Abstract
Serotonin is critical for shaping the development of neural circuits regulating emotion. Pet-1 (FEV-1) is an ETS-domain transcription factor essential for differentiation and forebrain targeting of serotonin neurons. Constitutive Pet-1 knockout (KO) causes major loss of serotonin neurons and forebrain serotonin availability, and behavioral abnormalities. We phenotyped Pet-1 KO mice for fear conditioning and extinction, and on a battery of assays for anxiety- and depression-related behaviors. Morphology of Golgi-stained neurons in basolateral amygdala (BLA) and prelimbic cortex was examined. Using human imaging genetics, a common variant (rs860573) in the PET-1 (FEV) gene was tested for effects on threat-related amygdala reactivity and psychopathology in 88 Asian-ancestry subjects. Pet-1 KO mice exhibited increased acquisition and expression of fear, and elevated fear recovery following extinction, relative to wild-type (WT). BLA dendrites of Pet-1 KO mice were significantly longer than in WT. Human PET-1 variation associated with differences in amygdala threat processing and psychopathology. This novel evidence for the role of Pet-1 on fear processing and dendritic organization of amygdala neurons and on human amygdala threat processing extends a growing literature demonstrating the influence of genetic variation in the serotonin system on emotional regulation via effects on structure and function of underlying corticolimbic circuitry.
Keywords: asolateral amygdala, medial prefrontal cortex, fear conditioning, serotonin, FEV
INTRODUCTION
The serotonin (5-hydroxytryptamine) neurotransmitter system plays a key role in regulating emotion, and genetic variations in the serotonin system influence individual differences in emotion and risk for emotional disorders (Holmes, 2008). Genetically-driven variation in regulators of serotonin signaling (e.g., tryptophan hydroxylase-2, Tph2; the serotonin transporter, 5-HTT) are associated with higher levels of anxiety-like behavior (Zhang et al., 2004) and deficient fear extinction (Wellman et al., 2007), as well as increased dendritic arborization in ventromedial prefrontal cortex (vmPFC) and higher spine density in basolateral amygdala (BLA) neurons (Nietzer et al., 2011; Wellman et al., 2007). In humans, a polymorphism in the 5-HTT promoter region is associated with functional uncoupling of prefrontal cortex (PFC) and amygdala and amygdala hyperactivity in response to threat (Hariri et al., 2002; Pezawas et al., 2005). Individuals with this polymorphism exhibit impaired fear extinction (Hartley et al., in revision) and are at increased risk for depression after a history of stressful life events (Caspi et al., 2010).
Given serotonin’s role in brain development (Gaspar et al., 2003), these effects may be driven by malformation of corticolimbic circuits mediating anxiety and fear (Ansorge et al., 2007; Esaki et al., 2005; Holmes et al., 2003). In this context, a major candidate for serotonergic influences on brain development is the ETS domain transcription factor Pet-1 (Pheochromocytoma 12 ets) aka FEV (Deneris, 2011). Pet-1 plays a critical role in differentiation and forebrain targeting of serotonin neurons, and expression of regulatory serotonin receptors on these neurons (Hendricks et al., 2003; Liu et al., 2010). Pet-1 knockout (KO) dramatically reduces the number of serotonin-immunoreactive neurons from embryonic development onwards, resulting in an ~80% reduction of serotonin in forebrain target regions (Deneris, 2011; Hendricks et al., 2003).
Increased anxiety-like behavior has been reported in mice with either constitutive Pet-1 KO (Hendricks et al., 2003) or Pet-1 KO restricted to adulthood (Kiyasova et al., 2011; Liu et al., 2010; Schaefer et al., 2009). Intriguingly, a preliminary report found that Pet-1 KO had enhanced conditioned fear behavior (Kiyasova et al., 2011). Serotonergic effects on fear extinction are of particular clinical relevance because deficits in fear extinction characterize anxiety disorders such as posttraumatic stress disorder (PTSD) (Milad et al., 2009). Indeed, disruption of serotonin genes produces morphological abnormalities in brain regions mediating fear extinction, notably the BLA (Herry et al., 2010) and vmPFC (Burgos-Robles et al., 2009; Graybeal et al., 2011; Wilber et al., 2011). However, the critical question of how lifelong loss of serotonin affects extinction of learned fear behavior remains unanswered.
Given the key role for the serotonergic systems in regulating emotional behavior, here we assessed the consequences of Pet-1 deletion for fear extinction as well as anxiety-like behaviors and stress responses. Further, emotional disorders are highly comorbid with alcohol abuse and the serotonin system modulates EtOH’s effects on behavior. For example, disruption of serotonin signaling, via 5-HTT KO, leads to exaggerated sensitivity to acute intoxicating effects of EtOH (Boyce-Rustay et al., 2006; Daws et al., 2006). Therefore, we also examined responses on an EtOH test battery. In addition, in a separate cohort of behaviorally naïve mice, we examined potential neural mechanisms at the level of dendritic arborization in BLA and vmPFC. We hypothesized that mice with genetic inactivation of Pet-1 would show alterations in emotional behavior and corticolimbic dendritic morphology relative to wild-type mice. We then interrogated the potential translational impact of our preclinical analyses by conducting a human neuroimaging genetics study of the association between a common PET-1 (aka FEV) single nucleotide polymorphism (rs860573) and threat-related amygdala reactivity, a human intermediate neural phenotype that reliably varies as a function of polymorphisms in serotonergic genes (Hariri and Holmes, 2006). The use of non-human animal models allows for explicit manipulation of Pet-1, while the extension in humans interrogating genetic variation within the PET-1 gene (FEV) allows for preliminary translational evidence for the importance of PET-1 in the emergence of individual differences in clinically relevant brain function and the related risk for psychopathology (Hariri, 2010). Thus, we hypothesized that genetic variation within the PET-1 gene (FEV) would associate with differences in amygdala reactivity; and these differences would parallel differences in emotional and fear behaviors in the Pet-1 knockout mice.
MATERIAL AND METHODS
Pet-1 KO
Subjects
Pet-1 null mutant mice were generated as previously described (Hendricks et al., 2003) and repeatedly backcrossed into the C57BL/6J strain for 10 generations. Wild-type (WT), heterozygous (HET), and KO mice were littermates generated from HET x HET matings (Lerch-Haner et al., 2008; Millstein et al., 2006). Mice were bred and maintained at The Jackson Laboratory (Bar Harbor, ME) and shipped to NIH at 7–9 weeks of age, or bred and maintained at NIH. Testing began when mice were ≥10 weeks old. Mice were group-housed with same-sex littermates in a temperature and humidity controlled vivarium under a 12 h light/dark cycle (lights on 0600 h). Approximately equal numbers of males and females of each genotype were used, with n=22–24 per genotype for behavioral phenotyping and n=10–11 mice per genotype for dendritic analyses. All experimental procedures were approved by the NIAAA Animal Care and Use Committee and followed the NIH guidelines ‘Using Animals in Intramural Research.’
Behavioral Phenotyping
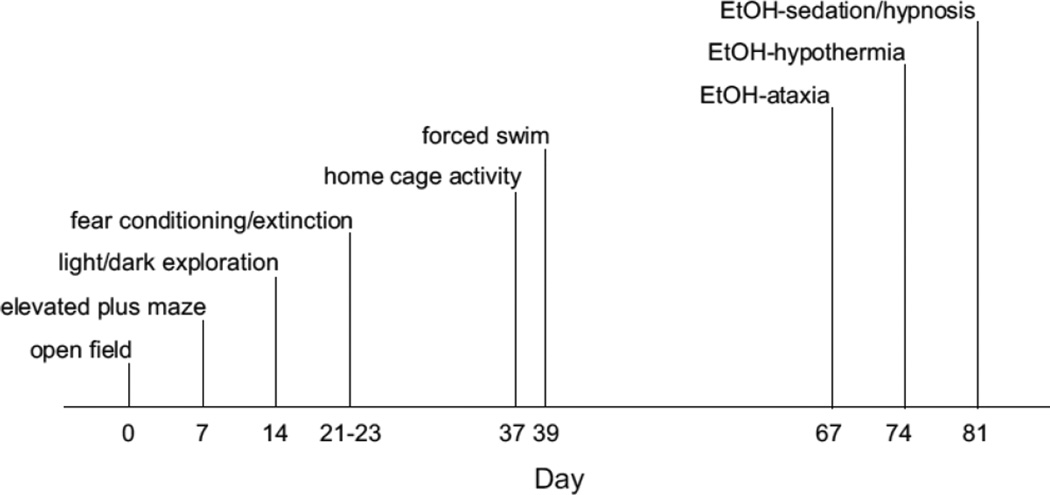
Testing was conducted with the putatively more stressful tests later in the sequence (order of testing: novel open field test, elevated plus-maze, light/dark exploration test, Pavlovian fear conditioning and extinction, home cage activity, and forced swim test). Seven days elapsed between tests, except for the intervals between Pavlovian fear conditioning and home cage activity (14 days) and between home cage activity and forced swim test (2 days). There was then an interval of 4 weeks before commencing the EtOH test battery. See Fig 1 for a summary of the time line of behavioral testing procedures. Except for home cage activity, mice were first acclimated to the test room for 1 hr. The experimenter remained blind to genotype during testing.
FIGURE 1. Time line of behavioral testing in WT and Pet-1 KO mice.
Testing was conducted with the putatively more stressful tests later in the sequence. Seven days elapsed between tests, except for the intervals between Pavlovian fear conditioning and home cage activity (14 days) and between home cage activity and forced swim test (2 days). There was then an interval of 4 weeks before commencing the EtOH test battery.
Pavlovian fear conditioning and extinction
Fear conditioning and extinction was assessed as previously described (Whittle et al., 2010; Yang et al., 2008). Mice were placed in a 27 × 27 × 11 cm chamber with transparent walls and a metal rod floor. To provide a distinctive olfactory environment, the chamber was cleaned between subjects with a 79% EtOH/20% water/1% vanilla extract solution. After a 180 sec acclimation period, mice received 3 pairings (60–120 sec interval after each pairing) of a tone (30 sec, 80 dB, white noise) and footshock (2 sec, 0.6 mA scrambled footshock), with the shock occurring during the last 2 sec of the tone. Presentation of stimuli was controlled by the Med Associates Freeze Monitor system (Med Associates Incorporated, Georgia, VT). Twenty-four h later, expression of fear to the tone and subsequent within-session extinction was tested in a novel context (Plexiglas cylinder with black/whitecheckered walls and a solid floor, cleaned with a 1% acetic acid/99% water solution) housed in a novel room. Following a 180 s acclimation period, mice received 50 × 30-s presentations of the tone alone (5 s no-stimulus interval). Twenty-four h later, extinction retrieval was probed with 3 tone presentations. Freezing (no visible movement except that required for respiration) to the tone was manually scored every 5 s and converted to a percentage ([number of freezing observations/total number of observations] × 100). Freezing during extinction trials was averaged into 10 × 5-trial blocks for analysis.
Anxiety-related behavior and stress responsivity
Anxiety-related behaviors were assessed using the novel open field, elevated plus maze, and light/dark exploration tests. Mice were tested for behavioral responses to stress using the forced swim test (Porsolt et al., 1978). Thirty min after the final forced swim trial, blood was collected for corticosterone assays. See Supplemental Materials for full details.
Home cage locomotor activity
Locomotor activity was assessed in mice individually-housed in a standard home cage under normal vivarium conditions and left undisturbed for a 48 hr acclimation period (Karlsson et al., 2008). Activity was then measured during the light and dark phases over 24 hr using the photocell-based Opto M3 activity monitor (Columbus Instruments, Columbus, OH).
Behavioral responses to EtOH
Emotional disorders are highly comorbid with alcohol abuse and the serotonin system modulates EtOH’s effects on behavior. For example, disruption of serotonin signaling, via 5-HTT KO, leads to exaggerated sensitivity to acute intoxicating effects of EtOH (Boyce-Rustay et al., 2006; Daws et al., 2006). Therefore we tested mice on measures of acute intoxication (ataxia, hypothermia, sedation/hypnosis in that order, each separated by a week). See Supplemental Materials for full details.
Dendritic morphology
In a separate set of behaviorally naïve mice, dendritic morphology of neurons in BLA and PL was assessed using a modification of Glaser and Van der Loos’ (1981) Golgi stain as described previously (e.g., Mozhui et al., 2010).
Neurons were reconstructed in 180 µm coronal sections. Analysis of BLA neurons was restricted to 0.8–2.0 mm posterior to bregma, where BLA is readily identified in Golgi-stained material. Pyramidal neurons were defined by the presence of a distinct, single apical dendritic tree, ≥2 basilar dendritic trees extending from the base of the soma, and dendritic spines.
Pyramidal neurons in layer II-III of the prelimbic region (PL) of vmPFC were drawn, as this region plays a role in the expression of learned fear during extinction (Burgos-Robles et al., 2009), and stimulation of PL impairs extinction (Vidal-Gonzalez et al., 2006). PL and layer II-III were identified based on position and cytoarchitecture as previously described (e.g., Mozhui et al., 2010). Pyramidal neurons in PL had ≥2 basilar dendritic trees with ≥third-order branches, a distinct, single apical dendritic tree extending towards the pial surface, and dendritic spines.
Neurons selected for reconstruction did not have truncated branches and were not obscured by neighboring neurons and glia, with dendrites that were easily discriminable by focusing through the depth of the tissue. In 4–6 sections evenly spaced through the rostral-caudal extent of each region, all pyramidal neurons meeting these criteria were identified. Eight neurons per mouse per region (4 from each hemisphere) were randomly selected from all identified neurons and reconstructed (final magnification, 600×). Morphology was quantified using a computerbased neuron tracing system (Neurolucida, MBF Bioscience, Williston, VT) with the experimenter blind to genotype. Total length and number of dendrites were measured. Amount and location of dendritic material was assessed using a Sholl analysis (Larkman, 1991) in which the number of intersections of dendrites with 10-µm concentric spheres centered on the soma was measured. For statistical and graphical purposes, counts of intersections were summed over pairs of radii.
Statistics
The effects of genotype x trial-block on freezing during extinction, genotype x trial on forced swim test immobility, genotype x time for open field and home cage activity, and genotype x distance from soma, were all analyzed using analysis of variance (ANOVA)with repeated measures for trial-block, trial, time point, and distance, respectively. For all other behavioral analyses, the effect of genotype was analyzed using one-way ANOVA, followed by Newman-Keuls post hoc tests where appropriate. For dendritic analyses, the effect of genotype was assessed using one-way ANOVA. For Sholl analyses, the effects of genotype x distance from soma were assessed using ANOVA with repeated measures. Statistical significance was set at p <.05.
Human PET-1 imaging genetics
Participants
Genetic and neuroimaging data were available from 375 participants who completed the Duke Neurogenetics Study (Nikolova and Hariri, 2012b), an ongoing protocol assessing a range of behavioral, experiential, and biological phenotypes among young adult volunteers. All participants provided written informed consent in accordance with Duke University guidelines and were in good general health. For completing the study, each participant received $120 remuneration. Study exclusion criteria included: 1) medical diagnoses of cancer, stroke, diabetes requiring insulin treatment, chronic kidney or liver disease, or lifetime history of psychotic symptoms; 2) use of psychotropic, glucocorticoid, or hypolipidemic medication; and/or 3) conditions affecting cerebral blood flow and metabolism (e.g., hypertension).
The Duke Neurogenetics Study seeks to measure variability in behavior and neurobiology across a broad spectrum recognizing the dimensional nature of these phenotypes. Accordingly, we thoroughly assess psychopathology following categorical nosology but do not exclude for it (with the exception of schizophrenia spectrum disorders). Diagnosis of current DSM-IV Axis I and select Axis II disorders (Antisocial Personality Disorder and Borderline Personality Disorder) was assessed with the electronic Mini International Neuropsychiatric Interview (eMINI; Sheehan et al., 1998) and Structured Clinical Interview for the DSM-IV (SCID; First et al., 1997). These disorders were not exclusionary (with the exception of schizophrenia spectrum disorders), as the DNS seeks to establish broad variability in multiple behavioral phenotypes related to psychopathology.
The final full sample consisted of 334 participants with amygdala reactivity data following quality control procedures. Specifically, data from 41 participants were excluded from analyses for the following reasons: incidental structural brain abnormalities (n = 2), significant movement outliers in fMRI data (n = 9; see preprocessing description below), inadequate signal in regions of interest (n = 7; see preprocessing description), technical difficulties during fMRI data collection or artifacts in data (e.g., coil problems; n = 3), or poor behavioral performance (i.e., less than 75% accuracy: n = 20).
We examined genotypes for rs860573, an A→G synonymous single nucleotide polymorphism (SNP) located in exon 3 of the PET-1 gene (FEV; Chromosome 2: 219,554,053-219,558,623; NCBI B36 assembly). This is the only FEV SNP available to us from our genome-wide assessment and the only variant tested in our current study. Because the minor allele frequency of our target locus (rs860573) is rare in many ethnic populations, the current analyses were restricted to 90 participants of self-identified Asian ancestry where the minor allele frequency (MAF) was 17.24% to permit adequate sample sizes across possible genotype groups, and to protect against confounding effects due population stratification. Genotyping at this locus failed for one Asian participant resulting in a final sample of 89 in the present study (Table 1 presents demographic and diagnostic data).
Table 1.
Demographic and psychopathology variables by rs860573 genotype.
| GG (n=60) | A Carriers (n=29) | Genotype effect | Total Sample (n=89) | |
|---|---|---|---|---|
| Gender (female n) | 34 | 16 | χ2 = 0018, p = 0.89 | 50 |
| Age | 19.55 ±1.35 | 19.66 ±1.45 | t(87) = 0.34, p = 0.74 | 19.60 ± 1.39 |
| Disorder present (n)a | 3 | 8 | Fisher’s exact: p = 0.005 | 11 |
| MASQ AA | 20.66 ± 5.40 | 22.95 ± 6.99 | t(87) = 1.70, p = 0.09 | 21.37 ± 6.00 |
| MASQ AD | 53.38 ± 11.27 | 52.97 ± 14.76 | t(87) = 0.15, p = 0.88 | 53.00 ± 12.58 |
| MASQ GDA | 16.67 ± 5.60 | 19.68 ± 6.50 | t(87) = 2.26, p < 0.03 | 17.64 ± 6.01 |
| MASQ GDD | 19.81 ± 7.78 | 23.13 ± 10.04 | t(87) = 1.71, p = 0.09 | 20.80 ± 8.66 |
| STAI | 38.30 ± 8.52 | 41.45 ± 10.75 | t(87) = 1.50, p = 0.14 | 39.24 ± 9.34 |
MASQ, Mood and Anxiety Symptom Questionnaire; AA, anxious arousal; AD, anhedonic depression; GDA, general distress-anxiety; GDD, general distress-depression
GG: marijuana abuse (n = 1); alcohol abuse (n = 2)
AG: generalized anxiety disorder (n = 2); alcohol dependence (n = 2); alcohol abuse (n = 1); alcohol dependence and comorbid marijuana abuse (n = 1); marijuana dependence and comorbid alcohol abuse (n = 1); borderline personality disorder and comorbid bipolar II, alcohol abuse, and marijuana abuse (n = 1)
Genotyping
Genotyping was conducted by 23andMe. Genomic DNA from all participants was isolated from buccal cells derived from Oragene DNA self-collection kits (DNA Genotek, Inc., Kanata, Ontario, Canada) customized for 23andMe. DNA extraction and genotyping were performed by the National Genetics Institute (NGI), a CLIA-certified clinical laboratory and subsidiary of Laboratory Corporation of America. The Illumina Omni Express chip and a custom array containing an additional ~300,000 SNPs were used to provide genome-wide data (Do et al., 2011; Eriksson et al., 2010; Tung et al., 2011). The other ethnic groups that compose the DNS had either too low MAF or too small sample size to be included in analyses (African American n=32, MAF 15.63%; European American n=166, MAF = 0.60%; Latino n=22, MAF 2.27%; Other n=23, MAF = 15.22%). Genotype frequencies for rs860573 did not deviate from Hardy-Weinberg Equilibrium in the Asian only sample (χ2=1.21, p=0.27). Because only one participant was a minor allele homozygote, participants were grouped as minor A allele carriers.
Self-report Measures
As part of a behavioral self-report battery, participants completed the Mood and Anxiety Symptoms Questionnaire (MASQ), which provides subscale measures specific for current depressive and anxiety symptoms (i.e., anhedonic depression and anxious arousal) as well as shared across depressive and anxiety symptoms (i.e., general distress anxiety and general distress depression) (Watson et al., 1995). Participants also completed the trait version of State Trait Anxiety Inventory (STAI-T), a widely used measure capturing both negative affect and anxiety (Spielberger et al., 1983).
Amygdala Reactivity Paradigm
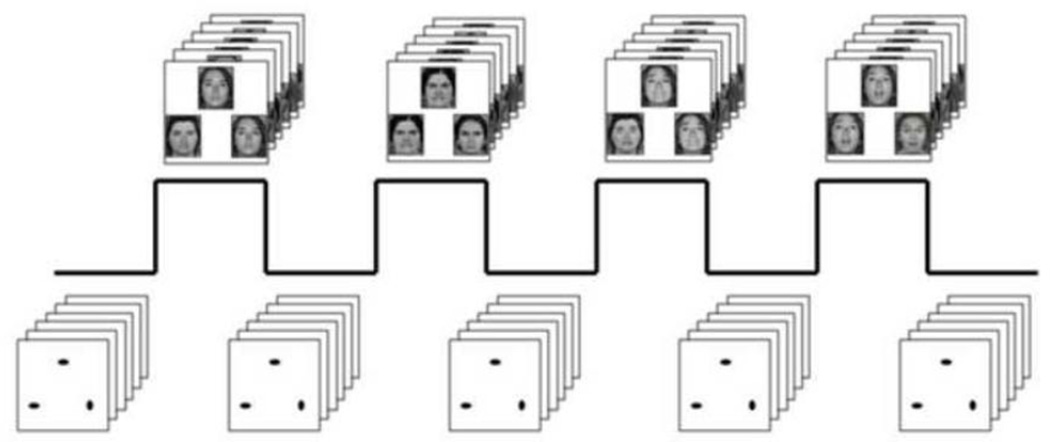
The experimental fMRI paradigm consists of 4 blocks of a face-processing task interleaved with 5 blocks of a sensorimotor control task (Brown et al., 2005; Hariri et al., 2009; see Fig. 2). Participant performance (accuracy and reaction time) is monitored during all scans using an MR-compatible button box. During task blocks, participants view a trio of faces and select one of two faces (bottom) identical to a target face (top). In the DNS version of the task, there are four task blocks with neutral, angry, fearful, or surprised facial expressions derived from a standard set of pictures of facial affect (Ekman and Friesen, 1976). The order of the expression-specific task blocks is counter-balanced across participants. During the sensorimotor control blocks, participants perform the same target-matching task with simple geometric shapes (circles and ellipses). Each sensorimotor control block consists of six different shape trios. All blocks are preceded by a brief instruction (“Match faces” or “Match shapes”) that lasts 2 seconds. In the task blocks, each of six face trios (three all male faces and three all female faces) is presented for 4 seconds with a variable interstimulus interval (ISI) of 2 to 6 seconds (mean, 4 seconds), for a total block length of 48 seconds. A variable ISI is used to minimize expectancy effects and resulting habituation, and maximize amygdala reactivity throughout the paradigm. In the control blocks, each of the six shape trios is presented for 4 seconds with a fixed interstimulus interval of 2 seconds, for a total block length of 36 seconds. Total task length is 390 seconds. Bilateral threat-related basolateral and centromedial amygdala reactivity to blocks containing angry and fearful facial expressions were extracted using the general linear model of SPM8 (p<.05, FWE corrected) across all participants.
FIGURE 2. Amygdala reactivity paradigm.
Participants match faces or geometric shapes. Face matching blocks contain neutral, angry, fearful or surprised expressions with block order counterbalanced across participants.
BOLD fMRI data acquisition
Participants were scanned using a research-dedicated GE MR750 3T scanner equipped with high-power high-duty-cycle 50-mT/m gradients at 200 T/m/s slew rate, and an eight-channel head coil for parallel imaging at high bandwidth up to 1MHz at the Duke-UNC Brain Imaging and Analysis Center. A semi-automated high-order shimming program was used to ensure global field homogeneity. A series of 34 interleaved axial functional slices aligned with the anterior commissure-posterior commissure (AC-PC) plane were acquired for full-brain coverage using an inverse-spiral pulse sequence to reduce susceptibility artifact (TR/TE/flip angle=2000 ms/30 ms/60; FOV=240 mm; 3.75×3.75×4 mm voxels; interslice skip=0). Four initial RF excitations were performed (and discarded) to achieve steady-state equilibrium. To allow for spatial registration of each participant’s data to a standard coordinate system, high-resolution three-dimensional structural images were acquired in 34 axial slices co-planar with the functional scans (TR/TE/flip angle=7.7 s/3.0 ms/12; voxel size=0.9×0.9×4 mm; FOV=240 mm, interslice skip=0).
BOLD fMRI data analysis
The general linear model of SPM8 (http://www.fil.ion.ucl.ac.uk/spm) was used for whole-brain image analysis. Individual subject data were realigned to the first volume in the time series to correct for head motion before being spatially normalized into the standard stereotactic space of the Montreal Neurological Institute template using a 12-parameter affine model. Data were then smoothed to minimize noise and residual differences in individual anatomy with a 6mm FWHM Gaussian filter. Next, the ARtifact detection Tool (ART) was used to generate regressors accounting for the possible confounding effects of volumes with large motion deflections (i.e., >0.6mm relative to the previous time frame) or spiking artifacts (i.e., global mean intensity 2.5 standard deviations from the entire time series). Nine participants, who had more than 5% of their acquisition volumes flagged by ART, were dropped from analyses. Because of the signal loss and noise often observed in amygdala and adjacent regions, single-subject BOLD fMRI data were included in subsequent analyses only if there was a minimum of 90% signal coverage in amygdala [using a bilateral ROI created from the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) using the Wake Forest University PickAtlas toolbox in SPM8]. Seven participants were excluded due to ≤90% coverage.
Following preprocessing, linear contrasts employing canonical hemodynamic response functions were used to estimate task-specific (i.e., Angry and Fearful Faces > Shapes) BOLD responses for each individual. Individual contrast images (i.e., weighted sum of the beta images) were then used in second-level random effects models accounting for scan-to-scan and participant-to-participant variability to determine mean task-specific regional responses using one-sample t-tests. A voxel-level statistical threshold of p<.05, FWE corrected for multiple comparisons across the amygdala regions of interest, and a cluster-level extent threshold of 10 contiguous voxels was applied to this analysis. Bilateral centromedial and basolateral amygdala regions of interest (ROIs) were defined using anatomical probability maps (Amunts et al., 2005). BOLD parameter estimates from clusters within right and left centromedial and basolateral amygdala ROIs exhibiting main effects of task were extracted using the VOI tool in SPM8 and exported for regression analyses in SPSS (v.18). Extracting parameter estimates from maximal clusters activated by our fMRI paradigm, rather than clusters specifically correlated with our independent variables of interest, is a more conservative analytic strategy that precludes the possibility of any correlation coefficient inflation that may result when an explanatory covariate is used to select a region of interest (Bogdan et al., 2012; Nikolova and Hariri, 2012a).
Statistical Analyses
To evaluate genotype effects on amygdala reactivity, linear regression analyses were conducted within SPSS (v18) to test the association between rs860573 genotype (i.e., G homozygotes, A allele carriers) and extracted threat-related amygdala reactivity (i.e., angry and fear > shapes contrast) using gender and the presence of a psychiatric disorder as covariates. For analyses of disorder status, as well as depressive and anxiety symptoms, independent samples t-tests or chi-square tests were used. For post-hoc exploratory mediational analyses we used the Process SPSS expansion (Hayes, 2013).
RESULTS
Pet-1 KO show increased acquisition, expression and post-extinction recovery of fear
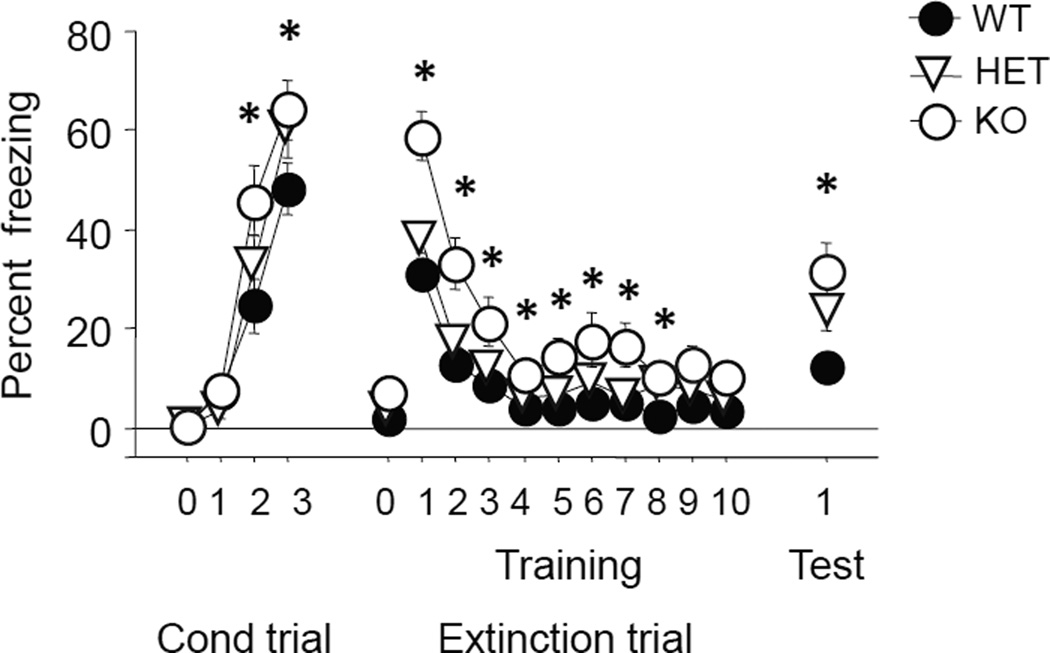
There were significant effects of genotype (F2,67=3.31, p<.05) and trial (F3,201=135.51, p<.01) and a borderline genotype x trial interaction (F6,201=2.10, p=.0546) for freezing during conditioning. Post hoc tests showed that freezing was not different between genotypes at baseline or during the first tone exposure prior to pairing with the first shock, but was significantly higher in Pet-1 KO mice than WT controls during the second and third tone exposures (Fig 3). There was a genotype x trial interaction for freezing during fear retrieval and extinction testing (F20,670=3.18, p<.01). Post hoc tests showed that freezing was significantly higher in Pet-1 KO mice than WT controls on all extinction trial-blocks except the last two (Fig 3). Freezing was again higher in Pet-1 KO mice than WT controls during extinction retrieval (main effect of genotype: F2,67=4.86, p<.05, followed by post hoc tests) (Fig 3).
FIGURE 3. Increased acquisition and maintenance of fear in Pet-1 KO mice.
Pet-1 KO mice and WT controls did not differ at baseline (trial 0), but Pet-1 KO mice showed higher freezing than WT controls on the second and third tone exposures during conditioning. Freezing was higher in Pet-1 KO mice than WT controls during initial fear retrieval and all extinction trial-blocks except the last two. During extinction retrieval, freezing was again higher in Pet-1 KO mice than WT controls. n=22–24 per genotype. Data are Means ±SEM. *p<05 vs. WT
Pet-1 KO show dendritic hypertrophy in BLA, but normal dendritic arborization in PL
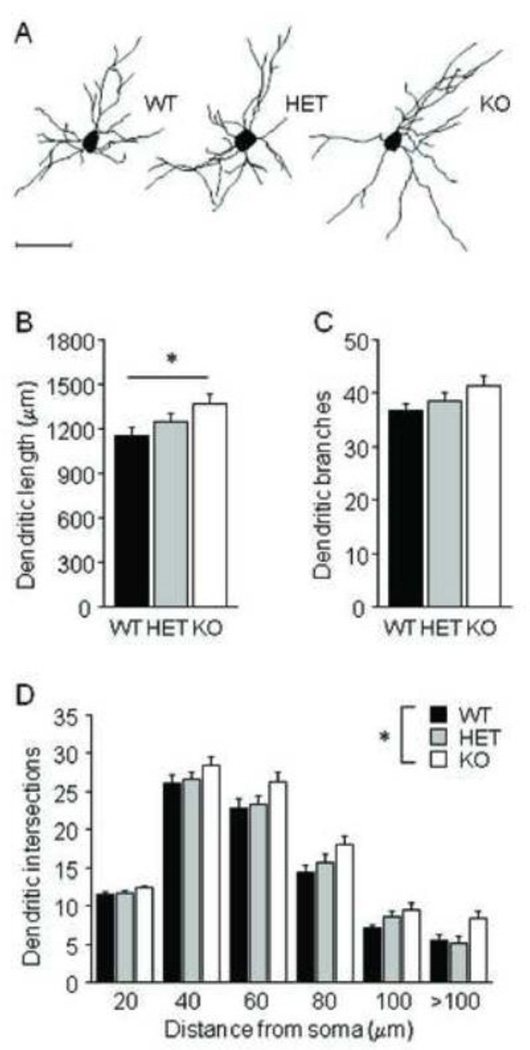
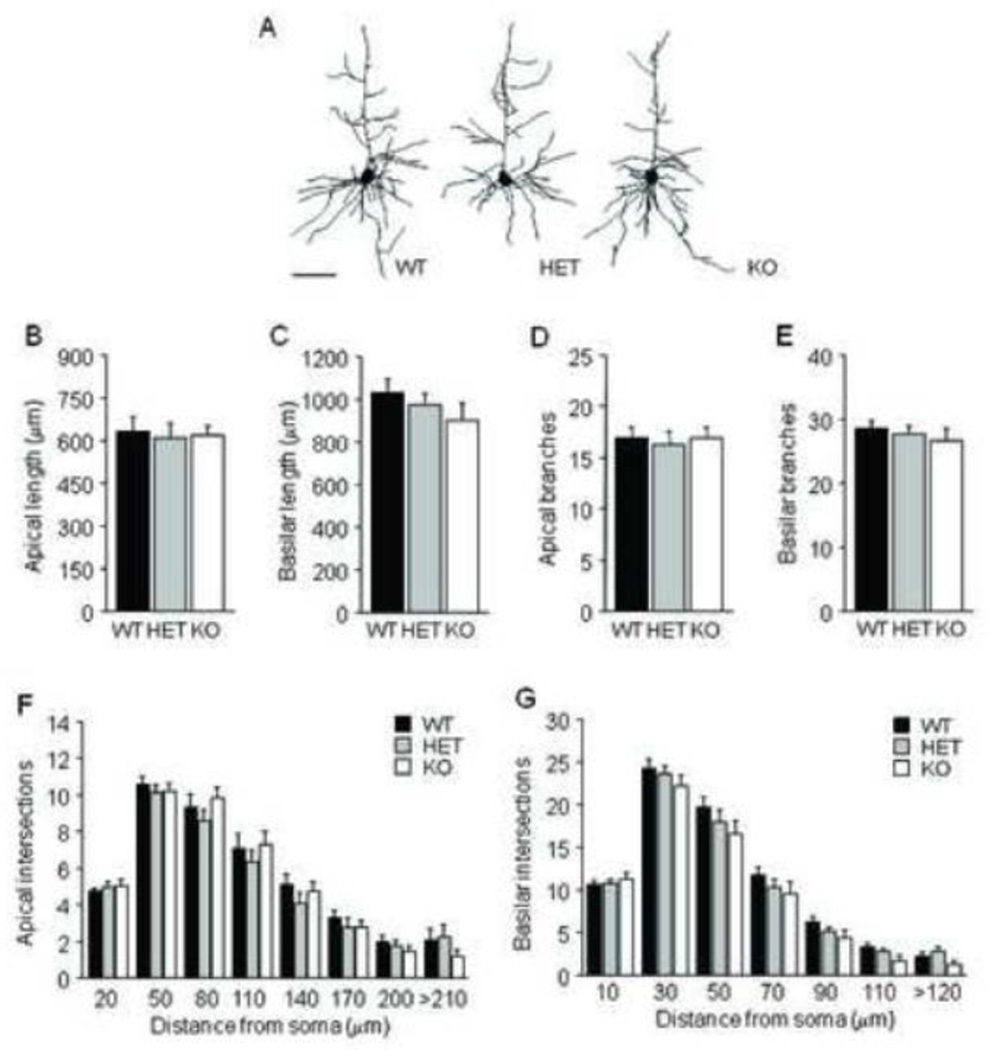
For BLA neurons, there was a significant effect of genotype on average dendritic length (F2,28=3.69, p<.05) (Fig 4B), but not on number of branches (Fig 4C). Post hoc tests showed that Pet-1 KO mice had longer dendritic arbors than WT controls. Sholl analysis of dendritic intersections found a significant effect of genotype (F2,28=4.28, p<.05) and distance from soma (F10,140=459.52, p<.01), but no interaction (Fig 4D). Simple main effects analysis showed that Pet-1 KO mice had more intersections than WT controls (F1,19=8.06, p<.05).
FIGURE 4. Dendritic hypertrophy in BLA of Pet-1 KO mice.
(A) Examples of BLA neuronal reconstructions. (B) Average dendritic length of BLA neurons was greater in Pet-1 KO mice as compared to WT controls (n=10–11 mice per genotype, n=8 neurons per mouse). (C) Genotypes did not differ in the number of BLA dendritic branches. (D) Increases in dendritic material were distributed uniformly throughout the dendritic arbor. Scale bar=50 µm. Data are Means ±SEM. *p <.05 vs. KO
In PL, there was no effect of genotype on average apical or basilar dendritic length or branch number (Fig 5B–E). Sholl analysis found a significant effect of distance from soma but not genotype (and no interaction) for apical (F7,196=187.80, p<.01) and basilar (F6,168=551.16, p<.01) intersections (Fig 5F–G).
FIGURE 5. Normal dendritic arborization in PL of Pet-1 KO mice.
(A) Examples of PL neuronal reconstructions. Genotypes did not differ in average apical (B) or basilar (C) dendritic length, average number of apical (D) or basilar (E) dendritic branches, or apical (F) or basilar (G) dendritic intersections (n=10–11 mice per genotype, n=8 neurons per mouse). Scale bar=50 µm. Data are Means ±SEM.
Pet-1 KO show normal anxiety-related behaviors and responses to forced swim
Total distance traveled and percent center time in the novel open field test did not vary with genotype (Supplementary Fig S1A–B). All genotypes showed habituation of locomotor activity in this test (main effect of timebin: F5,335=37.29, p<.01, timebin x genotype interaction: ns). In the elevated plus-maze, genotypes did not differ in percent open arm time, open arm entries (WT=8.64 ±1.13, HET=7.83 ±0.61, KO=8.50 ±0.93) or closed arm entries (Supplementary Fig S1C–D). Genotypes also showed similar percent time in the light compartment and light compartment entries during the first (Supplementary Fig S1E–F) and last (data not shown) 5 min of the light/dark exploration test.
There was no effect of genotype on immobility in the forced swim test (Supplementary Fig S2A). There was a significant overall increase in immobility from trial 1 to trial 2 (main effect of trial: F1,28=4.80, p<.05; trial x genotype interaction: ns). Genotypes did not differ in corticosterone responses to trial 2 (Supplementary Fig S2B) and all showed significantly higher corticosterone levels after swim, as compared to non-swum controls (main effect of swim: F2,55=31.06, p<.01, swim x genotype interaction: ns).
Pet-1 KO show normal home cage circadian activity, impaired rotarod motor coordination
Home cage activity significantly changed over the circadian cycle, but genotypes did not differ in the pattern or overall level of activity (Supplementary Fig S3A–B) (main effect of time of day: F95,1425=2.74, p <.01; main effect of genotype: ns; time of day x genotype interaction: ns) (for a fuller analysis of this behavior, see Paulus and Mintz, 2011).
In the accelerating rotarod, there was a significant effect of genotype (F2,43=3.60, p <.05) and trial (F9,387=12.66, p <.01), but no genotype x trial interaction, for latency to fall. Simple main effects analysis revealed that Pet-1 KO mice had lower latencies than WT controls (F1,27=4.82, p <.05) (Supplementary Fig S3C).
Pet-1 KO show normal responses to acute EtOH challenge
There was no effect of genotype on delta latency to fall from the accelerating rotarod following EtOH injection (Supplementary Fig S4A). EtOH produced a clear hypothermic response but this did not differ between genotypes (Supplementary Fig S4B). Sleep time responses were also similar across genotypes (Supplementary Fig S4C).
Human PET-1 gene variation predicts amygdala reactivity to threat
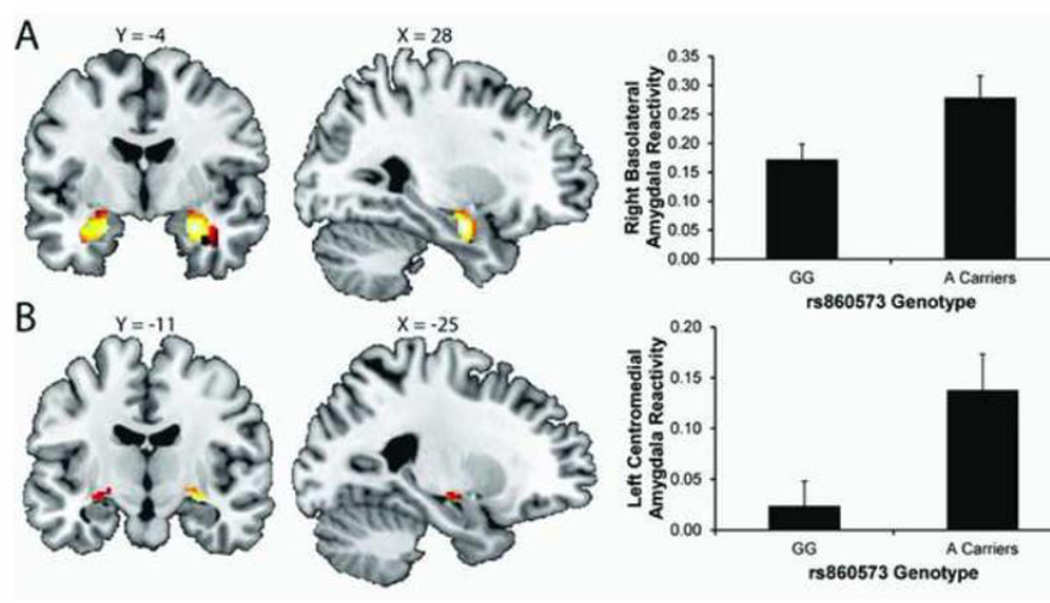
The imaging task robustly recruited bilateral basolateral and centromedial amygdala reactivity (Fig 6). Regression analyses revealed significant effects of rs860573 genotype on left centromedial and right basolateral threat-related amygdala reactivity (left centromedial: standardized beta=0.29; ΔF(1,84) = 6.72, ΔR2=.073, p<.02; right basolateral: standardized beta=0.25; ΔF(1,84) = 5.08, ΔR2=.056, p<.03). Specifically, carriers of the minor A allele exhibited significantly increased right basolateral and left centromedial amygdala reactivity in comparison with major G allele homozygotes (Fig 6). Genotype did not predict left basolateral (standardized beta=0.19; ΔF(1,84) = 2.97, ΔR2=.034, p>.08) or right centomedial (standardized beta=0.05; ΔF(1,84) = 0.90, ΔR2=.01, p>.34) reactivity. A allele carriers also had higher rates of psychopathology (Fisher’s exact: p<.005) and greater general distress anxiety as measured by the MASQ (p<.03; however, while anxiety as measured by the STAI was elevated in A allele carriers, the association did not reach statistical significance (p=.13; Table 1). There was no evidence that amygdala reactivity mediated self-reported differences in anxiety or depressive symptomatology.
FIGURE 6. Variation in the human PET-1 gene predicts threat-related amygdala reactivity.
Main effects of task (i.e., Angry & Fearful Faces > Shapes) on bilateral basolateral (A) and centromedial (B) amygdala ROIs in the entire sample (n = 332). Montreal Neurological Institute template coordinates and statistics for the left basolateral ROI: max voxel: x = −22 y = −6 z = −20; p < .001, cluster size = 189; right basolateral ROI: max voxel: x = 28 y = −6, z = −20; p < 0.001, cluster size = 219; left centromedial ROI: max voxel: x = −24 y = −10 z = −14; p < 0.001, cluster size = 38; right centromedial ROI: max voxel: x = 28 y = −12, z = −12; p < .001, cluster size = 67. A allele carriers at rs860573 within FEV (PET-1) had elevated right basolateral and left centromedial threat-related amygdala reactivity in the Asian subsample (n = 88).
DISCUSSION
The major findings of the current study were that 1) in mice, Pet-1 deletion produced amygdala dendritic hypertrophy and an augmented, extinction-resistant fear memory, and, 2) in humans, a PET-1 gene (FEV) variant associated with threat-driven amygdala reactivity and risk for psychopathology. Importantly, particularly in light of the importance of replication even with intermediate biological phenotypes (; but see also Goldman and Ducci, 2007; Hart et al., 2013), our human imaging genetics results should be viewed as preliminary. Our relatively small sample also limits the inferential value of our observed association with psychiatric disorder, which similarly require replication ideally in larger samples.
Increased, recovery-prone, fear in PET-1 KO mice
Pet-1 KO mice showed rapid, one-trial augmentation of fear memory acquisition, with higher freezing than WT controls as early as the second exposure to a conditioned stimulus during fear conditioning and increased fear the following day, which persisted during much of extinction training. Nonetheless, over extinction training trials, KO fear levels decreased to a level similar to WT. These patterns reflect sustained expression of an increased fear response in KO rather than a deficit in extinction learning. However, despite extinguishing to WT levels, KO showed significant fear recovery on an extinction-retrieval test the following day. Thus, lifelong loss of serotonin not only strengthens the formation and expression of fear, consistent with previous work (Kiyasova et al., 2011), but weakens the capacity for fear extinction training to produce lasting reductions in fear. This pattern is similar to that reported in anxiety and stress-related disorders, such as PTSD, where symptoms are liable to reoccur in the presence of trauma reminders, even after exposure therapy (Bradley et al., 2005; Felmingham and Bryant, 2012; Milad et al., 2009).
These findings are also reminiscent of the phenotype in other mouse models of constitutive serotonin loss (Fernandez and Gaspar, 2012). For instance, mutants with deletion of Lmx1b in the raphé nuclei exhibited increased acquisition and expression of conditioned contextual fear, although extinction was not tested (Dai et al., 2008). This convergence of findings across mouse models supports strong penetrance of effects of constitutive serotonin loss on fear behavior.
Amygdala dendritic hypertrophy in PET-1 KO mice
Pet-1 KO mice had significant expansion of BLA dendritic arbors. Although we did not detect abnormalities in PL dendritic morphology, activity of PL neurons positively correlates with fear and poor extinction in rats (Burgos-Robles et al., 2009; Wilber et al., 2011), and further studies are needed before discounting the contribution of abnormal PL function to Pet-1 KO fear behavior. Similar changes in BLA arborization are found in an inbred mouse strain exhibiting resistance to fear extinction (Camp et al., 2012), and increased BLA dendritic spine density is found in extinction-impaired mutant mice lacking 5-HTT (Nietzer et al., 2011; Wellman et al., 2007). Further, chronic stress causes BLA dendritic hypertrophy in rats, which may contribute to stress-induced changes in emotional behaviors (Roozendaal et al., 2009). Collectively, these findings show that BLA dendritic hypertrophy is a reliable correlate of excessive fear.
This is consistent with lesion studies demonstrating that the BLA complex is critical for the formation and expression of the Pavlovian fear memory (Anglada-Figueroa and Quirk, 2005; Ehrlich et al., 2009; LeDoux, 2000). Expansion of BLA dendritic arbors in Pet-1 KO mice could provide a neural substrate for enhanced encoding of fear memories, possibly by increasing the availability of synapses at which plasticity can occur. Given that serotonin is generally considered to be a trophic factor, dendritic hypertrophy in Pet-1 KO mice might seem paradoxical. However, consistent with our findings, a recent study demonstrated decreased dendritic length in BLA of MAO-A knockout mice (Bortolato et al., 2011). Serotonin axons innervate pyramidal neurons and inhibitory interneurons in the BLA (Muller et al., 2007), and multiple serotonin receptor subunits are expressed on both cell types. Therefore, the net effect of either excess or diminished serotonin in this region is likely to be highly complex. Parsing the mechanisms involved awaits future study.
Phenotypic specificity and genetic background modification
The fear phenotype in the Pet-1 KO mice was not associated with alterations in other anxiety-or stress-related phenotypes. This is consistent with recent analysis of Pet-1 KO mice in these same anxiety-related assays and forced swim test (Schaefer et al., 2009). However, other studies have reported increased anxiety-like behavior in Pet-1 KO mice in the elevated plus-maze (Hendricks et al., 2003; Liu et al., 2010), marble burying, and acoustic startle (Schaefer et al., 2009) tests. Conversely, others have reported decreased anxiety-like behavior in Pet-1 KO mice the elevated plus-maze and novelty-suppressed assays (Kiyasova et al., 2011), similar to the anxiolytic-like effects on marble-burying and the open field test seen in serotonin-depleted mice fed a tryptophan-deficient diet (Browne et al., 2012). In the present study as well as those cited above, mice underwent batteries of tests related to anxiety, stress, and emotional regulation; thus, it is possible that the effects of the stressors inherent in these tests differ across genotypes. Such an interaction could contribute to the discrepancies seen across studies in which both the specific tests used and the order in which they were given may vary (Browne et al., 2012; Fernandez and Gaspar, 2012).
On the other hand, genetic background may underlie variability in mutant anxiety-related phenotypes across studies (Crusio et al., 2009; Fernandez and Gaspar, 2012; Holmes and Hariri, 2003). Pet-1 KO mice have been examined on C57BL/6J x ‘129Sv’ hybrid and C57BL/6J-backcrossed (as in the current) genetic backgrounds. However, Hendricks et al. 2003 and Schaefer et al. 2009 both used hybrid backgrounds, but found different phenotypes. Therefore, the impact of Pet-1 KO on anxiety-like behavior is likely related to other factors. Given the importance of the serotonin system in modulating sensitivity to stress, one such factor could be variation in stress levels related to prior test history and local testing and housing conditions. This would echo other findings in mutants with altered serotonin function (e.g., TPH1 and Tph2 deletion, (Beaulieu et al., 2008) vs (Savelieva et al., 2008). Thus, in contrast to learned fear, complex environmental interactions and compensatory alterations may mitigate and mask effects of serotonin perturbation on unconditioned anxiety-like behavior. This could relate to anxiety’s recruitment of widespread and semi-redundant regions (Singewald et al., 2003), whereas fear learning is highly dependent on the function of a discrete circuit.
Translation to human amygdala reactivity and psychopathology
Based on our findings in Pet-1 KO mice, we extended our study to human neural processes. We found that a common SNP, rs860573, in the PET-1 gene (FEV) is associated with altered threat-related amygdala function. Carriers of the minor A allele exhibited increased amygdala reactivity to facial expressions signaling threat compared to homozygotes of the major G allele. While this effect is consistent with the increased and recovery-prone conditioned fear seen in Pet-1 KO mice, a future study fMRI exploring potential differences in fear conditioning and extinction in carriers of the minor A allele would be interesting. Importantly, the increased amygdala reactivity seen in carriers of the minor A allele was present against a common genetic background, as all individuals were of self-identified Asian ancestry. This provides another parallel with the modification of Pet-1 KO phenotypes by genetic background, and reinforces the notion that serotonin gene variants associated with emotionality interact strongly with genetic modifiers (Holmes and Hariri, 2003). While it would be interesting to test whether our observed effect is present against different genetic backgrounds, the relative rarity of the minor A allele in non-Asian populations will require sample sizes that exceed those of current studies.
PET-1 (FEV) genotype effects extended to behavioral and clinical phenotypes that have previously been associated with threat-related amygdala reactivity (Hariri, 2009). Carriers of the A allele, who exhibited increased amygdala reactivity, had significantly higher levels of psychopathology and increased trait anxiety. Future work should extend analyses into clinical populations to directly examine the potential influence of the PET-1 (FEV) variant on the prevalence of fear-related disorders. Regardless, the convergence of effects on neural, behavioral, and clinical phenotypes suggest that PET-1 rs860573 genotype may impact the development of human serotonin neurons and forebrain innervation patterns in a manner consistent with that observed in the Pet-1 KO. Studies utilizing receptor positron emission tomography to quantify serotonergic innervation in vivo will be valuable in exploring this possibility (Fisher and Hariri, 2012). Some important limitations of this human imaging genetics study warrant attention. First, the generalizability is limited to those of Asian ancestry. Second, while consisting of a relatively large sample for a neuroimaging study, our sample size is small for a genetic association study and we do not have access to a replication sample. As a result, we caution against over-interpretation of the reported findings until replication is established in larger samples (Duncan and Keller, 2011).
Conclusions
Loss of brain serotonin via gene deletion of Pet-1 produced an increased fear memory that was liable to recover following extinction and dendritic hypertrophy in a key brain region mediating fear and extinction. While previous studies have demonstrated how variation in genes regulating other major components of the serotonergic system alter corticolimbic morphology and associated fear extinction processing, they have focused on effects likely to produce hyperserotonergia – for instance, loss of SERT-mediated reuptake or the serotonin 5-HT1A autoreceptor, which controls serotonin neuronal firing (reviewed in Holmes, 2008). The current study demonstrates how these phenotypes are impacted by lifelong hyposerotonergia. Given this point, together with the complexity and multifaceted nature of the serotonin system, the current findings add appreciably to our understanding of the effects of genetic variation in the serotonergic system. This is underscored by our pattern of results, which are qualitatively different from that seen with, for example, SERT gene variation. For instance, PET1 disruption alters fear encoding and amygdala morphology, whereas SERT disruption alters extinction encoding and prefrontal morphology (Wellman et al., 2007). At the same time, the finding that gene variations producing either hyperserotonergia or hyposerotonergia both strongly modulate corticolimbic systems regulating emotion supports the broader conclusion that disruptions of the serotonin system in either direction can have remarkably convergent effects on corticolimbic systems regulating emotion.
Consistent with the effects of PET-1 deletion, in a sample of Asian-ancestry human subjects, a significant association was found between a common PET-1 genetic polymorphism and threat-related amygdala reactivity. The presence of this association, as well as an association with clinical diagnosis and with trait anxiety, suggests that the penetrance of this variant may be robust. This is consistent with the critical role of PET1 in the development of serotonergic neurons, and predicts that genetic variation in PET1 may manifest an even stronger influence on risk for mood and anxiety disorders when operating in interaction with, for example, variants in other serotonergic genes (e.g., 5-HTTLPR) and environmental triggers (e.g., childhood trauma).
Our data provide novel, convergent evidence of the consequences of Pet-1 gene deletion and variation on amygdala-mediated fear. These findings add to a growing literature demonstrating the lasting impact of genetic variation and ontogenic disruption of the serotonin system in regulating emotional behavior and influencing risk for mood and anxiety disorders, and will provide a foundation for future studies exploring the underlying biology in greater detail and the influence of PET1 variation in interaction with other risk factors in larger human populations.
Supplementary Material
HIGHLIGHTS.
Pet-1 knockout (KO) causes loss of serotonin neurons and forebrain serotonin availability
We phenotyped Pet-1 KO mice for fear conditioning and extinction, anxiety- and depression-related behaviors, and morphology of corticolimbic neurons
Using human imaging genetics, a common variant (rs860573) in the PET-1 (FEV) gene was tested for effects on threat-related amygdala reactivity and psychopathology in 88 Asian-ancestry subjects.
In mice, Pet-1 deletion produced amygdala dendritic hypertrophy and an augmented, extinction-resistant fear memory
In humans, a PET-1 gene variant associated with threat-driven amygdala reactivity and risk for psychopathology.
ACKNOWLEDGEMENTS
We are very grateful to Guoxiang Luo for genotyping and Drew Rosenbarger for technical assistance.
STATEMENT OF INTEREST
This work was supported by the US-Israel Binational Science Foundation (grant number 2007096 to AH, CLW, MM); the Intramural Research Program of the National Institute on Alcoholism and Alcohol Abuse (Z01-AA000411 to AH), and Duke University
ABBREVIATIONS
- BLA
basolateral amygdala
- EtOH
ethanol
- Pet-1, FEV
Pheochromocytoma 12 ets
- KO
knockout
- PTSD
posttraumatic stress disorder
- PL
prelimbic cortex
- 5-HTT
the serotonin transporter
- Tph2
tryptophan hydroxylase-2
- vmPFC
ventromedial prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and embryology. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25:9680–9685. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Hen R, Gingrich JA. Neurodevelopmental origins of depressive disorders. Curr Opin Pharmacol. 2007;7:8–17. doi: 10.1016/j.coph.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci U S A. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. The American journal of psychiatry. 2012;169:515–522. doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Chen K, Godar SC, Chen G, Wu W, Rebrin I, Farrell MR, Scott AL, Wellman CL, Shih JC. Social deficits and perseverative behaviors, but not overt aggression, in MAO-A hypomorphic mice. Neuropsychopharmacology. 2011;36:2674–2688. doi: 10.1038/npp.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, Holmes A. Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res. 2006;30:1957–1965. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Bradley R, Greene J, Russ E, Dutra L, Westen D. A Multidimensional Meta-Analysis of Psychotherapy for PTSD. Am. J. Psychiatr. 2005;162:214–227. doi: 10.1176/appi.ajp.162.2.214. [DOI] [PubMed] [Google Scholar]
- Brown SM, Peet E, Manuck SB, Williamson DE, Dahl RE, Ferrell RE, Hariri AR. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Molecular Psychiatry. 2005;10:884–888. doi: 10.1038/sj.mp.4001716. 805. [DOI] [PubMed] [Google Scholar]
- Browne CA, Clarke G, Dinan TG, Cryan JF. An effective dietary method for chronic tryptophan depletion in two mouse strains illuminates a role for 5-HT in nesting behaviour. Neuropharmacol. 2012;62:1903–1915. doi: 10.1016/j.neuropharm.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp MC, MacPherson KP, Lederle L, Graybeal C, Gaburro S, DeBrouse LM, Ihne JL, Bravo JA, O'Connor RM, Ciocchi S, Wellman CL, Luthi A, Cryan JF, Singewald N, Holmes A. Genetic Strain Differences in Learned Fear Inhibition Associated with Variation in Neuroendocrine, Autonomic, and Amygdala Dendritic Phenotypes. Neuropsychopharmacology. 2012;37:1534–1547. doi: 10.1038/npp.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusio WE, Goldowitz D, Holmes A, Wolfer D. Standards for the publication of mouse mutant studies. Genes Brain Behav. 2009;8:1–4. doi: 10.1111/j.1601-183X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- Dai JX, Han HL, Tian M, Cao J, Xiu JB, Song NN, Huang Y, Xu TL, Ding YQ, Xu L. Enhanced contextual fear memory in central serotonin-deficient mice. Proc Natl Acad Sci U S A. 2008;105:11981–11986. doi: 10.1073/pnas.0801329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Montanez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL, Holmes A. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci. 2006;26:6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneris ES. Molecular genetics of mouse serotonin neurons across the lifespan. Neuroscience. 2011;197:17–27. doi: 10.1016/j.neuroscience.2011.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, Francke U, Mountain JL, Goldman SM, Tanner CM, Langston JW, Wojcicki A, Eriksson N. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson's disease. PLoS genetics. 2011;7:e1002141. doi: 10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am. J. Psychiatr. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto: Consulting Psychologists Press; 1976. [Google Scholar]
- Eriksson N, Macpherson JM, Tung JY, Hon LS, Naughton B, Saxonov S, Avey L, Wojcicki A, Pe'er I, Mountain J. Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS genetics. 2010;6:e1000993. doi: 10.1371/journal.pgen.1000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaki T, Cook M, Shimoji K, Murphy DL, Sokoloff L, Holmes A. Developmental disruption of serotonin transporter function impairs cerebral responses to whisker stimulation in mice. Proc Natl Acad Sci U S A. 2005;102:5582–5587. doi: 10.1073/pnas.0501509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham KL, Bryant RA. Gender differences in the maintenance of response to cognitive behavior therapy for posttraumatic stress disorder. Journal of Consulting and Clinical Psychology. 2012;80:196–200. doi: 10.1037/a0027156. [DOI] [PubMed] [Google Scholar]
- Fernandez SP, Gaspar P. Investigating anxiety and depressive-like phenotypes in genetic mouse models of serotonin depletion. Neuropharmacol. 2012;62:144–154. doi: 10.1016/j.neuropharm.2011.08.049. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Washington, D.C.: American Psychiatric Press, Inc; 1997. [Google Scholar]
- Fisher P, Hariri AR. Linking variability in human serotonin signaling and threat-related corticolimbic circuit function through multimodal neuroimaging. Genes Brain Behav in press; 2012. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. J Neurosci Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Goldman D, Ducci F. Deconstruction of vulnerability to complex diseases: enhanced effect sizes and power of intermediate phenotypes. Scientific WorldJournal. 2007;7:124–130. doi: 10.1100/tsw.2007.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, Holmes A. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat Neurosci. 2011;14:1507–1509. doi: 10.1038/nn.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annu Rev Neurosci. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. Genetic polymorphisms: a cornerstone of translational biobehavioral research. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000811. 18ps16. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, Manuck SB. Divergent Effects of Genetic Variation in Endocannabinoid Signaling on Human Threat- and Reward-Related Brain function. Biol. Psychiatr. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hart AB, de Wit H, Palmer AA. Candidate gene studies of a promising intermediate phenotype: failure to replicate. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:802–816. doi: 10.1038/npp.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, McKEnna MC, Salman R, Holmes A, Casey BJ, Phelps EA, Glatt CE. Serotonin transporter polyadenylation polymorphism modulates the retention of fear extinction memory. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1202044109. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York City, NY, USA: Guilford Press; 2013. [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Hariri AR. The serotonin transporter gene-linked polymorphism and negative emotionality: placing single gene effects in the context of genetic background and environment. Genes Brain Behav. 2003;2:332–335. doi: 10.1046/j.1601-1848.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Hefner KR, Sibley DR, Holmes A. Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology (Berl) 2008;200:117–127. doi: 10.1007/s00213-008-1165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyasova V, Fernandez SP, Laine J, Stankovski L, Muzerelle A, Doly S, Gaspar P. A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J Neurosci. 2011;31:2756–2768. doi: 10.1523/JNEUROSCI.4080-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman AU. Dendritic morphology of pyramidal neurones of the visual cortex of the rat: I. Branching patterns. J Comp Neurol. 1991;306:307–319. doi: 10.1002/cne.903060207. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat Neurosci. 2008 doi: 10.1038/nn.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Maejima T, Wyler SC, Casadesus G, Herlitze S, Deneris ES. Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci. 2010;13:1190–1198. doi: 10.1038/nn.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millstein RA, Ralph RJ, Yang RJ, Holmes A. Effects of repeated maternal separation on prepulse inhibition of startle across inbred mouse strains. Genes Brain Behav. 2006;5:346–354. doi: 10.1111/j.1601-183X.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, Farrell MR, Hill EE, Graybeal C, Martin KP, Camp M, Fitzgerald PJ, Ciobanu DC, Sprengel R, Mishina M, Wellman CL, Winder DG, Williams RW, Holmes A. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;505:314–335. doi: 10.1002/cne.21486. [DOI] [PubMed] [Google Scholar]
- Nietzer SL, Bonn M, Jansen F, Heiming RS, Lewejohann L, Sachser N, Asan ES, Lesch KP, Schmitt AG. Serotonin transporter knockout and repeated social defeat stress: Impact on neuronal morphology and plasticity in limbic brain areas. Behav Brain Res. 2011;220:42–54. doi: 10.1016/j.bbr.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Hariri AR. Neural responses to threat and reward interact to predict stress-related problem drinking: A novel protective role of the amygdala. Biology of mood & anxiety disorders. 2012a;2:19. doi: 10.1186/2045-5380-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Hariri AR. Neural responses to threat and reward interact to predict stress-related problem drinking: A novel protective role of the amygdala. Biology of Mood and Anxiety Disorders. 2012b;2:19. doi: 10.1186/2045-5380-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus EV, Mintz EM. Developmental disruption of the serotonin system alters circadian rhythms. Physiol Behav. 2011;105:257–263. doi: 10.1016/j.physbeh.2011.08.032. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. “Behavioural despair” in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol. 1978;51:291–294. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, Lanthorn TH. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS One. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Vorhees CV, Williams MT. Mouse plasmacytoma-expressed transcript 1 knock out induced 5-HT disruption results in a lack of cognitive deficits and an anxiety phenotype complicated by hypoactivity and defensiveness. Neuroscience. 2009;164:1431–1443. doi: 10.1016/j.neuroscience.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl. 1998;20:22–33. quiz 34–57. [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Tung JY, Do CB, Hinds DA, Kiefer AK, Macpherson JM, Chowdry AB, Francke U, Naughton BT, Mountain JL, Wojcicki A, Eriksson N. Efficient replication of over 180 genetic associations with self-reported medical data. PloS one. 2011;6:e23473. doi: 10.1371/journal.pone.0023473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn. Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J. Abnormal Psychol. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garret JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle N, Hauschild M, Lubec G, Holmes A, Singewald N. Rescue of impaired fear extinction and normalization of cortico-amygdala circuit dysfunction in a genetic mouse model by dietary zinc restriction. J Neurosci. 2010;30:13586–13596. doi: 10.1523/JNEUROSCI.0849-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber AA, Walker AG, Southwood CJ, Farrell MR, Lin GL, Rebec GV, Wellman CL. Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neurosci. 2011;174:115–131. doi: 10.1016/j.neuroscience.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RJ, Mozhui K, Karlsson RM, Cameron HA, Williams RW, Holmes A. Variation in mouse basolateral amygdala volume is associated with differences in stress reactivity and fear learning. Neuropsychopharmacology. 2008;33:2595–2604. doi: 10.1038/sj.npp.1301665. [DOI] [PubMed] [Google Scholar]
- Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.