Abstract
Recent studies have shown mitochondrial dysfunction and increased production of reactive oxygen species in peripheral blood mononuclear cells (PBMC’s) and endothelial cells from patients with diabetes mellitus. Mitochondria oxygen consumption is coupled to ATP production and also occurs in an uncoupled fashion during formation of reactive oxygen species by components of the electron transport chain and other enzymatic sites. We therefore hypothesized that diabetes would be associated with higher total and uncoupled oxygen consumption in PBMC’s that would correlate with endothelial dysfunction. We developed a method to measure oxygen consumption in freshly isolated PBMC’s and applied it to 26 patients with type 2 diabetes mellitus and 28 non-diabetic controls. Basal (192±47 vs. 161±44 pMoles/min, P=0.01), uncoupled (64±16 vs. 53±16 pMoles/min, P=0.007), and maximal (795±87 vs. 715±128 pMoles/min, P=0.01) oxygen consumption rates were higher in diabetic patients compared to controls. There were no significant correlations between oxygen consumption rates and endothelium-dependent flow-mediated dilation measured by vascular ultrasound. Non-endothelium-dependent nitroglycerin-mediated dilation was lower in diabetics (10.1±6.6 vs. 15.8±4.8%, P=0.03) and correlated with maximal oxygen consumption (R= −0.64, P=0.001). In summary, we found that diabetes mellitus is associated with a pattern of mitochondrial oxygen consumption consistent with higher production of reactive oxygen species. The correlation between oxygen consumption and nitroglycerin-mediated dilation may suggest a link between mitochondrial dysfunction and vascular smooth muscle cell dysfunction that merits further study. Finally, the described method may have utility for assessment of mitochondrial function in larger scale observational and interventional studies in humans.
Keywords: mitochondria, oxygen consumption, diabetes mellitus, blood mononuclear cells
Introduction
In addition to generating ATP via oxidative phosphorylation, mitochondria are important for many other aspects of cellular function, including calcium flux, apoptosis, and intracellular cell signaling.1 It is now recognized that altered mitochondrial function contributes to the aging process and many common disease states, including neurodegenerative diseases, cancer, and atherosclerosis.2–5 In light of the ongoing obesity epidemic, there is also considerable interest in mitochondrial dysfunction in the pathogenesis of type 2 diabetes mellitus and its vascular complications.6–11 Recent work has demonstrated increased mitochondrial production of reactive oxygen species, oxidative stress, and altered mitochondrial morphology and dynamics in peripheral blood mononuclear cells and endothelial cells from patients with diabetes mellitus.12–15
Cellular oxygen consumption is increasingly recognized as a fundamental measure of mitochondrial function.16–18 Basal oxygen consumption rate reflects coupled mitochondrial respiration as well as uncoupled consumption of oxygen to form reactive oxygen species at mitochondrial and non-mitochondrial enzymatic sites. Coupled and uncoupled respiration can be distinguished by examining the effects of an inhibitor of ATP synthase (oligomycin) and/or Complex III (antimycin A). Maximal oxygen consumption rate, provoked by addition of a mitochondrial uncoupling agent such as carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP), provides an index of energetic reserve capacity. The Clark oxygen electrode19 and the Seahorse extracellular flux analyzer 16,17 can be used to measure oxygen consumption rates in cell suspensions and adherent cells in tissue culture, respectively. These approaches have provided insight into the importance of mitochondrial dysfunction in experimental models of disease.
Study of mitochondrial respiration in humans has previously been limited by a number of technical issues. The Clark electrode has been used to examine oxygen consumption in human leukocytes in suspension,20,21 but its requires a relatively large volume of blood. Attempts to measure oxygen consumption in blood cells using the Seahorse device have been limited by displacement of non-adherent cells during the automated movement of the oxygen sensor. For the present study, we developed a method to immobilize readily available peripheral blood mononuclear cells (PBMC’s) that allows us to measure mitochondrial oxygen consumption with the Seahorse XF-96 analyzer. We used the method to measure mitochondrial function in patients with type 2 diabetes mellitus and non-diabetic controls and related the results to vascular function. Prior studies have shown increased mitochondrial production of reactive oxygen species in PBMC’s, endothelial cells, and other cell types in diabetes, suggesting systemic mitochondrial dysfunction.12,13,22 On this basis, we hypothesized that diabetes mellitus would be associated with higher total and uncoupled oxygen consumption that would correlate with endothelial dysfunction, providing evidence in humans that mitochondrial dysfunction might be relevant to cardiovascular disease in humans.
Materials and Methods
Study subjects
Adult patients with type 2 diabetes mellitus and healthy volunteers were recruited at Boston Medical Center by advertisement. Diabetes was defined as fasting glucose ≥126 mg/dl or ongoing treatment for type 2 diabetes mellitus. Healthy volunteers were taking no medications, had fasting glucose <100 mg/dl, and had never smoked or had stopped smoking for at least a year prior to enrollment. A fasting blood sample was obtained in the morning by venipuncture, and glucose and lipids were measured in the Boston Medical Center Clinical Laboratory. Non-invasive measurement of endothelium and non-endothelium dependent vasodilator function was then completed, as described below. The study protocol was approved by the Boston Medical Center Institutional Review Board and all participants provided written informed consent.
Materials and reagents
The study used the following materials and reagents: FCCP (Sigma Life Science, St. Louis, MO, USA), oligomycin (Sigma life Science, St. Louis, MO, USA), poly- D-Lysine (EMD Millipore, Billerica, MA), Hanks’ balanced salt solution (HBSS)(Life technologies, Gibco, Grand Island, NY), Dulbecco’s modified eagle medium (DMEM)(Seahorse Bioscience, Billerica, MA), glucose (American Bioanalytical, Natick, MA), etomoxir (Calbiochem-EMD Biosciences, Billerica, MA), and sodium pyruvate (Life technologies, Gibco, Grand Island, NY).
Isolation of PBMC’s
Venous blood was collected into commercially available cell preparation tubes for PBMC’s (BD Vacutainer CPT with sodium citrate, Becton, Dickinson and Company, Franklin, NJ). The tubes were spun at 1650 X g for 30 min at room temperature and cell layers were collected and transferred into 25 mL of HBSS. This solution was centrifuged for additional 10 min at 250 X g at room temperature. The pellet was resuspended in 15 mL of fresh HBSS and cell count was determined using a glass hemocytometer (Double Neubauer Counting Chamber, Hausser Scientific, Horsham, PA). Fluorescence activated cell sorting (FACS) analysis in the Boston Medical Center Hematology Laboratory of samples from five subjects demonstrated that this isolation method yielded a cell population of 82±4% lymphocytes (CD3 positive cells) and 14±2% monocytes (CD14 positive cells) with the remaining 4% comprised of other mononuclear cells.
Plate Preparation
Prior to the addition of PBMC’s, XF-96 plates (Seahorse Bioscience, Billerica, MA) were treated with poly-D-lysine to improve cell adherence. Poly-D-lysine 50 μg/mL was prepared in sterile water and 30μL was added to each well for one hour. The solution was removed, the wells were washed with 300 μL sterile water, and the plate was dried in a laminar flow hood for 30 minutes.
PBMC’s were suspended in DMEM supplemented with pyruvate 1.0 mM and glucose 5.5 mM. DMEM was warmed to 37°C and the pH was adjusted to 7.4 prior to cell suspension. PBMC’s (600,000 per well) were seeded onto poly-D-lysine-treated XF-96 microplates (Seahorse Bioscience, Billerica, MA). After seeding, the plates were spun with slow acceleration (4 on a scale of 9) to a maximum of 450 rpm and then allowed to stop with zero braking (Model 5810R Centrifuge, Eppendorf North America, Hauppauge, NY). The plate orientation was reversed and the plate was spun again to 650 rpm in the same fashion. Plates were maintained at 37°C for approximately 20 min prior to loading during XF-96 analyzer calibration. During development of the method, we visualized the distribution of PBMC’s in XF-96 wells after plate preparation using an inverted microscope. PBMC’s were evenly distributed in a single layer with approximately 89% confluence. For each subject, we confirmed viability of cells by trypan blue exclusion prior to measurement of oxygen consumption.
Oxygen Consumption Measurement Protocol
Oxygen consumption rates were measured in accordance with manufacturer instructions (Seahorse Bioscience). The assay media (DMEM) was supplemented with glucose (5.5 mM) and pyruvate (1 mM) and the pH was adjusted to 7.4. In preliminary studies testing 200,000 to 1 million cells per well, we found an optimal signal and uniform distribution of cells on the bottom of the well with the use of 600,000 cells per well. Experiments were replicated in six wells and averaged for each experimental condition. The average coefficient of variation for basal oxygen consumption rate was 6.4±2.0%.
Oxygen consumption measurements were made approximately every 8 minutes under basal conditions, after the addition of a saturating concentration of oligomycin (5 μmoles/L),23 and after the addition of FCCP (1 μmoles/L). Basal and post-oligomycin respiration rates were calculated by averaging the last four measurements after achieving a steady state. Coupled respiration was expressed as the percent decrease from basal respiration. Maximal FCCP respiration was taken as the highest measurement after addition of FCCP.
Assessment of Vascular Function
We used vascular ultrasound to measure endothelium-dependent flow-mediated dilation of the brachial artery as previously described.24 Subjects fasted and withheld all medications on the morning of study. Two-dimensional ultrasound images of the brachial artery were recorded before and one minute after induction of reactive hyperemia by five-minute cuff occlusion of the upper arm. We measured Doppler flow velocity at baseline and immediately after cuff release. Non-endothelium-dependent dilation of the brachial artery was assessed by examining the change in brachial diameter induced by sublingual nitroglycerin (0.4 mg). The nitroglycerin portion of the vascular testing protocol was omitted if the participant had systolic blood pressure less than 100 mmHg or had a history of migraine headaches or nitrate intolerance.
Statistical Analyses
Clinical characteristics of the diabetic and healthy control groups were compared using the unpaired t-test or chi-square test for continuous and categorical variables respectively. Group differences (controls versus diabetic patients) in oxygen consumption were compared using the Wilcoxon signed rank test, because examination of histograms suggested that these variables lacked a normal distribution. Linear regression analysis was used to determine the relation between log-transformed oxygen consumption rates and diabetes mellitus while adjusting for age, gender, and black race. Changes in oxygen consumption over time according to group (controls and diabetic patients) were assessed using analysis of variance for repeated measures after log transformation of the oxygen consumption variables. We calculated Spearman’s correlation coefficients to test the relation between vascular function variables and oxygen consumption rates. P<0.05 was considered to be statistically significant. Data are expressed as mean ± SEM. Statistical analyses were completed using SPSS Version 20 (IBM, Inc.).
Results
Study Subjects
We enrolled 54 subjects (26 with diabetes mellitus and 28 controls). Their clinical characteristics are shown in Table 1. The two groups were similar in age and gender. As expected, the patients with diabetes mellitus had higher fasting glucose and body mass index, lower HDL cholesterol, and a trend toward higher triglyceride levels (P=0.05). Total and LDL cholesterol levels were lower in diabetic patients, likely reflecting the ongoing treatment with cholesterol lowering drugs. Also as expected, diabetic patients were taking drugs to lower blood glucose and blood pressure (Table 1).
Table 1.
Clinical Characteristics
| Controls n = 28 | Diabetes Mellitus n = 26 | |
|---|---|---|
| Age (years) | 52 ± 11 | 56 ± 9 |
| Gender, Female, n (%) | 13 (46%) | 16 (62%) |
| Black race, n (%) | 9 (32%) | 15 (58%) |
| Glucose (mg/dl) | 90 ± 11 | 134 ±70 * |
| Total cholesterol (mg/dl) | 200 ± 30 | 164 ± 35 * |
| HDL cholesterol (mg/dl) | 59 ± 17 | 45 ± 11 * |
| LDL cholesterol (mg/dl) | 122 ± 24 | 92 ± 28 * |
| Triglyceride (mg/dl) | 98 ± 61 | 136 ± 77 |
| BMI (kg/m2) | 25.7 ± 2.6 | 33.3 ± 8.0 * |
| Systolic blood pressure (mmHg) | 125 ± 11 | 131 ± 17 |
| Diastolic blood pressure (mmHg) | 75 ±7 | 72 ± 13 |
| Statin therapy, n (%) | 0 | 10 (38%) * |
| ACE/ARB therapy, n (%) | 0 | 17 (65%) * |
| Metformin therapy, n (%) | 0 | 17 (65%) * |
| Insulin therapy, n (%) | 0 | 15 (58%) * |
P <0.05 versus control group. Data are mean ± SD; BMI=body mass index;
ACE/ARB=angiotensin converting enzyme inhibitor/angiotensin receptor blocker.
Oxygen Consumption Rates in Controls and Diabetic Patients
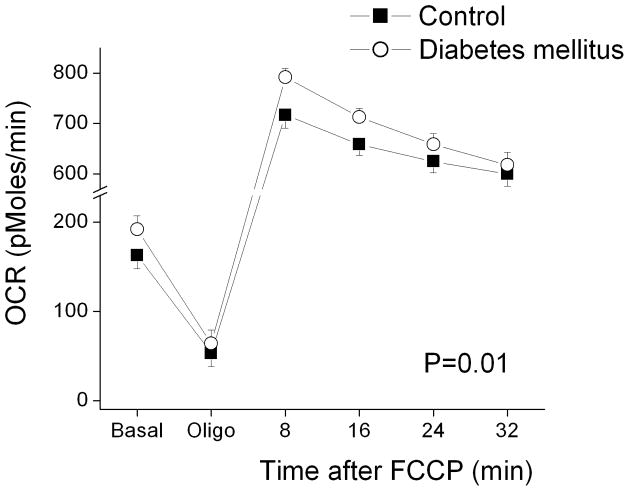
Table 2 displays oxygen consumption rates in PBMC’s from controls and diabetic patients. As shown, basal, post-oligomycin (uncoupled), and maximal after FCCP oxygen consumption rates were all higher in diabetic patients compared to controls. Interestingly, coupled respiration, calculated as the percent decrease after oligomycin, did not differ between groups. These findings are consistent with our hypothesis that diabetes would be associated with higher uncoupled respiration due to higher production of reactive oxygen species. The sequence of reagents and time course of oxygen consumption measurements is displayed in Figure 1. As shown, oxygen consumption was consistently higher in diabetic patients.
Table 2.
Diabetes Mellitus and Oxygen Consumption Rates in PBMC’s
| Control (n=28) | Diabetes Mellitus (n=26) | P value | |
|---|---|---|---|
| Basal OCR (pMoles/min) | 161 ± 44 | 192 ± 47 | 0.01 |
| OCR After Oligomycin (pMoles/min) | 53 ± 13 | 64 ± 16 | 0.007 |
| Coupled respiration (% decrease) | 67 ± 3% | 66 ± 4% | 0.73 |
| Maximal OCR (pMoles/min) | 715 ± 128 | 795 ± 87 | 0.01 |
Data are mean ± SD; PBMC’s = peripheral blood mononuclear cells; OCR = oxygen consumption rate.
Figure 1.
Oxygen consumption rates (OCR) in PBMC’s from control subjects (n=28) and patients with Type 2 diabetes mellitus (n=26). OCR was measured approximately every 8 minutes using an XF-96 analyzer as described in Methods. Basal and post-oligomycin (5 μmoles/L) rates were calculated by averaging the last four measurements after achieving a steady state. Sequential measurements were also made after addition of FCCP (1 μmoles/L), which depolarizes the mitochondrial membrane and induces maximal oxygen consumption. As shown, oxygen consumption rates were higher in PBMC’s from diabetic patients (P=0.01 for diabetes main effect and P=0.03 for diabetes by time interaction by repeated measures ANOVA).
Other Clinical Correlates of Oxygen Consumption Rates
We noted that there were non-significant tendencies for higher age (P=0.13), more females (P=0.27), and more black participants (P=0.06) in the diabetic group (Table 1), although none of these clinical characteristics correlated with oxygen consumption rates. As shown in Table 3, diabetes mellitus was associated with significantly higher basal, post-oligomycin, and maximal oxygen consumption rates in multivariable models adjusting for age, sex, and black race, suggesting that confounding effects of these factors do not explain our findings.
Table 3.
Age, Sex, and Race Adjusted Relations between Diabetes Mellitus and Oxygen Consumption Rates
| Diabetes Mellitus | ||
|---|---|---|
| β (SE) | P | |
| Basal (pMoles/min) | 30 (14) | 0.02 |
| After Oligomycin (pMole/min) | 10 (4) | 0.03 |
| Coupled (% decrease) | 0.4 (0.1) | 0.75 |
| Maximal (pMoles/min) | 96 (32) | 0.01 |
As shown in Table 1, some of the diabetic subjects were taking a statin, angiotensin converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB), metformin, or insulin. We compared basal, post-oligomycin, maximal, and coupled oxygen consumption rates according to medication status among the diabetic subjects. Diabetic patients taking insulin had slightly higher coupled respiration (68±4%, n=15) compared to diabetics not taking insulin (64±3%, n=11, P=0.04), possibly suggesting that insulin treatment promotes mitochondrial respiration. Insulin treatment did not relate to the other measures of oxygen consumption (data not shown). Oxygen consumption rates also did not differ in diabetic patients who were and were not taking a statin, ACEI/ARB, or metformin (data not shown).
We explored the other clinical correlates of oxygen consumption in the whole cohort. We observed no relation between basal, post-oligomycin, post FCCP, or coupled respiration with age, gender, blood glucose, blood lipids, body mass index, or blood pressure (data not shown).
Dependence of Oxygen Consumption Rates on Fatty Acid Transport
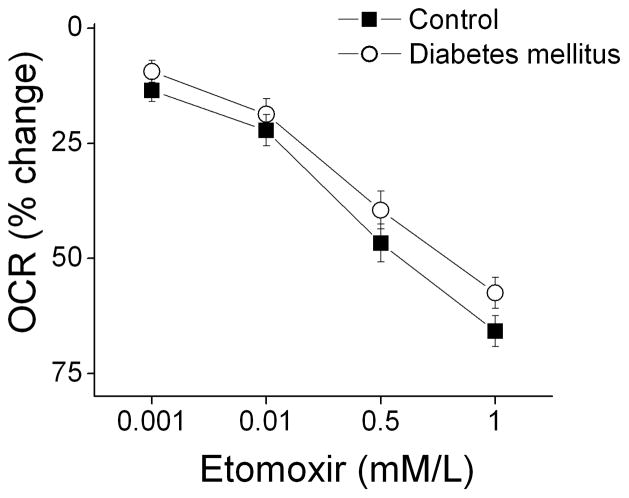
Type 2 diabetes mellitus is associated with insulin resistance and decreased expression of glucose transporters that might limit entry of glucose into PBMC’s.25 As shown in Table 1, we observed a strong trend toward higher serum triglycerides in our participants (P=0.05). A decrease in glucose transport and higher triglycerides would both be expected to cause a shift away from glucose and toward fatty acids as a source of fuel in mitochondria. We therefore investigated whether differences in fatty acid utilization might account for the higher oxygen consumption rate observed in our diabetic subjects. As shown in Figure 2, inhibiting fatty acid transport into mitochondria with the carnitine O-palmitoyltransferase-1 (CPT-1) inhibitor etomoxir produced a dose-dependent decrease in basal oxygen consumption that did not differ between control and diabetic subjects. This result argues against a disproportionate dependence on fatty acid oxidation in PBMC’s in diabetes mellitus.
Figure 2.
Etomoxir reduces basal oxygen consumption rate (OCR) to an equivalent extent in diabetic patients and controls. Oxygen consumption rate was measured in PBMC’s incubated with the indicated concentrations of etomoxir as described in Methods. As shown, inhibiting fatty acid uptake produced a dose-dependent decrease in oxygen consumption (P<0.001) in both diabetic (n=5) and control subjects (n=5), but the response did not differ between groups (P=0.64 by analysis of variance for repeated measures).
Relation of Oxygen Consumption Rates to Vascular Function
Table 4 displays vasodilator function in the control and diabetic subjects. As shown, endothelium-dependent flow-mediated dilation was lower in the diabetic subjects, whether expressed as percent change or absolute change (mm). Non-endothelium-dependent dilation of the brachial artery in response to sublingual nitroglycerin was also lower in the diabetic subjects. Finally, we observed a non-significant trend for lower reactive hyperemia, a measure of microvascular function, in the diabetic subjects. Since reactive hyperemia is the stimulus for flow-mediated dilation, reduced hyperemia might have accounted for our finding that flow-mediated dilation was lower in the diabetic subjects. However, diabetics had lower flow-mediated dilation after adjustment for reactive hyperemia, arguing against this possibility and providing addition evidence for impaired conduit artery endothelial dysfunction in the diabetic subjects.26,27
Table 4.
Diabetes Mellitus and Vascular Function
| Controls (n=22) | Diabetes Mellitus (n=21) | P value | |
|---|---|---|---|
| Flow-mediated dilation (%) | 9.9 ± 5.3 | 6.1 ± 4.5 | 0.01 |
| Flow-mediated dilation (mm) | 0.41 ± 0.19 | 0.26 ± 0.17 | 0.01 |
| Baseline diameter (mm) | 4.25 ± 0.58 | 4.57 ± 0.76 | 0.12 |
| Nitroglycerin-mediated dilation (%) | 15.8 ± 4.8 | 10.1 ± 6.6 | 0.03 |
| Hyperemic flow velocity (cm/sec) | 120 ± 25 | 108 ± 24 | 0.12 |
| FMD/hyperemic velocity (100*sec/cm) | 8.9 ± 5.3 | 5.8 ± 3.6 | 0.04 |
Data are mean ± SD. FMD = flow-mediated dilation
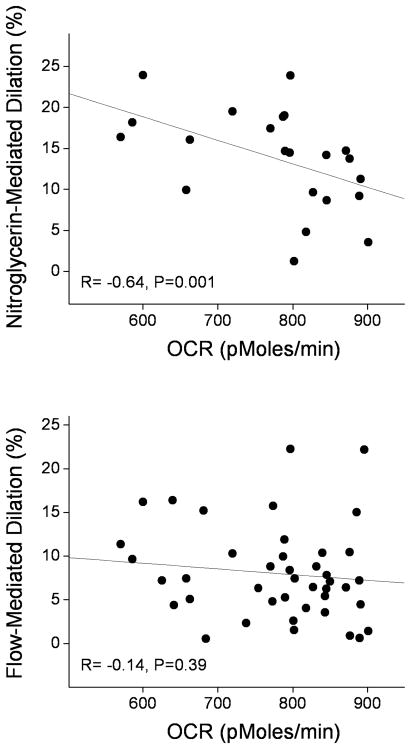
We investigated the relationships between oxygen consumption rates and vascular function. There were no significant correlations between brachial artery flow-mediated dilation (Figure 3, lower panel), hyperemic flow velocity, baseline diameter, and the measures of oxygen consumption (data not shown). Interestingly, there was a strong inverse correlation between nitroglycerin-mediated dilation and peak oxygen consumption rate (Figure 3 upper panel).
Figure 3.
Peak oxygen consumption in PBMC’s correlates with nitroglycerin-mediated dilation (upper panel), but not flow-mediated dilation (lower panel).
Discussion
In this study, we developed a method that allowed for measurement of mitochondrial oxygen consumption in PBMC’s and related the findings to vascular function in patients with type 2 diabetes mellitus. We observed higher basal, uncoupled, and maximal oxygen consumption in the diabetic group, findings consistent with our hypothesis that increased production of reactive species in diabetes mellitus would lead to an increase in uncoupled respiration. Inhibiting fatty acid transport into mitochondria with etomoxir lowered oxygen consumption to a similar extent in the two groups, suggesting that a shift toward fatty acid utilization does not explain our findings. Contrary to our initial hypothesis, we observed no significant correlation between measures of endothelial vasodilator function and oxygen consumption. There was a significant correlation between nitroglycerin-mediated dilation and maximal oxygen consumption in PBMC’s, raising the possibility that mitochondrial function and respiratory reserve might relate to the function of vascular smooth muscle cells. Overall, the study provides evidence that diabetes mellitus is associated with increased uncoupled and maximal respiration in PBMC’s that might be relevant to inflammation, altered immune function, and cardiovascular disease.
Our described method offers several advantages over other methods for assessing mitochondrial function in human subjects. Although the Clark electrode can be used to measure oxygen consumption rate in blood cells in suspension, the presented method uses fewer cells (6×105 vs. 107), reflecting the greater sensitivity of the Seahorse device.20,21,28 Other approaches for measuring mitochondrial function in humans have included measurement of mitochondrial enzyme activity in biopsied tissue and by magnetic resonance spectroscopy.29,30 Such studies are invasive or require expensive equipment, and are poorly suited to the larger scale epidemiological or intervention studies that would be required to understand how mitochondrial function relates to disease risk or management.
Prior studies have shown that mitochondrial function is relevant to leukocyte function. For example, mitochondrial membrane hyperpolarization and the production of reactive oxygen species (ROS) contribute to immune activation of monocytes and lymphocytes.31,32 Maturation of memory T-cells involves mitochondrial biogenesis and an increase in mitochondrial reserve capacity.33 Several prior studies used a Clark electrode to examine oxygen consumption in PBMC’s. Belikova and colleagues demonstrated higher and more uncoupled oxygen consumption in patients with sepsis.20 Kuhnke and colleagues showed that patients with rheumatologic disease have higher basal oxygen consumption rates that correlate with activity of disease, increase with mitogenic stimulation, and decrease following steroid treatment.21 It is possible that systemic inflammation and activation of PBMC’s in diabetes mellitus might relate to increased oxygen consumption in the present study. It is important to acknowledge that we assessed quiescent cells and a mixed population of lymphocytes and monocytes in the present study. As has recently been emphasized, it would be interesting to examine activated cells and to compare results in monocyte and lymphocyte subsets to gain further insight into the importance of mitochondrial function for leukocyte biology.34
Several prior studies specifically measured mitochondrial function in other types of blood cells from patients with diabetes mellitus. Hernandez-Mijares and colleagues reported lower oxygen consumption rates measured by Clark electrode in neutrophils from diabetic patients.35 Avila and colleagues reported lower oxygen consumption rates in platelets from diabetic patients measured with the Seahorse XF-24 analyzer.36 In that study, Cell-Tak (BD Biosciences) was used to enhance platelet adherence to the wells. Cell-Tak was also used in a very recent report examining oxygen consumption rates in human leukocytes and platelets34 and appears to be a reasonable alternative to poly-D-lysine, which was used in our study.
We previously reported higher mitochondrial ROS production, higher membrane potential, a shift toward mitochondrial fission, and lower mitochondrial mass in PBMC’s that were isolated from diabetic patients by the method used in the present study.12 Our new findings add to that prior work and provide additional evidence for altered mitochondrial function in diabetes using an emerging and potentially more clinically relevant approach. Higher ROS production likely accounts for the higher basal, post-oligomycin, and maximal oxygen consumption rates observed in the present study. It is a limitation of our study that we were not able to simultaneously measure ROS production and oxygen consumption rates to investigate this hypothesis. It is notable that we observed higher oxygen consumption despite our prior finding of lower mitochondrial mass. Studies with isolated mitochondria will be needed to differentiate the relative importance of mitochondrial mass, energy demand, coupled respiration, and ROS production for net cellular oxygen consumption in diabetes mellitus. In regard to mechanism, our finding that a CPT-1 inhibitor had similar effects in cells from both groups argues against an alteration in fatty acid utilization. Further study will be required to elucidate the mechanisms accounting for higher oxygen consumption and other mitochondrial abnormalities in diabetes mellitus. Overall, these studies in PBMC’s are consistent with a large body of work suggesting altered mitochondrial respiratory capacity and function in other tissues, including skeletal muscle, liver, islet cells, and myocardium.8,30,37 Since they are readily available, oxygen consumption in PBMC’s may be a useful endpoint to study cross-sectional correlates and the effects of interventions on mitochondrial function in systemic diseases, such as type 2 diabetes mellitus.
Our study also adds to the prior literature by examining the relationship between oxygen consumption in circulating PBMC’s and vascular function. We observed that patients with diabetes mellitus had markedly impaired vasodilator function of the conduit brachial artery and a trend toward blunted reactive hyperemia, findings that are consistent with many prior studies (see Tabit el al. for a review).22 Despite the apparent systemic nature of mitochondrial dysfunction in diabetes and a large body of experimental work implicating these mechanisms in endothelial dysfunction,5 we found no significant correlation between oxygen consumption rates in PBMC’s and endothelial function. We did observe a significant correlation between maximal oxygen consumption and non-endothelium-dependent nitroglycerin-mediated dilation. It is known that mitochondrial ROS contribute to dysfunction of vascular smooth muscle cells in cardiovascular disease.38 One might speculate that common mechanisms might account for altered oxygen consumption rates in PBMC’s and vascular smooth muscle cells in diabetes, but investigation of this possibility goes far beyond the scope of the present study. A more likely explanation for the lack of correlation between oxygen consumption rates and endothelial function is the relatively high degree of variability associated with non-invasive vascular function measurements and the relatively modest sample size. Finally, these results emphasize the importance of direct examination of endothelial cells to understand mechanisms of endothelial dysfunction, and it is notable that such studies are now feasible in humans.13,15
Our study has a number of limitations. First, the sample size was relatively modest and we had limited power to adjust for potential confounders or show correlations between the measured variables. Second, the diabetic patients were taking medications that might influence mitochondrial function and confound the results, although we observed no difference in oxygen consumption rates in diabetic patients who were or were not taking a statin, ACEI/ARB, or metformin. Finally, further studies will be needed to elucidate the mechanisms that account for the findings of this initial report.
In regard to the oxygen consumption methodology, several additional points are worth noting. First, the approach requires study of freshly isolated, live cells, which would make it less useful than a test that could be performed using frozen samples. Second, we used an XF-96 Seahorse analyzer and had only had two ports available for addition of oligomycin and FCCP to the well. As a result, we could not test the effects of antimycin A and rotenone to distinguish the contributions of proton leak and non-mitochondrial respiration to basal oxygen consumption. We did not have access to an XF-24 analyzer, which has additional ports and might be used in future studies, however the larger well size in that device would require a larger number of cells. These limitations are balanced by the potential utility of this method for clinical research and clinical care, because it provides for the measurement of a key aspect of mitochondrial function in readily accessible PBMC’s.
In summary, we described a method to evaluate oxygen consumption in freshly isolated PBMC’s and used it to examine mitochondrial function in patients with diabetes mellitus. We observed higher basal, maximal, and uncoupled oxygen consumption in the diabetic patients, findings that are consistent with prior work showing increased mitochondrial ROS production in PBMC’s. Oxygen consumption in circulating blood cells did not correlate with endothelial vasodilator function, although we did observe a correlation between nitroglycerin-mediated dilation and maximal oxygen consumption that may merit further study. These findings add to our understanding of mitochondrial dysfunction in circulating leukocytes that may be relevant to inflammation, immune dysfunction and cardiovascular disease in diabetes mellitus. Furthermore, our described method might be applied to epidemiological studies and intervention studies and might eventually prove useful for clinical care.
Acknowledgments
We gratefully acknowledge Seahorse Bioscience, Inc. for the loan of an XF-96 analyzer and donation of plates to conduct the described experiments. We also acknowledge the technical assistance of Mai-Anne Carey and Jonathan Aasen with the non-invasive testing of vascular function.
Funding
The project was supported by NIH grants HL081587, HL083801, HL083269, and HL115391 from the National Heart, Lung and Blood Institute. Drs. Vita and Hamburg receive support from the NIH-sponsored Boston University Medical Center Leadership Program in Vascular Medicine (K12 HL083781). Dr. Fetterman receives support from T32 HL007224. Dr. Hamburg is supported by NIH grants HL102299.
References
- 1.Bailey SM, Landar A, Darley-Usmar V. Mitochondrial proteomics in free radical research. Free Radic Biol Med. 2005;38:175–188. doi: 10.1016/j.freeradbiomed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Ames BN, Shigenaga MK, Hagen TM. Mitochondrial decay in aging. Biochim Biophys Acta. 1995;1271:165–170. doi: 10.1016/0925-4439(95)00024-x. [DOI] [PubMed] [Google Scholar]
- 3.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swerdlow RH. Mitochondrial Medicine and the Neurodegenerative Mitochondriopathies. Pharmaceuticals (Basel) 2009;2:150–167. doi: 10.3390/ph2030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res. 2013;112:1171–1188. doi: 10.1161/CIRCRESAHA.111.300233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 7.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 8.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 9.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patti ME, Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev. 2010;31:364–395. doi: 10.1210/er.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Widlansky ME, Wang J, Shenouda SM, Hagen TM, Smith AR, Kizhakekuttu TJ, Kluge MA, Gutterman DD, Vita JA. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl Res. 2010;156:15–25. doi: 10.1016/j.trsl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess M-A, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, Vita JA. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kizhakekuttu TJ, Wang J, Dharmashankar K, Ying R, Gutterman DD, Vita JA, Widlansky ME. Adverse alterations in mitochondrial function contribute to type 2 diabetes mellitus-related endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol. 2012;32:2531–2539. doi: 10.1161/ATVBAHA.112.256024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabit CE, Shenouda SM, Holbrook M, Fetterman JL, Kiani S, Frame AA, Kluge MA, Held A, Dohadwala MM, Gokce N, Farb MG, Rosenzweig J, Ruderman NB, Vita JA, Hamburg NM. Protein kinase-c beta contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation. 2013;127:86–95. doi: 10.1161/CIRCULATIONAHA.112.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Dranka BP, Benavides GA, Diers AR, Giordano S, Zelickson BR, Reily C, Zou L, Chatham JC, Hill BG, Zhang J, Landar A, Darley-Usmar VM. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic Biol Med. 2011;51:1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry CG, Kane DA, Lanza IR, Neufer PD. Methods for assessing mitochondrial function in diabetes. Diabetes. 2013;62:1041–1053. doi: 10.2337/db12-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Graham BH. Measurement of mitochondrial oxygen consumption using a Clark electrode. Methods Mol Biol. 2012;837:63–72. doi: 10.1007/978-1-61779-504-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belikova I, Lukaszewicz AC, Faivre V, Damoisel C, Singer M, Payen D. Oxygen consumption of human peripheral blood mononuclear cells in severe human sepsis. Crit Care Med. 2007;35:2702–2708. doi: 10.1097/01.ccm.0000295593.25106.c4. [DOI] [PubMed] [Google Scholar]
- 21.Kuhnke A, Burmester GR, Krauss S, Buttgereit F. Bioenergetics of immune cells to assess rheumatic disease activity and efficacy of glucocorticoid treatment. Ann Rheum Dis. 2003;62:133–139. doi: 10.1136/ard.62.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grover GJ, Marone PA, Koetzner L, Seto-Young D. Energetic signalling in the control of mitochondrial F1F0 ATP synthase activity in health and disease. Int J Biochem Cell Biol. 2008;40:2698–2701. doi: 10.1016/j.biocel.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 24.McMackin CJ, Vita JA. Update on nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2005;396:541–553. doi: 10.1016/S0076-6879(05)960-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kipmen-Korgun D, Bilmen-Sarikcioglu S, Altunbas H, Demir R, Korgun ET. Type-2 diabetes down-regulates glucose transporter proteins and genes of the human blood leukocytes. Scand J Clin Lab Invest. 2009;69:350–358. doi: 10.1080/00365510802632163. [DOI] [PubMed] [Google Scholar]
- 26.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: The Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 28.Horan MP, Pichaud N, Ballard JW. Review: Quantifying Mitochondrial Dysfunction in Complex Diseases of Aging. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/glr263. [DOI] [PubMed] [Google Scholar]
- 29.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med. 2005;2:e233. doi: 10.1371/journal.pmed.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perl A, Gergely P, Jr, Nagy G, Koncz A, Banki K. Mitochondrial hyperpolarization: a checkpoint of T-cell life, death and autoimmunity. Trends Immunol. 2004;25:360–367. doi: 10.1016/j.it.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emre Y, Hurtaud C, Nubel T, Criscuolo F, Ricquier D, Cassard-Doulcier AM. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem J. 2007;402:271–278. doi: 10.1042/BJ20061430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chacko BK, Kramer PA, Ravi S, Johnson MS, Hardy RW, Ballinger SW, Darley-Usmar VM. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab Invest. 2013;93:690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez-Mijares A, Rocha M, Apostolova N, Borras C, Jover A, Banuls C, Sola E, Victor VM. Mitochondrial complex I impairment in leukocytes from type 2 diabetic patients. Free Radic Biol Med. 2011;50:1215–1221. doi: 10.1016/j.freeradbiomed.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Avila C, Huang RJ, Stevens MV, Aponte AM, Tripodi D, Kim KY, Sack MN. Platelet Mitochondrial Dysfunction is Evident in Type 2 Diabetes in Association with Modifications of Mitochondrial Anti-Oxidant Stress Proteins. Exp Clin Endocrinol Diabetes. 2012;120:248–251. doi: 10.1055/s-0031-1285833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bachschmid MM, Schildknecht S, Matsui R, Zee R, Haeussler D, Cohen RA, Pimental D, Loo B. Vascular aging: chronic oxidative stress and impairment of redox signaling-consequences for vascular homeostasis and disease. Ann Med. 2013;45:17–36. doi: 10.3109/07853890.2011.645498. [DOI] [PMC free article] [PubMed] [Google Scholar]