, 4·79] | 0·359 | |
| 7·0–8·9 | 1·98 | [0·76, 5·18] | 0·165 | |
| 9·0+ | Reference | - | - | |
|
| ||||
| Pre-operative diabetes medications | Other* | 2·71 | [1·32, 5·56] | 0·0064 |
| ISA+Sulf‡ | Reference | - | - | |
|
| ||||
| Serum Insulin (μU/mL) | <17 | Reference | - | - |
| 17–30 | 1·77 | [0·96, 3·27] | 0·070 | |
| 30+ | 2·13 | [1·06, 4·29] | 0·035 | |
Other: none, Metformin (Met) only, Sulfonylurea (Sulf) only, insulin sensitizing agent other than metformin (ISA), Met+Sulf, Met+ISA.
ISA+Sulf: insulin sensitizing agent other than metformin + sulfonylurea therapy combined.
Table 3. Predictors of early and late T2D remission after RYGB surgery for patients using insulin (T2D+I) preoperatively.
Early remission (partial + complete) was defined by reaching diabetes-free status during the first two months after surgery and late remission (partial + complete) was defined by reaching diabetes-free status more than two months after surgery. In univariate analysis for early remission, there were 26 variables with p-value < 0·10 (14 with p-values < 0·05). In univariate analysis for late remission, there were 33 variables with p-value < 0·10 (17 with p-values < 0·05). Individual variables were added until the models below were developed. (% EWL: percent excess body weight loss).
| Early T2D remission
| ||||
|---|---|---|---|---|
| Odds ratio | 95% Confidence interval | p-value | ||
|
| ||||
| Age (years) | Each 10 year decrease | 2·21 | [1·32, 3·70] | 0·0024 |
|
| ||||
| Pre-operative HbA1c (%) | <6·5 | 6·81 | [1·95, 23·86] | 0·027 |
| 6·5–6·9 | 3·20 | [0·73, 13·98] | 0·122 | |
| 7·0–8·9 | 1·27 | [0·37, 4·44] | 0·706 | |
| 9·0+ | Reference | - | - | |
|
| ||||
| Hypertension diagnosis | Yes | 0·40 | [0·16, 0·99] | 0·046 |
| No | Reference | - | - | |
|
| ||||
| Use of incretin mimetic agent | Yes | 3·61 | [1·08, 12·14] | 0·038 |
| No | Reference | - | - | |
|
| ||||
|
Late T2D remission
| ||||
| Hazard ratio | 95% Confidence interval | p-value | ||
|
| ||||
| Post-op % EWL | Each 10% increase | 1·16 | [1·05, 1·29] | 0·0029 |
|
| ||||
| Age (years) | Each 10 year decrease | 1·79 | [1·20, 2·63] | 0·0036 |
|
| ||||
| Pre-operative HbA1c (%) | <6·5 | 3·31 | [1·19, 9·18] | 0·022 |
| 6·5–6·9 | 1·67 | [0·42, 6·75] | 0·469 | |
| 7·0–8·9 | 1·97 | [0·81, 4·83] | 0·137 | |
| 9·0+ | Reference | - | - | |
Weighting of variables used in predicting T2D remission prior to RYGB surgery
Two preoperative variables, age (every 10 years) and HbA1c (< 6.5%), were associated with both early and late remission of diabetes in all T2D patients irrespective of insulin use. In addition, combined use of an insulin sensitizing agent other than metformin with sulfonylurea (i.e., ISA+Sulf) correlated with both early and late remission in the non-insulin T2D group of patients. These 3 variables (i.e., age, HbA1c, and type of antidiabetic medication), along with preoperative treatment with insulin (defined as use or no use of insulin) were used in a Cox regression model (Table 4). The hazard ratios from this model were used for developing the scoring algorithm that penalized for older age (i.e., 40–90 years: 1 point, 50–59: 2 points, etc.), high HbA1c (i.e., 6.5–6.9: 2 points, 7.0–8.9: 4 points, etc.), use of sulfonylureas and another ISA (3 points), and use of insulin (10 points) which was the heaviest penalty applied (Table 5).
Table 4. DiaRem score weights with corresponding elements and their hazard ratios (HR) used for weighting each variable contributing to the DiaRem score.
The hazard ratios represent failure to remit T2D (partial + complete). The hazard ratio for each DiaRem score (or penalty) was as follows: Score=1: Age 40–50 (HR=1·08); Score=2: Age 50–59 (HR=1·31), HbA1c 6·5%–6·9% (HR=1·46); Score=3: Age 60+ (HR=1·78), ISA+Sulf (HR=2·07); Score=4: HbA1c 7·0–8·9 (HR=2·51); Score=6: HbA1c 9+ (HR=3·35); Score=10: Insulin meds (HR=5·90). The Full Cox regression model was used to estimate the hazard ratios using all T2D patients (N=690).
| Failure of T2D remission
| ||||
|---|---|---|---|---|
| Hazard ratio | 95% Confidence interval | P-value | ||
|
| ||||
| Pre-operative Insulin medication | Yes | 5·90 | [4·41, 7·90] | <0·0001 |
| No | Reference | - | - | |
|
| ||||
| Age (years) | <40 | Reference | - | |
| 40–50 | 1·08 | [0·82, 1·41] | 0·602 | |
| 50–60 | 1·31 | [1·00, 1·73] | 0·053 | |
| 60+ | 1·78 | [1·27, 2·49] | 0·0009 | |
|
| ||||
| Pre-operative HbA1c (%) | <6·5 | Reference | - | - |
| 6·5–6·9 | 1·46 | [1·12, 1·89] | 0·0045 | |
| 7·0–8·9 | 2·51 | [1·96, 3·23] | <0·0001 | |
| 9·0+ | 3·35 | [2·24, 5·03] | <0·0001 | |
|
| ||||
| Pre-operative diabetes medications | Other* | Reference | - | - |
| ISA+Sulf‡ | 2·07 | [1·50, 2·84] | <0·0001 | |
Other: none, Metformin (Met) only, Sulfonylurea (Sulf) only, insulin sensitizing agent other than metformin (ISA), Met+Sulf, Met+ISA.
ISA+Sulf: insulin sensitizing agent other than metformin + sulfonylurea therapy combined.
Table 5. A pre-operative diabetes remission (DiaRem) score predicting the probability of diabetes remission after RYGB surgery.
Our analysis identified two variables that were associated with remission (partial or complete) of diabetes in all T2D patients irrespective of insulin use (i.e., age and pre-operative HbA1c). In addition, antidiabetic medication was significantly associated with early as well as late remission in the non-insulin T2D group of patients (i.e., use of ISA+Sulf). These 3 variables and treatment with Insulin were used to develop the DiaRem score based on a weighting system for each variable (Table 4). The DiaRem prediction score has a range of 0–22 and was stratified into 5 groups: 0–2 (highest probability), 3–7, 8–12, 13–17, 18–22 (lowest probability). ISA: insulin sensitizing agent other than metformin.
| Prediction factor | Score | |
|---|---|---|
| Age (years) | If age < 40, enter 0 → If age 40–49, enter 1 → If age 50–59, enter 2 → If age 60+, enter 3 → |
|
| HbA1c (%) | If HbA1c < 6.5, enter 0 → If HbA1c 6.5–6.9, enter 2 → If HbA1c 7.0–8.9, enter 4 → If HbA1c 9.0+, enter 6 → |
|
| Other diabetes medications | If not using sulfonylureas or not using ISA, enter 0 → If on sulfonylureas and ISA, enter 3 → |
|
| Treatment with Insulin | If not using insulin, enter 0 → | |
| If using insulin, enter 10 → | ||
| DiaRem Score (sum of individual components) → | ||
Using the DiaRem score for predicting the probability for T2D remission
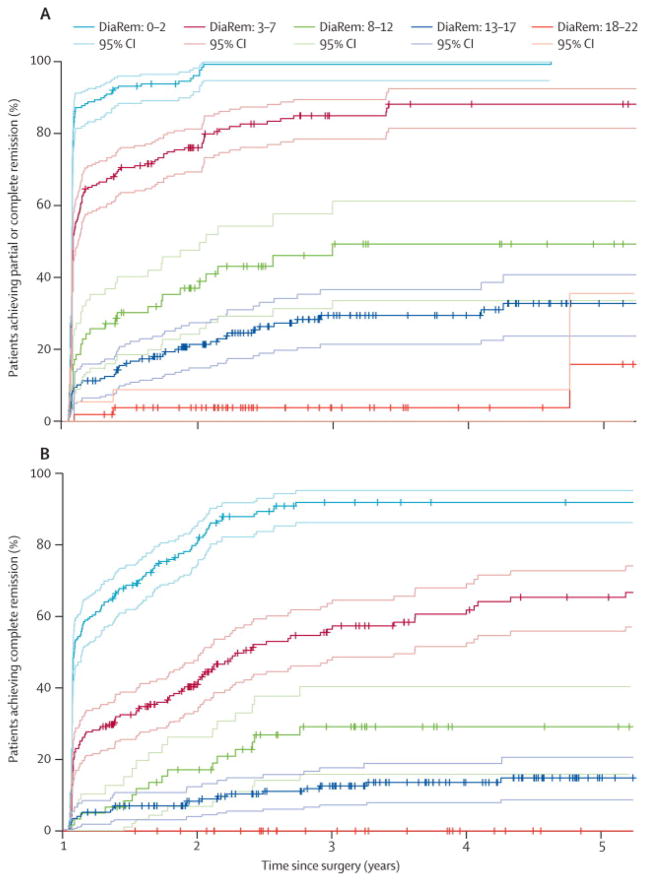
We performed Kaplan-Meier estimates over five years to determine the percent (%) probability for T2D remission after RYGB surgery, stratified by the DiaRem score into five groups: 0–2 (88%–99%), 3–7 (64%–88%), 8–12 (23%–49%), 13–17 (11%–33%), 18–22 (2%–16%) (Table S6). Low DiaRem scores (i.e., the 0–2 grouping) predicted 88%–99% probability for T2D remission, while, high DiaRem scores (18–22) predicted low probability for T2D remission (Figure 3A).
Figure 3. Kaplan-Meier survival estimates with 95% confidence intervals (CIs) over five years showing the percent (%) probability for T2D remission after RYGB surgery, stratified by the DiaRem score.
(A) According to the definitions of “partial + complete” diabetes remission, the lowest DiaRem score (i.e., the 0–2 grouping) predicted high probability for T2D remission (88%–99%), while, the highest DiaRem score (18–22) predicted low probability for going into T2D remission (2%). Intermediate DiaRem scores predicted intermediate probabilities for T2D remission. (B) According to the definition of “complete” diabetes remission, the lowest DiaRem score again predicted high probability for T2D remission (61%–94%), while, the highest DiaRem score predicted no remission (0%). Intermediate DiaRem scores predicted intermediate probabilities for remission. Each DiaRem score line is shown in black color and the corresponding CIs are shown in alternating dotted or dashed gray lines. More cohort information is provided in the Supplemental information section (Tables S6 & S7).
In our primary cohort of patients that had remission, 22% had partial and 78% had complete remission. We thus performed further Kaplan-Meier estimates and re-derived the DiaRem score over 5-years using our primary cohort and strictly the ADA-recommended criteria only for “complete” remission (Table S7). The DiaRem score trended similarly to our standard model of “partial + complete” remissions combined but yielded lower probabilities of remission: DiaRem 0–2 (61%–94%), 3–7 (32%–72%), 8–12 (10%–34%), 13–17 (5%–16%), 18–22 (0%) (Figure 3B).
Replication of the DiaRem score
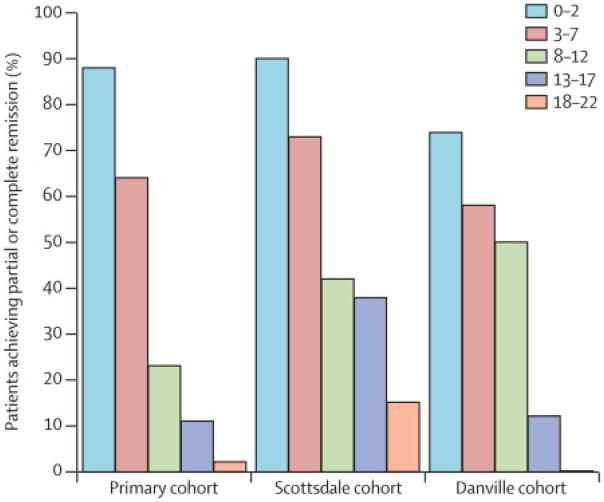
The performance of the DiaRem score was evaluated using two geographically independent RYGB cohorts from Scottsdale, Arizona (partial T2D remission: HbA1c <6.0%, no antidiabetic medication) and a new, previously unused, subset from Danville, Pennsylvania (partial T2D remission: HbA1c < 6·5, fasting glucose < 125 mg/dL, no antidiabetic medications) with data available for the first 14 months after surgery (Table 1). As expected, there were significant differences between DiaRem scores within each cohort (Cochran-Armitage trend test, P < 0·0001), while, the DiaRem scores of the three (i.e., the primary and the two replication) cohorts trended similarly (Figure 4). When pooling the data from all three cohorts, the predicted probabilities for 14-month remission by the DiaRem score, were as follows: DiaRem 0–2 (87%), 3–7 (66%), 8–12 (32%), 13–17 (16%), 18–22 (5%), which are close to or within the 5-year DiaRem score probability ranges described earlier: DiaRem 0–2 (88%–99%), 3–7 (64%–88%), 8–12 (23%–49%), 13–17 (11%–33%), 18–22 (2%–16%).
Figure 4. DiaRem scores predicting percent (partial + complete) T2D remission in three independent cohorts, 14 months after RYGB surgery.
Primary: the main cohort from central Pennsylvania that was used to develop the DiaRem score. AZ: the first replication cohort from Scottsdale, Arizona. PA: the second replication cohort also from Central Pennsylvania. Cochran-Armitage trend tests were used to confirm that lower DiaRem scores were associated with higher chance of remission in each cohort (P < 0·0001). When pooling together the DiaRem scores from the three cohorts, the following mean probability ranges were obtained: DiaRem 0–2: 87%, 3–7: 66%, 8–12: 32%, 13–17: 16%, 18–22: 5%. Basic characteristics of the three cohorts are provided in Table 1.
We also re-derived in the Arizona cohort DiaRem scores according to five different definitions of diabetes remission (classified as “partial” according to ADA recommendations) by using fasting glucose levels (FG), and/or HbA1c percent levels, and/or the use of antidiabetic medication, and corresponding to five different remission rates (percent), as follows: [AZ-1 (59·4%): FG <100 mg/dL, no medication, AZ-2 (55·6%): HbA1c <6.0%, no medication, AZ-3 (46·9%): HbA1c <5.7%, no medication, AZ-4 (46·7%): FG <100 mg/dL, HbA1c <6.0%, AZ-5: FG <100 mg/dL, HbA1c <5.7%] at 14 months after RYGB surgery.5 We found that the DiaRem scores trended similarly among the five different remission models (Figure S1).
DISCUSSION
The goal of this study was to develop a simple and accurate method for predicting T2D remission resulting from RYGB surgery using preoperative clinical measures. First, a Cox regression analysis showed that T2D+I patients were 7·25 less likely to have diabetes remission (modeled as “partial + complete” remission combined) and therefore we classified T2D patients into two categories: non-insulin users (T2D) and insulin users (T2D+I). This confirmed a previous report5, while, the Kaplan-Meier survival estimates showed that only 10·3% of T2D+I patients had early T2D remission compared to the non-insulin using T2D patients of whom 70·6% had early T2D remission. When using the definition of “complete” diabetes remission, the predicted probabilities of remission were even lower for both categories of patients. As a result, our weighting system penalized insulin use with the most severe (highest) score (“10”) in the DiaRem scoring system. Secondly, we accounted for short- and long-term effects of RYGB on improving T2D remission. In the T2D patients, factors that were associated with increased early remission (partial + complete) were younger age, lower pre-operative HbA1c, also shown by others22, pre-operative use of less complex therapy, and high pre-operative serum insulin. Increased late remission was associated with the same measures and also with greater postoperative % EWL, which has been associated with T2D remission after RYGB23. % EWL, however, was not a strong enough variable in our weighting models and was not included in developing the DiaRem score. And neither was preoperative body weight. Preoperative BMI was recently proposed as an inappropriate selection criterion for offering RYGB surgery24 as a means for resolving diabetes even for patients with low BMI (25–35 Kg/m2).25 In agreement with this notion, our weighting system did not find preoperative BMI as a sufficiently strong predictor and therefore it was not included in the DiaRem Score.
Long duration of diabetes has been associated with decreased remission rates.22 Duration of diabetes was not available in our EMR. Age, however, was available and we found that older individuals had lower chances for diabetes remission. We hypothesize that older, severely obese, patients with diabetes may have had the disease for a longer period of time. This is likely to have negative effects on beta cell function and require complex pharmacotherapy including insulin, which as we show here, can significantly diminish remission rates. In the T2D+I group, increased early remission was also associated with younger age, lower pre-operative HbA1c and, for the first time in any group, with pre-operative use of incretin mimetic agents. We also replicated in the T2D+I group the association of a pre-operative diagnosis of hypertension with decreased early remission, as previously reported.14, 17
The DiaRem score was thus developed using four preoperative clinical variables and was divided into five groups corresponding to five probability-ranges for T2D remission. It should be noted that the DiaRem score predicts T2D remission irrespective of early or late occurrence and includes patients in “partial” remission who may be progressing to “complete” remission. In our cohort with T2D remission, 22% of patients had partial and 78% had complete remission, according to ADA-recommended criteria.21 The performance of the DiaRem score at 14 months was further evaluated in two replication cohorts using the definition of “partial” remission of diabetes. Overall, DiaRem scores from the three (i.e., primary and the two replication) cohorts followed similar trends suggesting that the model was faithfully replicated despite of differences in ethnicity, diet, pre- and post-operative management of diabetes, geography/climate, etc. In general, perfect replication probability within an explicit model is usually unattainable because of statistical uncertainty regarding the size of the initial observed effect.26
There is some discordance between centers in the use of the definition of diabetes remission depending on the chosen levels of HbA1c, fasting glucose, and the duration of remission.5, 27 We therefore re-derived Kaplan-Meier estimates using the “complete” definition of diabetes remission which trended similarly to our “partial + complete” model of diabetes remission but predicted lower probabilities. This was to be expected because the former model is more stringent in evaluating improved glycemic control. In addition, we recalculated the DiaRem score probabilities in the Arizona cohort at 14 months after surgery using five different definitions of “partial” diabetes remission, which this did not adversely affect the performance of the DiaRem algorithm. We favor our primary cohort model of “partial + complete” diabetes remission because it captures the transitory state from partial to complete remission of diabetes and reflects the overall improvement in glycemic control as a result of RYGB surgery.
Our study has some limitations such as the high mean BMI of the primary cohort and that the majority of patients (97%) were White Caucasian. Moreover, the DiaRem score was developed only for RYGB surgery. Separate DiaRem scores may need to be developed for other types of surgeries such as sleeve gastrectomy. The DiaRem score, however, offers for the first time a preoperative tool for predicting diabetes remission after RYGB surgery by using four readily obtainable clinical variables. For example, an individual with a BMI of 39 kg/m2 and a DiaRem score of 22 may benefit in terms of body weight loss but would have low probability of diabetes remission from RYGB surgery, and may thus opt to using intensive lifestyle changes or incretins prior to surgery, which as we show here may improve the odds of remission for individuals taking insulin. Further research is warranted to confirm the preoperative use of incretin mimetics in the improvement of glycemic control after RYGB surgery, as reported here. In conclusion, the DiaRem score is a tool for accurately predicting preoperatively the utility of RYGB surgery in remitting diabetes.
Supplementary Material
Acknowledgments
We would like to thank the thousands of participating RYGB surgery patients at Geisinger Health System. This research was supported by research funds from the Geisinger Clinic and the National Institutes of Health grants DK072488 (GSG, CDS, GA) and DK088231 (GSG) and DK091601 (GSG).
Footnotes
Conflicts of interests
The authors have no conflicts of interest to declare.
Author contributions
CDS admitted all the patients to the weight loss bariatric program at Geisinger Clinic. CGW performed all the analytical aspects of the study including the design of the DiaRem score algorithm. WES, ATP, JG, and AI performed all RYGB surgeries. GSG, PB, JS, and BI helped with the patient participation, the design of the study, and the manuscript preparation. MPC and RB provided one of the replication cohorts (Scottsdale, Arizona) and analytical support. GA conceived and designed the study, participated in patient selection, and wrote the manuscript with help from all the coauthors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes care. 2004;27(5):1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Gill RS, Sharma AM, Al-Adra DP, Birch DW, Karmali S. The impact of bariatric surgery in patients with type-2 diabetes mellitus. Current diabetes reviews. 2001;7(3):185–9. doi: 10.2174/157339911795843087. [DOI] [PubMed] [Google Scholar]
- 3.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–85. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 4.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackstone R, Bunt JC, Cortes MC, Sugerman HJ. Type 2 diabetes after gastric bypass: remission in five models using HbA1c, fasting blood glucose, and medication status. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2012;8(5):548–55. doi: 10.1016/j.soard.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Pournaras DJ, Osborne A, Hawkins SC, et al. Remission of type 2 diabetes after gastric bypass and banding: mechanisms and 2 year outcomes. Annals of surgery. 2010;252(6):966–71. doi: 10.1097/SLA.0b013e3181efc49a. [DOI] [PubMed] [Google Scholar]
- 7.Varela JE. Bariatric surgery: a cure for diabetes? Curr Opin Clin Nutr Metab Care. 2011;14 (4):396–401. doi: 10.1097/MCO.0b013e3283468e50. [DOI] [PubMed] [Google Scholar]
- 8.Coffin S, Konduru C, Schwarcz M, Frishman W. Surgical approaches for the prevention and treatment of type 2 diabetes mellitus. Cardiol Rev. 2009;17(6):275–9. doi: 10.1097/CRD.0b013e3181bc23d1. [DOI] [PubMed] [Google Scholar]
- 9.Lee WJ, Hur KY, Lakadawala M, Kasama K, Wong SK, Lee YC. Gastrointestinal metabolic surgery for the treatment of diabetic patients: a multi-institutional international study. J Gastrointest Surg. 2012;16(1):45–51. doi: 10.1007/s11605-011-1740-2. discussion -2. [DOI] [PubMed] [Google Scholar]
- 10.Huang CK, Shabbir A, Lo CH, Tai CM, Chen YS, Houng JY. Laparoscopic Roux-en-Y gastric bypass for the treatment of type II diabetes mellitus in Chinese patients with body mass index of 25–35. Obesity surgery. 2011;21(9):1344–9. doi: 10.1007/s11695-011-0408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chikunguwo SM, Wolfe LG, Dodson P, et al. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2010;6(3):254–9. doi: 10.1016/j.soard.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Hamza N, Abbas MH, Darwish A, Shafeek Z, New J, Ammori BJ. Predictors of remission of type 2 diabetes mellitus after laparoscopic gastric banding and bypass. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2011;7(6):691–6. doi: 10.1016/j.soard.2010.03.292. [DOI] [PubMed] [Google Scholar]
- 13.Deitel M. Update: Why diabetes does not resolve in some patients after bariatric surgery. Obesity surgery. 2011;21(6):794–6. doi: 10.1007/s11695-010-0329-2. [DOI] [PubMed] [Google Scholar]
- 14.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Annals of surgery. 2003;238(4):467–84. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 84–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon JB, Chuang LM, Chong K, et al. Predicting the Glycemic Response to Gastric Bypass Surgery in Patients With Type 2 Diabetes. Diabetes care. 2012 doi: 10.2337/dc12-0779. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall TC, Pellen MG, Sedman PC, Jain PK. Preoperative factors predicting remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery for obesity. Obesity surgery. 2010;20(9):1245–50. doi: 10.1007/s11695-010-0198-8. [DOI] [PubMed] [Google Scholar]
- 17.Hayes MT, Hunt LA, Foo J, Tychinskaya Y, Stubbs RS. A model for predicting the resolution of type 2 diabetes in severely obese subjects following Roux-en Y gastric bypass surgery. Obesity surgery. 2011;21(7):910–6. doi: 10.1007/s11695-011-0370-9. [DOI] [PubMed] [Google Scholar]
- 18.Wood GC, Chu X, Manney C, et al. An electronic health record-enabled obesity database. BMC medical informatics and decision making. 2012;12:45. doi: 10.1186/1472-6947-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerhard GS, Styer AM, Wood GC, et al. A Role for Fibroblast Growth Factor 19 and Bile Acids in Diabetes Remission After Roux-en-Y Gastric Bypass. Diabetes care. 2013;36(7):1859–64. doi: 10.2337/dc12-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes care. 2012;35 (Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes care. 2009;32(11):2133–5. doi: 10.2337/dc09-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arterburn DE, Bogart A, Sherwood NE, et al. A Multisite Study of Long-term Remission and Relapse of Type 2 Diabetes Mellitus Following Gastric Bypass. Obesity surgery. 2013;23 (1):93–102. doi: 10.1007/s11695-012-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadera BE, Lum K, Grant J, Pryor AD, Portenier DD, DeMaria EJ. Remission of type 2 diabetes after Roux-en-Y gastric bypass is associated with greater weight loss. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2009;5(3):305–9. doi: 10.1016/j.soard.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Livingston EH. Pitfalls in using BMI as a selection criterion for bariatric surgery. Current opinion in endocrinology, diabetes, and obesity. 2012;19(5):347–51. doi: 10.1097/MED.0b013e328357f0b8. [DOI] [PubMed] [Google Scholar]
- 25.Lanzarini E, Csendes A, Gutierrez L, et al. Type 2 Diabetes Mellitus in Patients with Mild Obesity: Preliminary Results of Surgical Treatment. Obesity surgery. 2012 doi: 10.1007/s11695-012-0780-3. [DOI] [PubMed] [Google Scholar]
- 26.Miller J, Schwarz W. Aggregate and individual replication probability within an explicit model of the research process. Psychological methods. 2011;16(3):337–60. doi: 10.1037/a0023347. [DOI] [PubMed] [Google Scholar]
- 27.Pournaras DJ, Aasheim ET, Sovik TT, et al. Effect of the definition of type II diabetes remission in the evaluation of bariatric surgery for metabolic disorders. The British journal of surgery. 2012;99(1):100–3. doi: 10.1002/bjs.7704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.