Abstract
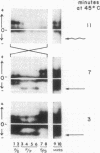
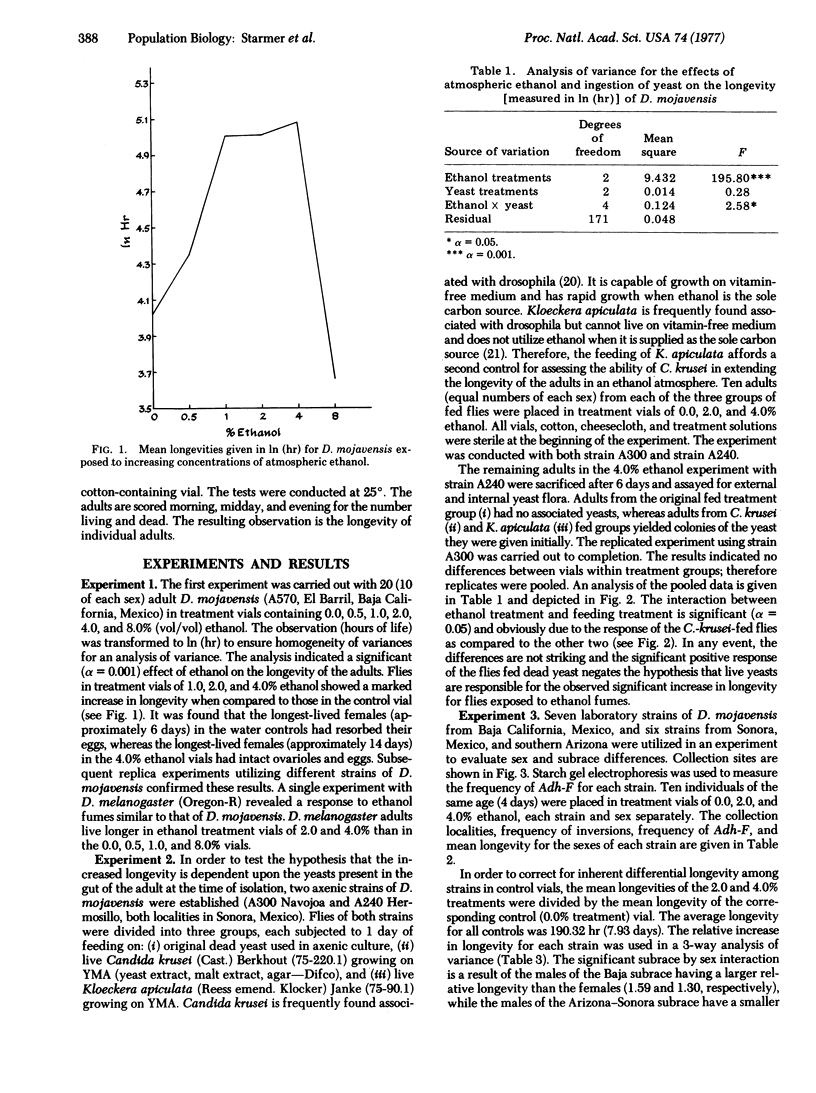
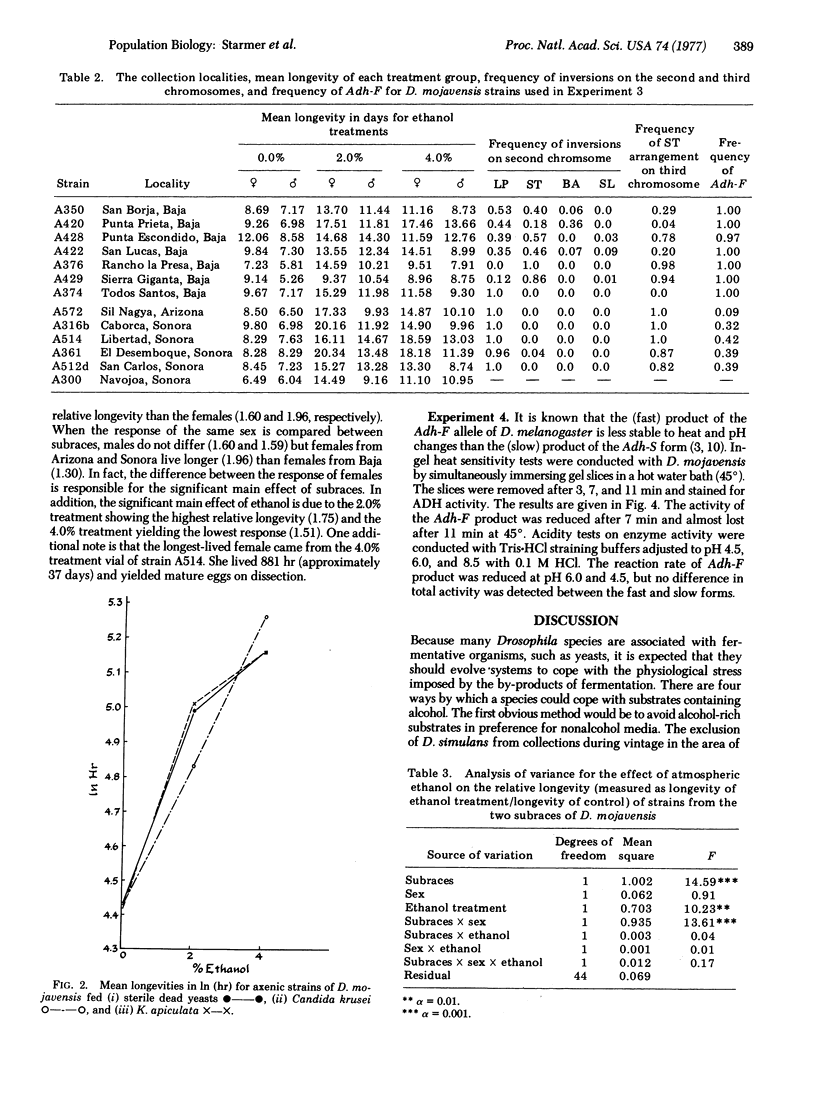
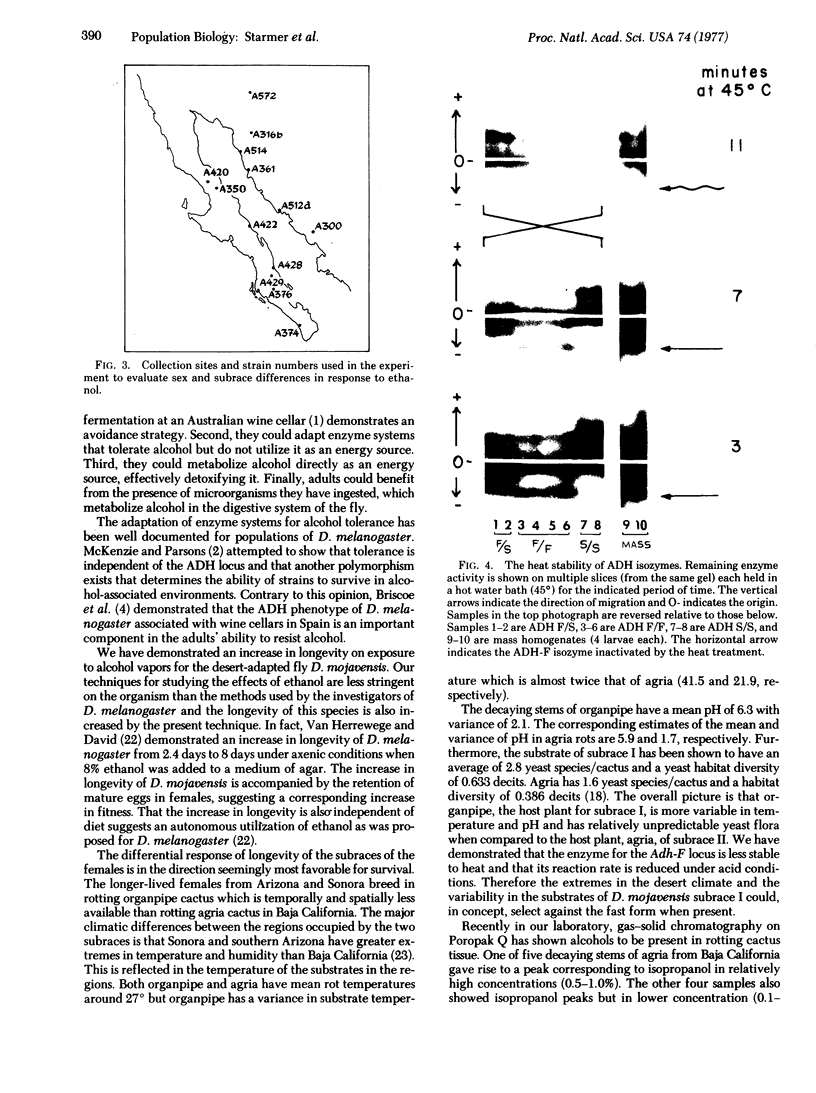
Drosophila mojavensis adults, which breed and feed on necrotic cacti, show an increase in longevity when exposed to atmospheric ethanol. The increase in longevity is accompanied by retention of mature ovarioles and is independent of diet. Differences in longevity among strains from different localities were detected for females. Strains from Arizona and Sonora, Mexico, showed the greatest increase in longevity, while strains from Baja California, Mexico, showed the least increase. These differences may be controlled by the alcohol dehydrogenase locus, the octanol dehydrogenase locus, and modifier genes, because the aduld response is correlated with the frequency of alcohol dehydrogenase alleles, as well as second chromosomal inversions containing the octanol dehydrogenase locus. The longevity response is also consistent with the more uneven distribution and availability of the host plant in Arizona and Sonora, Mexico. Strains from Arizona and Sonora, Mexico, have a high frequency of Adh-S, the allele whose product is heat and pH tolerant. The host plant, organpipe cactus, exhibits extremes in temperature and pH in the same geographic region. Strains from Baja California, Mexico, possess a high frequency of Adh-F, whose product is heat and pH sensitive. The substrate in this region, agria cactus, has moderate temperature and pH extremes and contains relatively high concentrations of isopropanol. Isopropanol is presumable a selective agen favorable to Adh-F. The environmental heterogeneity that is proposed for maintaining the alleles at the alcohol dehydrogenase locus is the interaction of substrate alcohol content with temperature and pH. Substrates that do not contain appreciable amounts of isopropanol and are exposed to high temperatures and exhibit variable pH favor Adh-S, while substrates containing isopropanol and having moderate temperatures and pH favor Adh-F.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briscoe D. A., Robertson A., Malpica J. M. Dominance at Adh locus in response of adult Drosophila melanogaster to environmental alcohol. Nature. 1975 May 8;255(5504):148–149. doi: 10.1038/255148a0. [DOI] [PubMed] [Google Scholar]
- Clarke B. The contribution of ecological genetics to evolutionary theory: detecting the direct effects of natural selection on particular polymorphic loci. Genetics. 1975 Jun;79 (Suppl):101–113. [PubMed] [Google Scholar]
- David J. R., Bocquet C. Similarities and differences in latitudinal adaptation of two Drosophila sibling species. Nature. 1975 Oct 16;257(5527):588–590. doi: 10.1038/257588a0. [DOI] [PubMed] [Google Scholar]
- Day T. H., Hillier P. C., Clarke B. Properties of genetically polymorphic isozymes of alcohol dehydrogenase in Drosophila melanogaster. Biochem Genet. 1974 Feb;11(2):141–153. doi: 10.1007/BF00485770. [DOI] [PubMed] [Google Scholar]
- Day T. H., Hillier P. C., Clarke B. The relative quantities and catalytic activities of enzymes produced by alleles at the alcohol dehydrogenase locus in Drosophila melanogaster. Biochem Genet. 1974 Feb;11(2):155–165. doi: 10.1007/BF00485771. [DOI] [PubMed] [Google Scholar]
- Gibson J. B., Miklovich R. Modes of variation in alcohol dehydrogenase in Drosophila melanogaster. Experientia. 1971 Jan 15;27(1):99–100. doi: 10.1007/BF02137764. [DOI] [PubMed] [Google Scholar]
- Hewitt N. E., Pipkin S. B., Williams N., Chakrabartty P. K. Variation in ADH activity in class I and class II strains of Drosophila. J Hered. 1974 May-Jun;65(3):141–148. doi: 10.1093/oxfordjournals.jhered.a108485. [DOI] [PubMed] [Google Scholar]
- McKenzie J. A., Parsons P. A. Microdifferentiation in a natural population of Drosophila melanogaster to alcohol in the environment. Genetics. 1974 Jun;77(2):385–394. doi: 10.1093/genetics/77.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson R. H., Smouse P. E. Patterns of molecular variation. I. Interspecific comparisons of electromorphs in the Drosophila mulleri complex. Biochem Genet. 1976 Jun;14(5-6):447–466. doi: 10.1007/BF00486126. [DOI] [PubMed] [Google Scholar]
- Singh R. S. Substrate-specific enzyme variation in natural populations of Drosophila pseudoobscura. Genetics. 1976 Mar 25;82(3):507–526. doi: 10.1093/genetics/82.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Herrewege J., David J. Utilisation de l'alcool éthylique dans le métabolisme énergétique d'un insecte: influence sur la durée de survie des adultes de Drosophila melanogaster. C R Acad Sci Hebd Seances Acad Sci D. 1974 Jul 22;279(4):335–338. [PubMed] [Google Scholar]
- Vigue C. L., Johnson F. M. Isozyme variability in species of the genus Drosophila. VI. Frequency-property-environment relationships of allelic alcohol dehydrogenases in D. melanogaster. Biochem Genet. 1973 Jul;9(3):213–227. doi: 10.1007/BF00485735. [DOI] [PubMed] [Google Scholar]
- Zouros E. The distribution of enzyme and inversion polymorphism over the genome of Drosophila: evidence against balancing selection. Genetics. 1976 May;83(1):169–179. doi: 10.1093/genetics/83.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]