Abstract
Neurodegenerative diseases are attributed to impairment of the ubiquitin-proteasome system (UPS). Oxidative stress has been considered a contributing factor in the pathology of impaired UPS by promoting protein misfolding and subsequent protein aggregate formation. Increasing evidence suggests that NADPH oxidase is a likely source of excessive oxidative stress in neurodegenerative disorders. However, the mechanism of activation and its role in impaired UPS is not understood. We show that activation of NADPH oxidase in a neuroblastoma cell line (SHSY-5Y) resulted in increased oxidative and nitrosative stress, elevated cytosolic calcium, ER-stress, impaired UPS, and apoptosis. Rac1 inhibition mitigated the oxidative/nitrosative stress, prevented calcium-dependent ER-stress, and partially rescued UPS function. These findings demonstrate that Rac1 and NADPH oxidase play an important role in rotenone neurotoxicity.
Keywords: Neurodegenerative diseases, NADPH oxidase, Nox, Rotenone, Rac1, Impaired UPS
1. Introduction
The ubiquitin-proteasome system (UPS) is a dynamic cellular pathway involved in the deaggregation of misfolded proteins through proteasome degradation [1]. Defective UPS function is frequently observed in patients afflicted with neurodegenerative diseases, such as Alzheimer disease, Parkinson disease, and Huntington disease [1]. Increased oxidative stress has long been implicated in the pathogenesis of impaired UPS function by promoting protein misfolding and subsequent protein aggregate formation [1]. However, the source of ROS and the mechanisms by which ROS is elevated have not been elucidated. Maintaining the integrity of UPS function will likely prove beneficial in these neurodegenerative diseases; and therefore, understanding the mechanisms by which oxidative stress impairs UPS is very crucial to the development of treatments.
Nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase, Nox) is a plasma membrane-bound superoxide producing enzyme complex that is essential to maintaining functionality of the host defense mechanism [2]. There is an increasing body of work suggesting that activation of Nox leads to the pathogenesis of progressive neurodegeneration and chronic inflammation [2–4]. Activation of Nox leads to excess oxidative stress, resulting in aggregate formation and neuronal cell death [5–7]. Exposure to rotenone, a widely used pesticide, increases the risk to develop neurodegenerative disorders [1;8;9]. Rotenone is known to inhibit complex 1 of the mitochondrial respiratory chain and has been widely used in human neuroblastoma cells (SHSY-5Y) as an in-vitro experimental model of Parkinson’s disease [1]. More recently, rotenone has been implicated as a potential regulator of Nox activity, augmenting oxidative stress in neuronal cells and leading to neurodegeneration [3;10].
The Nox complex consists of membrane-bound subunits gp91phox and p22phox, and cytosolic subunits p47phox, p67phox, and p40phox [11]. Assembly of these multiple subunits into a membrane complex is required for enzyme activity [12]. Upon stimulation, p47phox is phosphorylated and translocates from the cytosol to the plasma membrane where it binds with the gp91phox/p22phox complex [13]. Rotenone directly binds with the catalytic subunit gp91phox; and this interaction enhances p67phox translocation from the cytosol to the membrane complex [3]. Earlier studies have demonstrated that the Rho-like GTPase, Rac1, is necessary for structural and functional activation of the Nox complex by ameliorating the gp91phox-p67phox interaction [3]. The inactive form of Rac1 remains in the cytosol as a Rac1-GDP complex [14]. Upon dissociation from its cytosolic complex, Rac1 translocates to the membrane with guanosine diphosphate (GDP) and guanine nucleotide dissociation inhibitor (GDI) [14]. The conversion of the inactive GDP-bound Rac1 to active GTP-bound Rac1, with GDP/GTP conversion, is regulated by the guanine nucleotide exchange factors (GEFs), Tiam1 and Trio, which are located on the membrane [3]. Active-Rac1 induces activation of the Nox complex by enhancing or promoting the interaction between gp91phox and p67phox [3]. Activation of Nox transfers electrons from intracellular NADPH across the plasma membrane to extracellular molecular oxygen, generating extracellular superoxide and its downstream reactive oxygen species (ROS) [15].
Excess superoxide production has also been implicated in the activation of plasma membrane calcium channels, resulting in increased intracellular calcium levels [16]. Intracellular calcium levels may also be enhanced by pathophysiologic calcium release from intracellular stores, the endoplasmic reticulum (ER) [16]. Elevated cytosolic calcium activates neuronal NO-synthase (nNOS), increasing reactive nitrogen species (RNS, *NO) production [17]. Together, reactive oxygen and nitrogen species alter cellular homeostasis and eventually elicit impaired UPS function.
As there is mounting evidence that increased oxidative stress is associated with impaired UPS function and neurodegeneration, understanding the mechanism(s) by which Nox-induced oxidative stress leads to impaired UPS function is critical as we seek therapeutic strategies for these neurodegenerative disorders. In this study, we found that rotenone increased Nox-induced oxidative stress and protein aggregate formation via impaired UPS in SHSY-5Y cells. Inhibition of Rac1 mitigated Nox-dependent oxidative stress, diminished protein aggregate formation, and partially rescued UPS function. Collectively, these findings demonstrate that Rac1 plays an important role in oxidative stress-induced impaired UPS function in SHSY-5Y cells. Targeting either Rac1 or Nox may prove to be therapeutically beneficial for treating neurodegenerative disorders.
2. Materials and methods
2.1. Redox sensitive constructs
The Nox-specific redox sensor p47-roGFP and the glutathione redox potential-specific sensor Grx1-roGFP2 were designed as previously described by our lab [18]. To generate an ER targeted- redox-sensitive-GFP (ER-roGFP), the calreticulin signal sequence MLLPVLLLGLLGAAAD was added 5’ and an ER retention sequence, KDEL, was added 3’ to the modified roGFP1 (roGFP1-iL, kind gift from Dr. James Remington [19]) using the forward primer (roERNotI)5’AAGCTTGCGGCCGCCACATGCTGCTGCCCGTCCCCCTGCTGCTGGGCCTGCTGGGCGCCGCCGCCGACAGTAAAGGAGAAGAACTTTTC 3’ and reverse primer (roERXbaI)5’ACTCGATCTAGATTACAGCTCGTCCTTTTTGTATAGTTCATCCATGCC3’, and fused to the pCDNA3 vector
2.2. Reagents and antibodies
Rotenone (RT), antimycin-A, pegulated-catalase (PEG-cat) and catalase (Cat) were purchased from Sigma-Aldrich. Rac1 inhibitor (Rac1 (−), C24H35N7.3HCl) was from TOCRIS bioscience. Fura-2/AM, Fluo-4/AM, DAF-FM, Amplex-red, mitoSOX and DCFH-DA (6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate) were from Invitrogen. Phosphate buffered saline (PBS) was from GIBCO. The Nox-specific peptide inhibitor gp91 ds was from Biosynthesis, Lewisville, TX. Anti-Ubiquitin was from Dako, anti-poly (ADP-ribose) polymerase (PARP) was from Cell Signaling Technology, anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was from Millipore, and anti-PDI (protein disulfide isomerase) was from Abcam.
2.3. Cell culture and treatment
SHSY-5Y cell line is used as a model of in vitro neurodegenerative disease studies because this cell line has biochemical properties of human neurons in vivo. Moreover, since these cells are tumor derived, they can continuously grow and divide [20]. The cells were differentiated to provide mature neuron-like phenotype [21]. SHSY-5Y cells (ATCC® CRL2266™) were grown in DMEM-F12 (1:1, Gibco) supplemented with 10% heat inactivated fetal bovine serum (FBS, Atlanta Biologicals), 2 mM L-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin (Gibco). Cells were seeded on 96-well plates (Costar) at a density of 12000 cells/well. Cells were treated with rotenone (500 nM, 24 h), Rac1 inhibitor (50 µM, 1 h), gp91 ds (5 µM, 1 h), and catalase (5 µM, 1 h) as indicated.
2.4. Live cell imaging
Cells were seeded (2000/well) on 96-well plates for either intracellular (DCFH-DA) or extracellular (Amplex-red H2O2 assay) ROS measurements as suggested by the manufacturer’s protocol. For assessment of changes in the glutathione redox potential or ER redox potential cells were transiently transfected with either Grx1-roGFP2 or ER-roGFP respectively. For assessment of Nox activity cells were transfected with our Nox specific redox biosensor p47-roGFP [18]. Transfection was done using X-tremeGENE HP DNA Transfection Reagent (Roche) at a ratio of 3:1 (Reagent to DNA). Changes in the redox sensors were assessed as we have previously described [18].
2.6. Assessment of calcium influx by Mn2+ quench
Cells were seeded (2000 cells/well) in 96-well plates for calcium influx measurements. Calcium influx was assessed by a well-established Mn2+ quench of Fura-2 fluorescence [22]. Due to the negligible concentration of intracellular Mn2+ and that Mn2+ permeates through calcium channels, the rate of quench of Fura-2 fluorescence was assessed in the presence of extracellular MnCl2 as a measure of plasma membrane calcium permeability. Rotenone treated or untreated cells were washed with Ringer solution containing in m M: 146 NaCl, 4.7 KCl, 1.8 CaCl2, 0.6 MgSO4, 1.6 NaHCO3, 0.13 NaH2PO4, 7.8 Glucose and 20 HEPES. Cells were loaded with 5 µM Fura-2 and incubated for 30 minutes at room temperature. Cells were then washed with Ringer solution and incubated for 20 minutes at room temperature to allow for dye de-esterification. Fluorescence signals were monitored at the calcium-isosbestic (360 nm) and the calcium-free excitation (380 nm) wavelengths in either normal Ringer (0–120 s) or Ringer in which CaCl2 was replaced by MnCl2 (1.8 mM), The rate of Mn2+ quench of the 360 nm signal was calculated over the last 60 s.
2.7. Intracellular calcium measurements and RNS measurement
Cells were seeded (12000 /well) in a 96-well plate in DMEM for intracellular calcium measurements. Cells were washed with PBS followed by incubation with the fluorescent calcium indicator Fluo- 4/AM (5 µM) for 30 minutes at room temperature. Cells were washed with PBS and the dye allowed to de-esterify for 20 minutes at room temperature prior to microscopy. Fluorescent images were captured with a Zeiss LSM 5 Live inverted confocal microscope equipped with a 40×, 1.2 NA water immersion objective. Excitation was at 488 nm and emission was captured with a linear detector after passing through a 495–555 nm band pass filter. Intensity values were calculated in ImageJ by outlining cells and calculating average basal Fluo-4 fluorescence. The single cell data was background subtracted and the fluorescence of all cells (25 cells) from a single frame were averaged. RNS was measured using DAF-FM dye. Excitation was at 488 nm and emission was captured with a linear detector after passing through a 495–555 nm band pass filter.
2.8. Mitochondrial ROS measurement
We investigated mitochondrial ROS in SHSY-5Y cells using mitoSOX. Cells treated with rotenone or antimycin-A (mitochondrial complex-III chain inhibitor) for 24 h. For measurements of ROS derived from the mitochondria, SHSY-5Y cells were loaded with 5 µm mitoSOX for 10 min at 37° C. Cells were excited at 488 nm and the emitted fluorescence was recoded at 560–615 nm.
2.9. Immunoblotting
Cells were seeded onto 60-mm Petri dishes and incubated overnight to promote adherence. Cells were treated with rotenone (500 nM) in the presence or absence of Rac1 inhibitor (50 µM). Cells were harvested and lysed in RIPA buffer (50 mM Tris-HCl, ph 7.4, 1% NP40, 0.5% Na-deoxycholate, 0.1% SDS, 150 mM NaCl, 2 mM EDTA, and 50 mM NaF) including a cocktail of protease (Roche) and phosphatase (SIGMA) inhibitors. Immunoblotting was carried out with soluble and insoluble protein mixture as previously described [23]. Densitometric analysis was done using ImageJ software.
2.9. Data Analysis
Data are reported as mean ± SEM, unless otherwise specified. Statistical differences between groups were determined using ANOVA with Tukey’s post-hoc test. Statistical analysis was performed in Origin Pro (OriginLab Corporation, Northhampton, MA) with a significance level of *p < 0.05 and **p<0.01.
3. Results
3.1. Inhibition of Rac1 decreases rotenone-induced ROS production
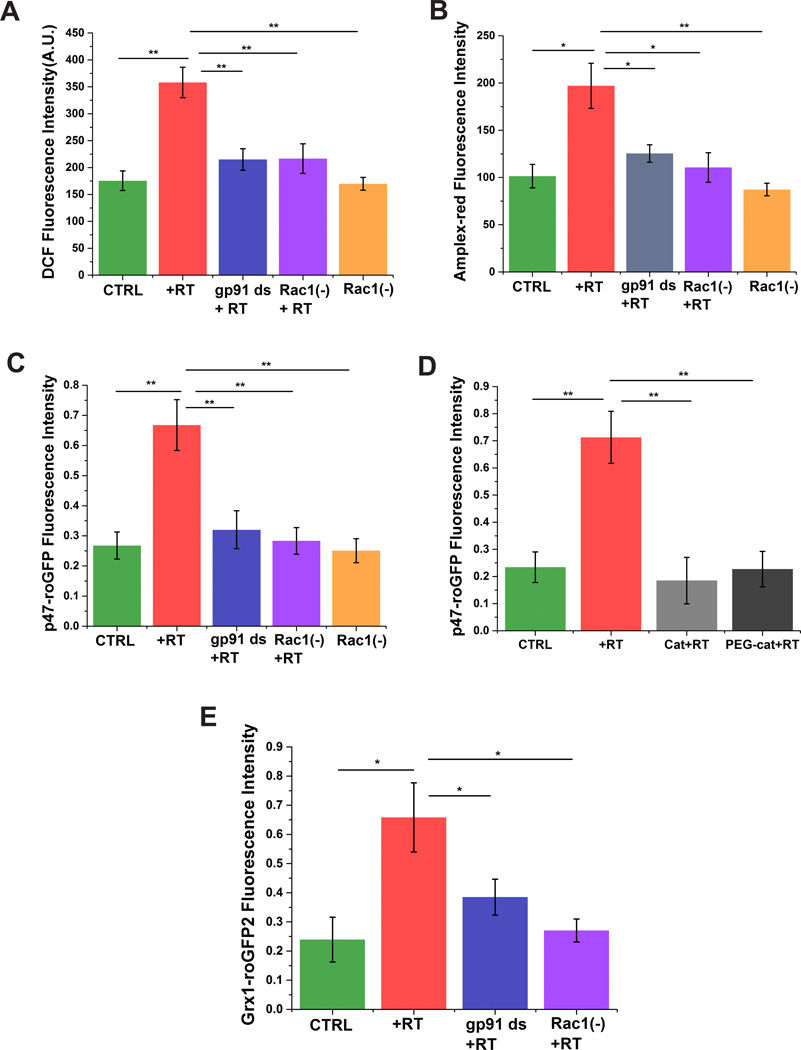
To investigate whether rotenone induces ROS production via the Rac1-Nox axis, we measured intra- and extra-cellular ROS using SHSY-5Y cells upon rotenone administration as a model system. General intracellular ROS was measured by assessment of changes in DCF fluorescence (Fig. 1A). SHSY-5Y cells treated with rotenone showed a ~2 fold increase in DCF fluorescence compared to untreated cells. Preincubation with the Rac1 inhibitor or the Nox-specific inhibitor gp91 ds significantly inhibited the rotenone induced ROS production (Fig. 1A), which were not significantly different from controls. The Rac1 inhibitor alone had no effect on intracellular ROS production, indicating minimal Nox formation under basal conditions. Production of extracellular ROS was assessed using the peroxidase-based assay for H2O2 by monitoring the oxidation of membrane impermeable Amplex-red. Rotenone increased the oxidation of Amplex-red ~1.5 fold compared to untreated cells (Fig. 1B). Cells preincubated with Rac1 inhibitor and gp91 ds showed marked decreases in oxidation of Amplex-red (Fig. 1B), which were not significantly different from controls. Addition of extracellular catalase prevented the oxidation of Amplex-red upon rotenone exposure (data not shown), suggesting that the Nox complex produces ROS into the extracellular space, which diffuses across the plasma membrane to promote intracellular oxidative stress. Our results indicate that Nox is a major source of ROS production and that Rac1 plays an important role in activating this enzyme complex in response to rotenone.
Figure 1. Rac1-inhibition decreases oxidative stress in SHSY-5Y cells.
Rotenone increases overall intracellular (A) and extracellular (B) ROS, which is attenuated by inhibition of Nox activity (gp91 ds) or Rac1 (Rac1(−))Rotenone enhances Nox-specific ROS production, which is prevented by inhibition of Nox activity with gp91 ds or a Rac1 inhibitor (C), and catalase (D). Rotenone induces oxidation of cellular glutathione, which is prevented by inhibition of either Nox activity or Rac1(E). Data are representative of three replicates. *p < 0.05 and **p<0.01.
3.2. Role of Rac1 in rotenone-induced Nox-specific ROS production and alterations in the glutathione redox potential
Nox-specific ROS has recently gained attention as a major source of ROS in the pathology of several neuronal dysfunctions [12, 14]. Nox-specific ROS production was assessed using the p47-roGFP redox biosensor in which redox sensitive GFP is linked to p47phox [18]. Previous reports have shown that 500 nM rotenone is able to induce a significant amount of apoptosis, by inducing activation of morphological markers of apoptosis, after 24 h of treatment [24]. We assessed the dose and time-dependent effect of rotenone on Nox-specific ROS generation (Supplementary Figure 1). Rotenone at 300 and 500 nM significantly increased the oxidation of p47-roGFP after 24 h. No significant difference was observed between 24 h and 48 h rotenone treatment. Using higher concentrations of rotenone (>500 nM) induced high levels of cytotoxicity (>40%), data not shown. Therefore, we chose 500 nM rotenone for 24 hours in our studies to mimic an acute model of neurodegeneration.
Rotenone treated cells showed a 2.5 fold higher Nox-specific ROS generation than untreated cells (Fig. 1C), suggesting that rotenone activates Nox. Cells preincubated with the Nox-specific inhibitor, gp91 ds, prior to rotenone treatment showed marked decrease in ROS generation (Fig. 1C) back to controls levels. Inhibition of Rac1 significantly mitigated p47-roGFP oxidation (Fig. 1C), suggesting that Rac1 plays an important role in Nox complex formation and activation. Application of extracellular catalase or PEG-catalase completely abolished the rotentone induced oxidation of p47-roGFP (Fig. 1D), providing further evidence that Nox produces superoxide into the extracellular space, which likely dismutes into H2O2, and H2O2 penetrates into the intracellular space to promote oxidative stress. As cellular oxidative stress leads to alterations in the glutathione redox potential we used Grx1-roGFP2, a translational fusion between glutaredoxin-1(Grx1) and roGFP2, to monitor the glutathione redox potential [25;26]. Rotenone treatment shifted the glutathione redox potential of SHSY-5Y cells to a more oxidized state (3.5–4 fold) compared to untreated cells (Fig. 1E). Inhibition of either Nox or Rac1 decreased the rotenone-induced oxidation of glutathione back to control levels (Fig. 1E).
3.3. Inhibition of Rac1 decreases Nox-mediated calcium influx and alterations of intracellular calcium
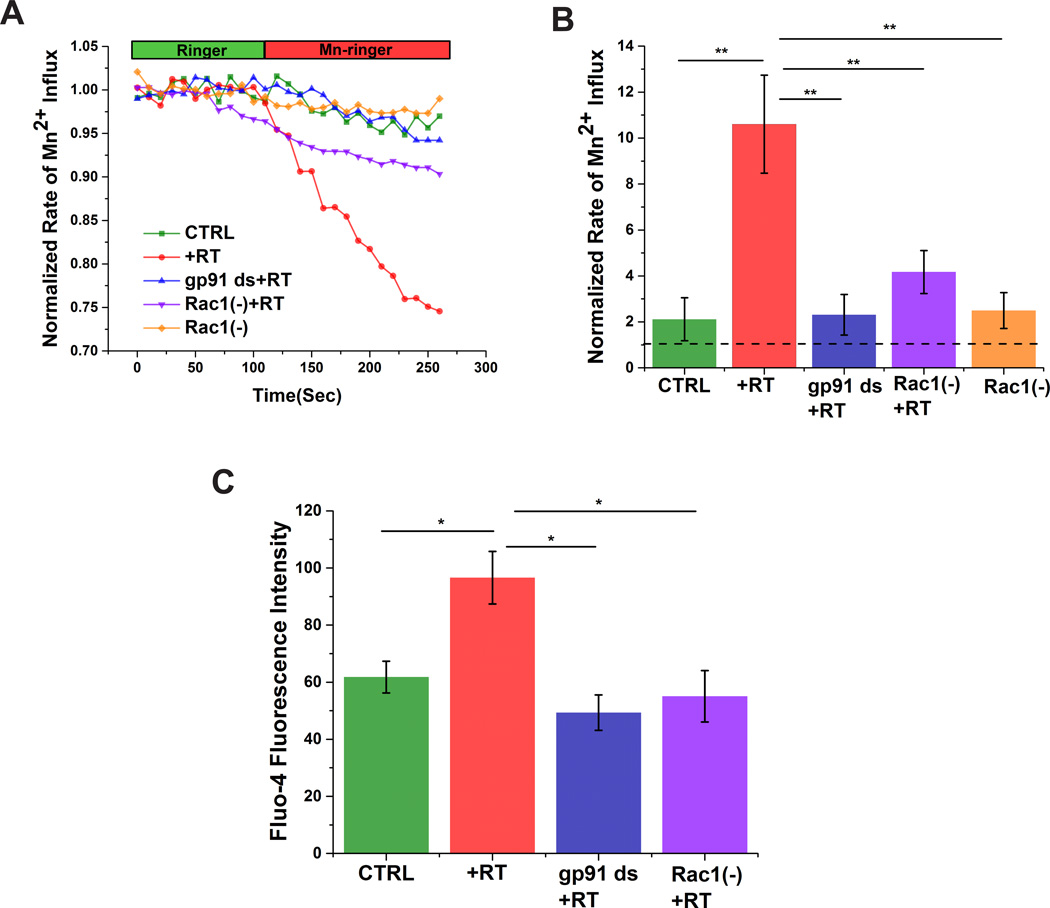
To investigate the relationship between altered Nox function and calcium homeostasis in our model system we monitored intracellular calcium influx under different conditions. Plasma membrane calcium influx was measured by analyzing the quench of Fura-2 fluorescence upon addition of extracellular Mn2+ (Fig. 2) [27]. Rotenone treatment enhanced membrane calcium permeability, which was prevented by preincubation with the Nox-specific inhibitor gp91 ds (Fig. 2A&B). In addition, rotenone-induced calcium influx was significantly reduced upon Rac1 inhibition (Fig. 2A&B). We also assessed changes in intracellular calcium using Fluo-4 AM [28–30] Rotenone increased Fluo-4 fluorescence, which was prevented by inhibition of either Nox or Rac1 (Fig. 2C).Taken together, our data strongly support a role for Rac1-dependent activation of Nox in the alterations of intracellular calcium homeostasis.
Figure 2. Rac1-inhibition mitigates Nox-meadiated calcium influx and elevated intracellular calcium in SHSY-5Y cells.
Rotenone induces Nox-dependent plasma membrane calcium influx (A&B) and subsequent increases in intracellular calcium (C) in SHSY-5Y cells, which is prevented by inhibition of Nox activity with gp91 ds or a Rac1 inhibitor. Dashed line in B indicates normalized value of calcium influx prior addition of extracellular Mn2+. Data are representative of four replicates. *p < 0.05 and **p<0.01.
3.4. Inhibition of Rac1 attenuates reactive nitrogen species production
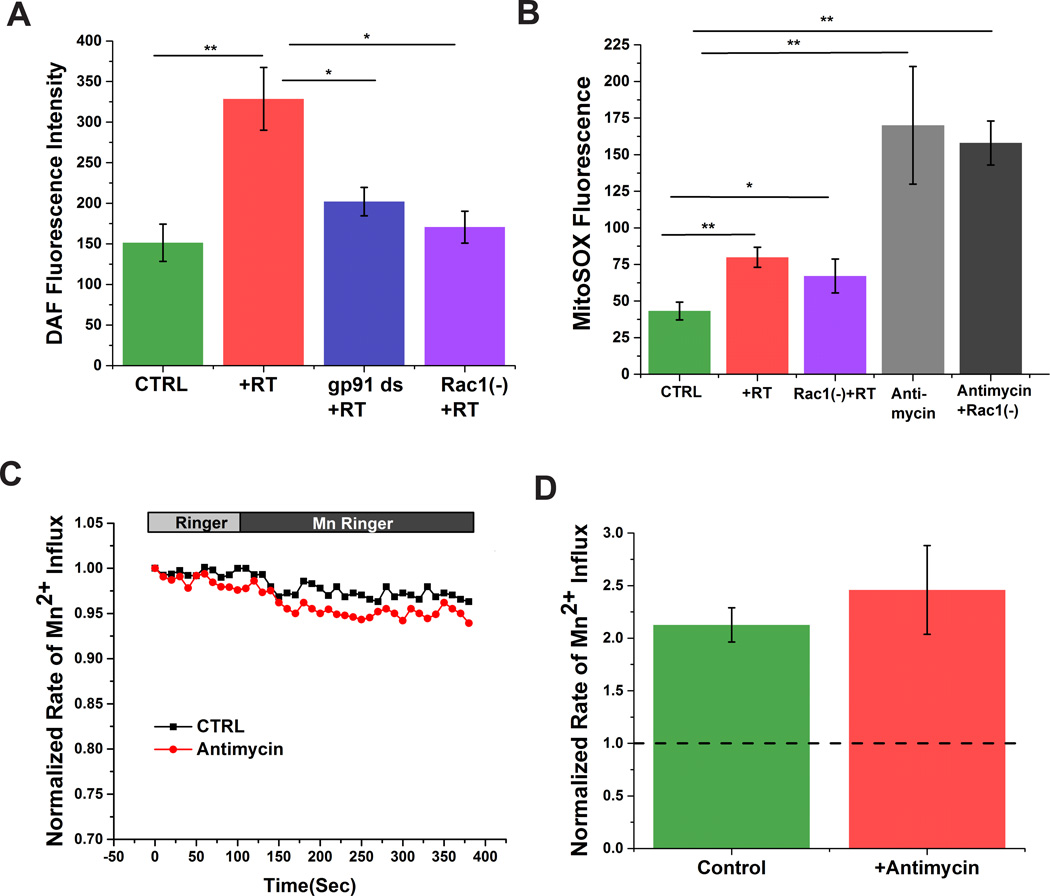
Earlier studies have established a link between increased intracellular calcium and excess RNS production, which can lead to oxidative stress [1;5]. Therefore, we wanted to know whether the Rac1/Nox-dependent increase in intracellular calcium could lead to RNS production. We measured *NO with DAF-FM. Rotenone treatment increased DAF fluorescence, and therefore *NO production, which was markedly attenuated by inhibition of Nox activity or Rac1 (Fig. 3A).
Figure 3. Rac1-inhibition attenuates reactive nitrogen species (RNS) production in SHSY-5Y cells.
Rotenone increases RNS production in SHSY-5Y cells, which is mitigated by inhibition of Nox activity with gp91 ds or a Rac1 inhibitor (A). Rotenone stimulated mitochondrial ROS production, which is not inhibited by a Rac1 inhibitor (B). Production of mitochondrial ROS by antimysin-A does not induce calcium influx in SHSY-5y cells (C&D)Dashed line in D indicates normalized value of calcium influx prior addition of extracellular Mn2+ Data are representative of three replicates. *p < 0.05 and **p<0.01.
3.5. Mitochondrial-ROS does not alter plasma membrane-calcium influx in SHSY-5Y cells
Rotenone is a well-known mitochondrial complex I chain inhibitor. While our data strongly support a role for rotenone in activating Nox, we cannot rule out a contribution of rotenone-induced oxidative stress via the mitochondria. Rotenone (500 nM) or antimycin-A (5 µM) increased mitochondrial-ROS ~1.8 fold and ~4.0 fold, respectively (Fig. 3B, Supplementary Figure 2). Cells preincubated with Rac1 inhibitor did not show any significant alteration in mitochondrial-ROS generation compared to RT treated cells (Fig. 3B). Since ROS is involved in activation of plasma membrane calcium influx and increased calcium is associated with RNS production, we investigated whether mitochondrial-ROS could increase sarcolemmal calcium influx (Fig. 3C&D). Cells treated with antimycin-A, which resulted in the greatest change in MitoSOX, did not show any significant difference in calcium influx compared to untreated cells, suggesting that ROS derived specifically from Nox increased sarcolemmal calcium influx and subsequently RNS production.
3.6. Inhibition of Rac1 rescues cells from rotenone-mediated ER stress
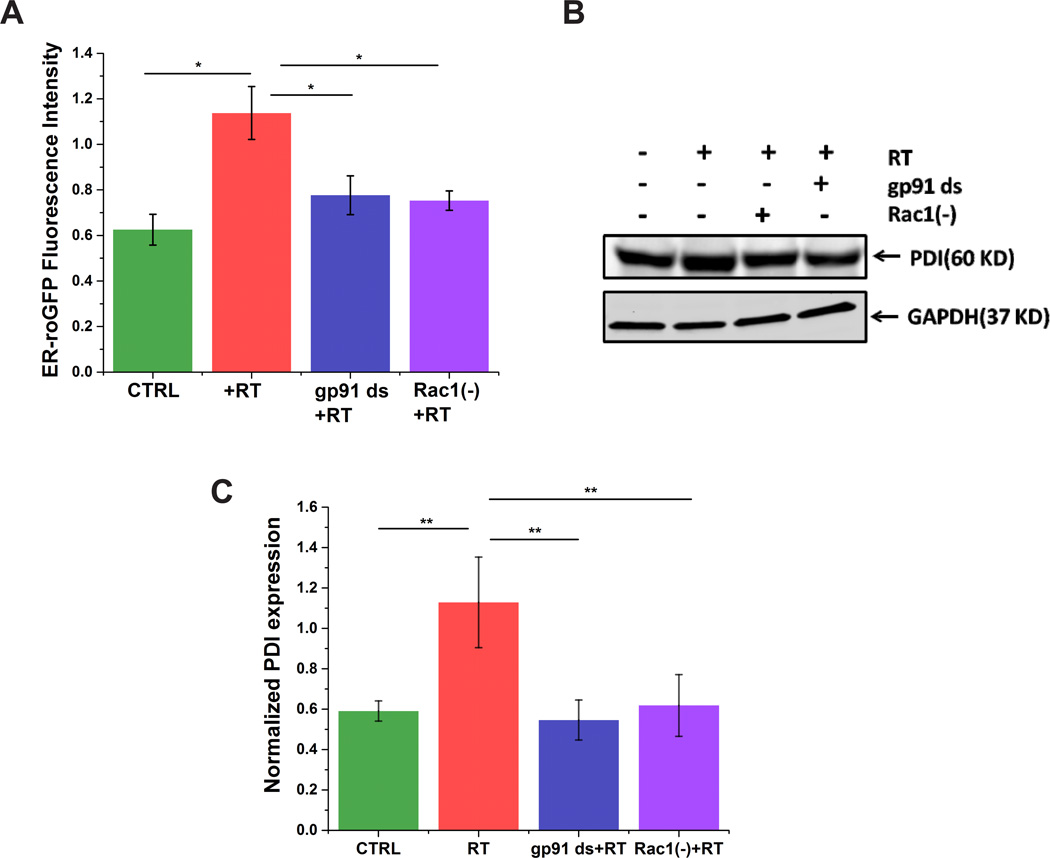
ROS and RNS together exacerbate oxidative stress, which stimulates ER-stress. Increased intracellular calcium can activate ER calcium release, leading to ER calcium depletion and ER stress [27]. To investigate whether rotenone induces ER stress via the Rac1-Nox axis, we measured oxidative stress within the ER by assessing redox changes with an ER targeted redox biosensor (ER-roGFP) in our model system (Fig. 4A). Cells transfected with ER-roGFP showed a significant increase in oxidation upon rotenone treatment, which was prevented by preincubation with either gp91 ds or Rac1 inhibitor (Fig. 4A). Earlier studies have established that ER stress initiates an unfolded protein response (UPR); which is characterized by a rapid increase in the expression of PDI, an UPR sensors and marker of ER stress [31;32]. Therefore, we investigated the effect of rotenone on PDI expression (Fig. 4B&C). Cells treated with rotenone showed a marked increase in PDI expression compared to untreated cells, which was significantly mitigated by preincubation with either gp91 ds or Rac1 inhibitor. These results suggest that Nox is capable of inducing ER-stress and that Rac1 plays an important role in rotenone-induced oxidative stress in the ER of SHSY-5Y cells.
Figure 4. Rac1 plays a major role in Nox-induced ER stress in SHSY-5Y cells.
Rotenone induces ER stress as assessed by the oxidation of the ER targeted redox probe ER-roGFP (A) or by immunoblotting with anti-PDI antibody (B&C). Inhibition of Nox activity or Rac1 prevents ER stress. Data are representative of three independent experiments. *p < 0.05 and **p<0.01.
3.7. Inhibition of Rac1 prevents rotenone-induced protein aggregation and apoptotic cell death
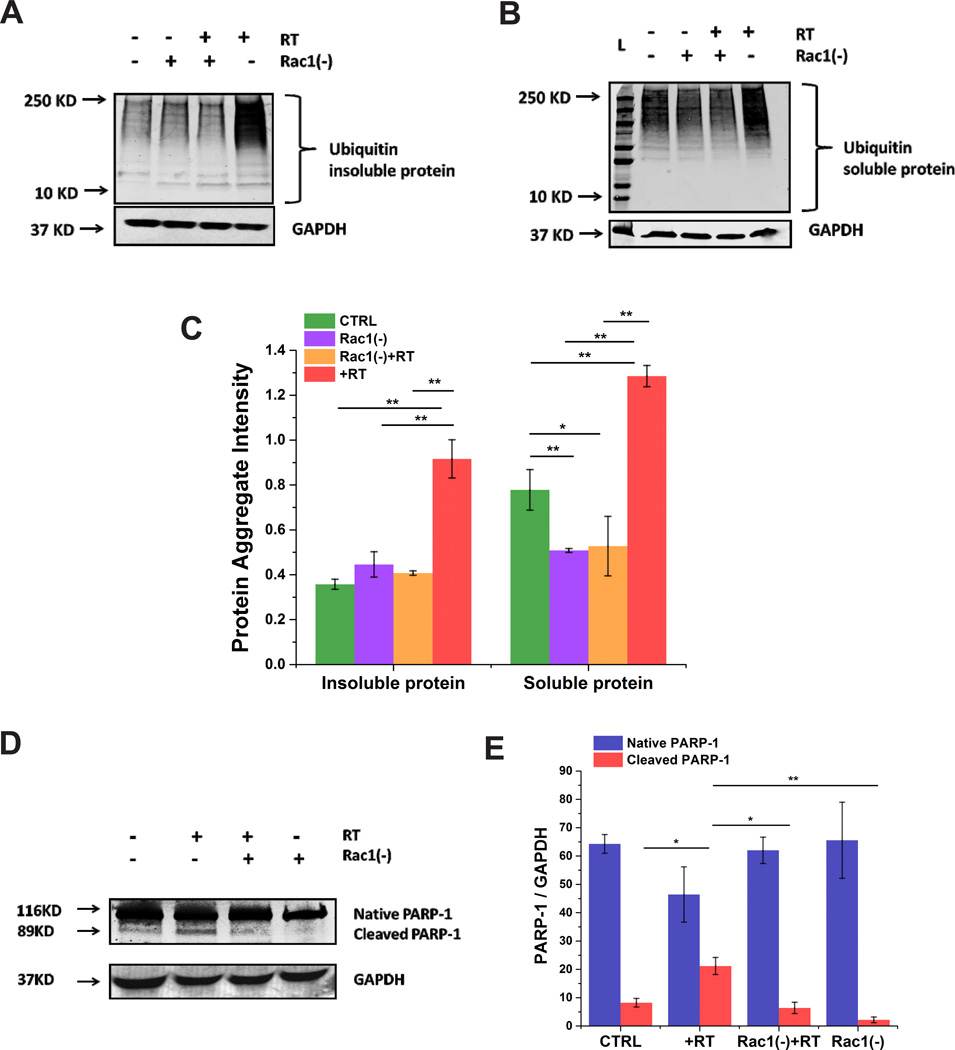
Oxidative/nitrosative stress can lead to misfolded protein aggregation [33]. Therefore, we were interested whether rotenone induced Nox mediated oxidative stress could lead to impaired UPS. Using an anti-ubiquitin antibody we found that rotenone treated cells showed accumulation of ubiquitinated protein in both the soluble and insoluble protein fractions (Fig. 5A–C). However, cells preincubated with Rac1 inhibitor prior to rotenone treatment showed a marked decrease in accumulation of high molecular weight bands in both fractions (Fig. 5A–C). We found that rotenone also induced apoptosis as evidenced by an increase in cleaved PARP, which was attenuated upon inhibition of Rac1 (Fig. 5D&E). Cells treated with Rac1 inhibitor itself showed no significant increase in protein aggregation or cleaved PARP-1. Taken together, our results indicate that Nox-specific oxidative stress plays a major role in impaired UPS in SHSY-5Y cells and that Rac1 plays an important role in maintaining the homeostasis of the UPS.
Figure 5. Rac1 plays a major role in Nox-induced misfolded protein aggregation and apoptotic cell death in SHSY-5Y cells.
Rotenone induces misfolded protein aggregation in the insoluble (A&C) and soluble (B&C) protein fractions as measured by accumulation of ubiquitinated proteins. Rotenone also increases cleaved PARP-1, indicating increased apoptosis (D&E). Both misfolded protein aggregation and apoptosis was prevented by inhibition of Rac1. Data are representative of three independent experiments. *p < 0.05 and **p<0.01.
4. Discussion
Oxidative stress and impaired UPS are the two most common features frequently observed in neurodegenerative diseases [1]. Activation of Nox complex is attributed as a major source of oxidative and inflammatory responses in neurodegeneration [5–7]. Previously Zhou et al. demonstrated that rotenone activates Nox by promoting a stronger interaction between the membrane subunit gp91phox and the cytosolic subunit p67phox [3]. However, the mechanism of action of Nox-derived ROS in the pathology of impaired UPS is still unclear. Therefore, understanding the interface between the onset of Nox-mediated ROS generation and impaired UPS is important for therapeutic aspects of neurodegenerative diseases. In this study we have shown that rotenone-induced activation of Nox increases calcium influx, enhances calcium-dependent ER-stress and ultimately leads to impaired UPS and apoptotic cell death. Furthermore, we have also demonstrated that inhibition of Rac1, a key regulatory factor in the formation of the Nox complex, mitigates Nox-mediated increases in intracellular calcium, prevents calcium-dependent ER-stress and rescues the homeostasis of UPS function.
Consistent with a previous report [3], we found that rotenone leads to activation of Nox and ROS generation. Inhibition of Rac1 prevents the excess ROS generation by Nox and stabilizes the homeostasis of cellular glutathione redox balance, which is imperative for proper protein folding. Our data suggest that Rac1 is a key regulator of Nox-dependent ROS production and that targeting either Rac1 or Nox may prove beneficial in neurodegenerative diseases.
Previous studies have suggested that Nox-specific superoxide production enhances plasma membrane calcium permeability [34;35]. We found that activation of Nox increased calcium influx and intracellular calcium levels, both of which were significantly blunted upon Rac1 inhibition. Alterations in intracellular calcium homeostasis can lead to excessive RNS generation via nNOS-activation (for review see [17]). Our data suggest that rotenone-induced activation of Nox leads to increased *NO production, which was markedly attenuated upon inhibition of Rac1. While the mechanisms by which Nox induces increased calcium influx and RNS are still under investigation, our findings indicate that Rac1 is a key regulatory factor in excessive RNS production.
The DCFH assay and DAF-FM have been widely used to monitor ROS and RNS production, respectively. DCF is non-specific and can be oxidized by a number of reactive oxygen and nitrogen species and is also susceptible to light induced oxidation [36]. While DAF is more specific to RNS, it can be oxidized by peroxidases in the presence of H2O2 [37]. To overcome some of these limitations we have used genetically encoded redox sensitive green fluorescent protein [38]to monitor subcellular redox potential from NADPH oxidase using p47-roGFP [18], within the ER using our newly constructed ER-roGFP, or the GSH/GSSG redox pair using Grx1-roGFP2 [25]. While we are making great strides in the development of genetic probes to measure ROS, there is still a need for more selective indicators with subcellular specificity for RNS.
Excessive ROS production is a major mediator of ER-stress among numerous pathological conditions [39]. ER-stress response is afflicted with an adaptive signaling cascade known as the unfolded protein response (UPR) [39]. Excess ROS generation mediates unfolded or misfolded protein aggregation, which stimulates the prolonged UPR [39]. ER-stress-mediated prolonged UPR leads to general translational attenuation and hindrance in ER-associated degradation of unfolded or misfolded proteins [39;40]. This phenomenon promotes accumulation of high molecular ubiquitinated proteins and impaired UPS [40]. PDI is a chaperone protein that remains in the ER-lumen and assists in the maturation and transportation of unfolded protein via thiol disulfide exchange. Previous work has shown that rotenone can lead to S-nitrosylation of PDI (catalytically inactive form), initiating misfolded protein aggregation [1]. We found that inhibition of the Rac1-Nox axis abrogated both oxidation within the ER and increased PDI expression, strongly supporting a role for Nox-dependent ROS in UPS.
Disruption of cellular GSH status can trigger UPS and apoptosis (for review see[41]). In addition, Shamoto-Nagai et al have shown that prolonged exposure (>48 hours) of SHSY-5Y cells to rotenone leads to apoptosis due to inactivation of the proteasome as a result of oxidative/nitrosative modifications [33]. We found that rotenone shifted the GSH redox status to a more oxidized state, impaired UPS and induced apoptosis. Oxidative inactivation of the proteasome would be consistent with our findings. Intriguingly, we found that inhibition of Rac1 did not prevent mitochondrial ROS generation but it did prevent the shift in GSH redox balance, significantly decrease ER-stress, mitigate accumulation of ubiquitinated proteins, and prevent the impaired UPS-mediated apoptotic cell death. Together these results suggest that Rac1 plays a major role in impaired UPS by facilitating the formation and activation of the NADPH oxidase complex. Future studies investigating whether Nox-dependent ROS can lead to inactivation of the proteasome are warranted.
In summary, using a cell model of neurodegenerative disease we demonstrate that Rac1 is an important regulatory factor which promotes activation of Nox. Activation of Nox induces excessive ROS generation, which influences signaling cascades such as calcium and prolonged UPR, resulting in impaired UPS. Inhibition of Rac1 can rescue UPS function and prevent apoptotic cell death. Our findings suggest that Rac1 could be a potential therapeutic target for common neurodegenerative diseases.
Supplementary Material
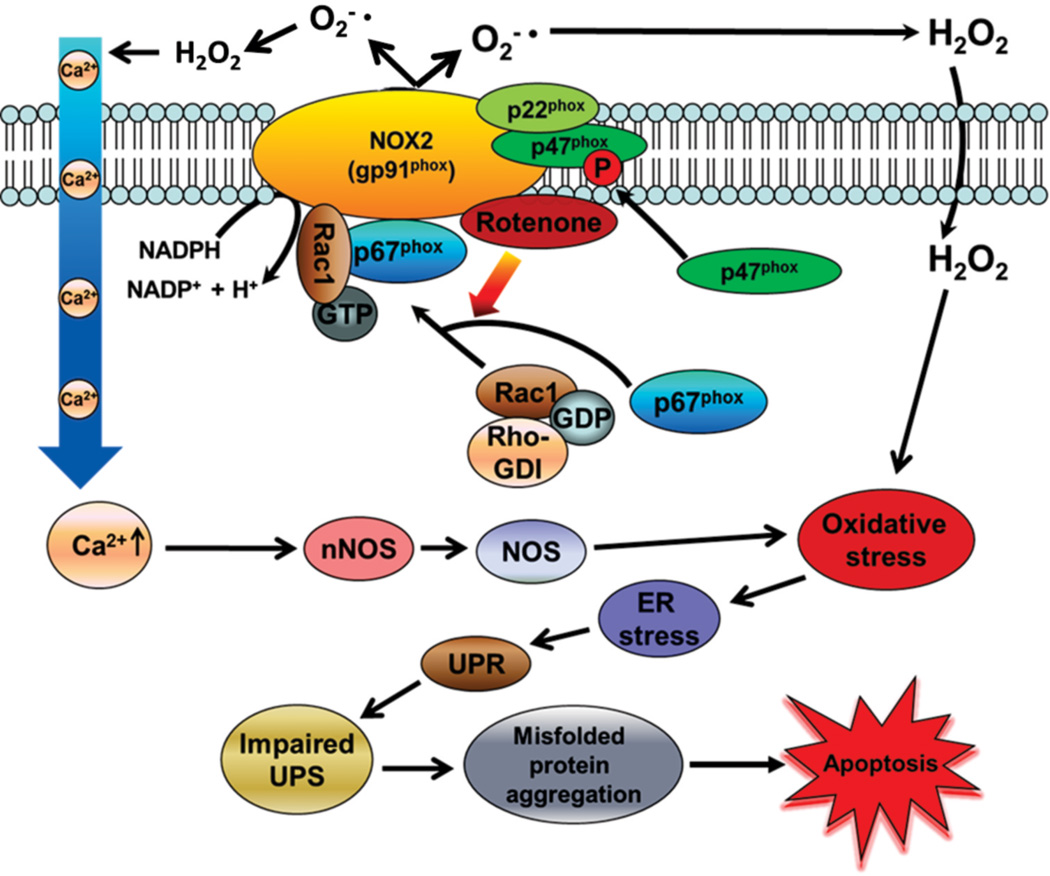
Figure 6. Proposed mechanism for the role of the small GTPase Rac1 in Nox-mediated oxidative stress and neuronal cell death via impaired UPS function.
The environmental pesticide rotenone induces Rac1-dependent activation of Nox complex, which increases oxidative stress via excess ROS and RNS production. Increased oxidative stress mediates misfolded protein aggregation, ER stress, impaired UPS and subsequent cell death.
Acknowledgement
We thank James A. Loehr for critically reading the manuscript. This study was supported in part by a grant from The National Institutes of Health, R01 AR061370, to G.G.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 2.Gao HM, Zhou H, Hong JS. NADPH oxidases: novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol. Sci. 2012;33:295–303. doi: 10.1016/j.tips.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou H, Zhang F, Chen Sh, Zhang D, Wilson B, Hong Js, Gao HM. Rotenone activates phagocyte NADPH oxidase by binding to its membrane subunit gp91phox. Free Radical Biology and Medicine. 2012;52:303–313. doi: 10.1016/j.freeradbiomed.2011.10.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajayi A, Yu X, Strom AL. The role of NADPH oxidase (NOX) enzymes in neurodegenerative disease. Front. Biol. 2013;8:175–188. [Google Scholar]
- 5.Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat. Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin. Invest. 2007;117:910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang QG, Laird MD, Han D, Nguyen K, Scott E, Dong Y, Dhandapani KM, Brann DW. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS ONE. 2012;7:e34504. doi: 10.1371/journal.pone.0034504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HJ, Khoshaghideh F, Patel S, Lee SJ. Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci. 2004;24:1888–1896. doi: 10.1523/JNEUROSCI.3809-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pal R, Miranda M, Narayan M. Nitrosative stress-induced Parkinsonian Lewy-like aggregates prevented through polyphenolic phytochemical analog intervention. Biochem Biophys. Res. Commun. 2011;404:324–329. doi: 10.1016/j.bbrc.2010.11.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 11.Li JM, Shah AM. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J Biol. Chem. 2003;278:12094–12100. doi: 10.1074/jbc.M209793200. [DOI] [PubMed] [Google Scholar]
- 12.Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu GY, Quinn MT, Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol. Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taura M, Miyano K, Minakami R, Kamakura S, Takeya R, Sumimoto H. A region N-terminal to the tandem SH3 domain of p47phox plays a crucial role in the activation of the phagocyte NADPH oxidase. Biochem J. 2009;419:329–338. doi: 10.1042/BJ20082028. [DOI] [PubMed] [Google Scholar]
- 14.Raz L, Zhang QG, Zhou CF, Han D, Gulati P, Yang LC, Yang F, Wang RM, Brann DW. Role of Rac1 GTPase in NADPH oxidase activation and cognitive impairment following cerebral ischemia in the rat. PLoS ONE. 2010;5:e12606. doi: 10.1371/journal.pone.0012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher AB. Redox signaling across cell membranes. Antioxid. Redox. Signal. 2009;11:1349–1356. doi: 10.1089/ars.2008.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X, Wen K, Yuan D, Ai L, He P. Calcium influx-dependent differential actions of superoxide and hydrogen peroxide on microvessel permeability. Am. J Physiol Heart Circ. Physiol. 2009;296:H1096–H1107. doi: 10.1152/ajpheart.01037.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura T, Lipton SA. Preventing Ca2+-mediated nitrosative stress in neurodegenerative diseases: possible pharmacological strategies. Cell Calcium. 2010;47:190–197. doi: 10.1016/j.ceca.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal R, Basu Thakur P, Li S, Minard C, Rodney GG. Real-Time Imaging of NADPH Oxidase Activity in Living Cells Using a Novel Fluorescent Protein Reporter. PLoS ONE. 2013;8:e63989. doi: 10.1371/journal.pone.0063989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohman JR, Remington SJ. Development of a Family of Redox-Sensitive Green Fluorescent Protein Indicators for Use in Relatively Oxidizing Subcellular Environments. Biochemistry. 2008;47:8678–8688. doi: 10.1021/bi800498g. [DOI] [PubMed] [Google Scholar]
- 20.Constantinescu R, Constantinescu AT, Reichmann H, Janetzky B. Neuronal differentiation and long-term culture of the human neuroblastoma line SH-SY5Y. J. Neural Transm. Suppl. 2007:17–28. doi: 10.1007/978-3-211-73574-9_3. [DOI] [PubMed] [Google Scholar]
- 21.Kovalevich J, Langford D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 2013;1078:9–21. doi: 10.1007/978-1-62703-640-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marrelli SP, O'neil RG, Brown RC, Bryan RM., Jr PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am. J Physiol Heart Circ. Physiol. 2007;292:H1390–H1397. doi: 10.1152/ajpheart.01006.2006. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Li X, Xie W, Jankovic J, Le W, Pan T. Neuroprotection of deferoxamine on rotenone-induced injury via accumulation of HIF-1 alpha and induction of autophagy in SH-SY5Y cells. Neurochem. Int. 2010;57:198–205. doi: 10.1016/j.neuint.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Qin ZH, Leng Y, Wang Y, Jin X, Chase TN, Bennett MC. Prostaglandin A1 inhibits rotenone-induced apoptosis in SH-SY5Y cells. J. Neurochem. 2002;83:1094–1102. doi: 10.1046/j.1471-4159.2002.01224.x. [DOI] [PubMed] [Google Scholar]
- 25.Gutscher M, Pauleau AL, Marty L, Brach T, Wabnitz GH, Samstag Y, Meyer AJ, Dick TP. Real-time imaging of the intracellular glutathione redox potential. Nat. Med. 2008;5:553–559. doi: 10.1038/nmeth.1212. [DOI] [PubMed] [Google Scholar]
- 26.Albrecht SC, Sobotta MC, Bausewein D, Aller I, Hell R, Dick TP, Meyer AJ. Redesign of Genetically Encoded Biosensors for Monitoring Mitochondrial Redox Status in a Broad Range of Model Eukaryotes. J Biomol. Screen. 2013 doi: 10.1177/1087057113499634. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Berkels R, Dachs C, Roesen R, Klaus W. Simultaneous measurement of intracellular Ca(2+) and nitric oxide: a new method. Cell Calcium. 2000;27:281–286. doi: 10.1054/ceca.2000.0119. [DOI] [PubMed] [Google Scholar]
- 28.Rodney GG, Wilson GM, Schneider MF. A calmodulin binding domain of RyR increases activation of spontaneous Ca2+ sparks in frog skeletal muscle. J Biol Chem. 2005;280:11713–11722. doi: 10.1074/jbc.M408189200. [DOI] [PubMed] [Google Scholar]
- 29.Rodney GG, Schneider MF. Calmodulin Modulates Initiation but Not Termination of Spontaneous Ca2+ Sparks in Frog Skeletal Muscle. Biophys. J. 2003;85:921. doi: 10.1016/S0006-3495(03)74531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodney GG. Calmodulin in Adult Mammalian Skeletal Muscle: Localization and Effect on Sarcoplasmic Reticulum Ca2+ Release. Am J Physiol Cell Physiol. 2008;294:C1288–C1297. doi: 10.1152/ajpcell.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas CG, Spyrou G. ERdj5 sensitizes neuroblastoma cells to endoplasmic reticulum stress-induced apoptosis. J. Biol. Chem. 2009;284:6282–6290. doi: 10.1074/jbc.M806189200. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Lee SY, Neely E, Nandar W, Moyo M, Simmons Z, Connor JR. Mutant HFE H63D protein is associated with prolonged endoplasmic reticulum stress and increased neuronal vulnerability. J. Biol. Chem. 2011;286:13161–13170. doi: 10.1074/jbc.M110.170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shamoto-Nagai M, Maruyama W, Kato Y, Isobe K, Tanaka M, Naoi M, Osawa T. An inhibitor of mitochondrial complex I, rotenone, inactivates proteasome by oxidative modification and induces aggregation of oxidized proteins in SH-SY5Y cells. J. Neurosci. Res. 2003;74:589–597. doi: 10.1002/jnr.10777. [DOI] [PubMed] [Google Scholar]
- 34.Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319:1241–1244. doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]
- 35.Wong K, Li XB, Hunchuk N. N-acetylsphingosine (C2-ceramide) inhibited neutrophil superoxide formation and calcium influx. J. Biol. Chem. 1995;270:3056–3062. doi: 10.1074/jbc.270.7.3056. [DOI] [PubMed] [Google Scholar]
- 36.Myhre O, Andersen JM, Aarnes H, Fonnum F. Evaluation of the probes 2',7'-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem. Pharmacol. 2003;65:1575–1582. doi: 10.1016/s0006-2952(03)00083-2. [DOI] [PubMed] [Google Scholar]
- 37.Jourd'heuil D. Increased nitric oxide-dependent nitrosylation of 4,5-diaminofluorescein by oxidants: implications for the measurement of intracellular nitric oxide. Free Radic. Biol. Med. 2002;33:676–684. doi: 10.1016/s0891-5849(02)00955-3. [DOI] [PubMed] [Google Scholar]
- 38.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 39.Hotamisligil GkS. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin. Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Circu ML, Aw TY. Glutathione and modulation of cell apoptosis. Biochim. Biophys. Acta. 2012;1823:1767–1777. doi: 10.1016/j.bbamcr.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.