Abstract
Background and Purpose
Recently independent studies reported an association between coronary heart disease and single-nucleotide polymorphisms (SNPs) located at chromosome 9p21, near CDKN2A and CDKN2B genes. Given that stroke is a common complication after myocardial infarction, we investigated if the same SNPs were associated with ischemic stroke in our population.
Methods
We recently initiated a whole genome analysis of ischemic stroke and published the first stage of a case control study using >400 000 SNPs from Illumina Infinium Human-1 and HumanHap300 assays. We focused on SNPs recently associated with heart disease by Helgadottir and colleagues and SNPs from the same haplotype block.
Results
In analyses both unadjusted and adjusted for stroke risk factors, significant associations with ischemic stroke were observed for SNPs from the same haplotype block previously associated with myocardial infarction. Significant association was also seen between disease and haplotypes involving these SNPs, both with and without adjustment for stroke risk factors (odd ratios: 1.01 to 2.65).
Conclusions
These data are important for 3 reasons: first, they suggest a genetic association for stroke; second, they suggest that this association shares pathogenic mechanisms with heart disease and diabetes; and third, they illustrate, that public release of data can facilitate rapid risk locus discovery.
Keywords: ischemic stroke, genetics, heart disease, diabetes
Recently, independent studies have reported an association between myocardial infarction (MI) and common genetic variation on chromosome 9p21. The variants associated with MI are located in a linkage disequilibrium (LD) block near the genes CDKN2A and CDKN2B.1–4 At the same time, genetic variants around the same genes were associated with an increased risk of type 2 diabetes mellitus5–7 and atherothrombosis.8 All these data suggest there may be common pathogenic mechanisms involved in these apparently disparate diseases.
Coronary disease increases the risk of stroke, and stroke is associated with a large increase in the risk for death after MI. Furthermore, both diseases share common risk factors and treatments9; because of this we sought to determine whether the CDKN2A/CDKN2B locus associated with MI may also modulate risk for IS. To do this we analyzed data from our recently completed first stage of a whole genome analysis of IS that used more than 400 000 single-nucleotide polymorphisms (SNPs) from Illumina Infinium Human-1 and HumanHap300 assays, on a cohort of 249 samples with ischemic stroke (IS) and 268 controls.10 Here we present the results of these analyses.
Subjects and Methods
Participants
All stroke samples used in the present study were collected by the Ischemic Stroke Genetics Study (ISGS), which is a prospective 5-center North American case-control study. Control samples were from the National Institute of Neurological Disorders and Stroke Neurogenetics Repository. The protocol for ISGS and controls has been reported previously.10,11 Briefly, stroke was defined according to World Health Organization criteria, and index strokes were confirmed to be ischemic by CT or MRI of the head. Index strokes were classified according to the prespecified Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria and were blinded to genotype.
Genotyping
All samples were assayed with the Illumina Infinium Human-1 and HumanHap300 SNP chips (Illumina Inc). Details have been reported previously.10
Statistical Methods
Statistical analysis of the raw genotype data and moving window haplotype tests were done with the software SNPGWA.
Using the case-control data, a series of generalized estimating equations were used that permitted inclusion of recognized stroke risk factors as covariates (age, sex, race, hypertension status, presence of atrial fibrillation, history of MI, smoking status, presence of diabetes mellitus, and family history of stroke).
For adjusted and unadjusted analyses, odds ratios in dominant, additive and recessive models and 95% CIs were computed.
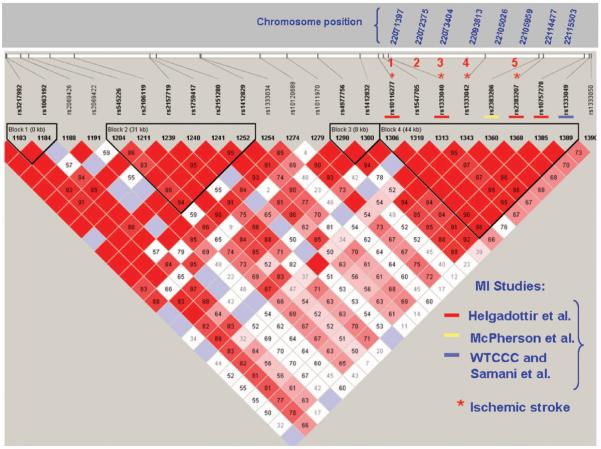
Here we have focused on chr9p21 SNPs that were significantly associated with MI (rs10116277-rs1333040-rs2383207) in the work by Helgadottir and colleagues, and other SNPs from the same haplotype block (Figure).
Figure.
Pairwise LD in the region 21.9 to 22.1 Mb (NCBI build 36.2) on chromosome 9 downloaded from the HapMap database (www.hapmap.org) for the CEU population. This plot shows SNPs assayed in the present study using Illumina Infinium Human-1 and HumanHap300 assays (numbers 1 to 5) and SNPs previously associated with MI.1–4 Numbers within the diamonds are D' values (a measure of LD strength) for the respective SNP pairs. Solid red diamonds represent absolute LD (D'=1), blue diamonds represent strong LD with low level of significance. Numbers in gray within white diamonds represent a high probability or evidence of historical recombination. The figure shows clearly how one LD block comprising all SNPs, significantly associated with MI (squares) or IS (asterisks). WTCCC indicates Wellcome Trust Case Control Consortium.
Results
In unadjusted analyses, significant associations were observed for rs10116277, rs1333040, rs1333042, and rs2383207. The latter 3 SNPs showed an association under a dominant model; two of these SNPs, rs1333040 and rs2383207, were previously associated with MI and all exist within the same LD block. After adjustment for stroke risk factors, the risk allele in the SNP rs1333042 showed an opposite association being protective against disease. Significant association was also seen between IS and almost all haplotypes involving these SNPs in additive and dominant models, before and after adjustment for stroke risk factors. See Tables 1 and 2.
Table 1.
Association Between SNPs at 9p21 and Risk for IS
| Unadjust.-A | Unadjust.-D | Unadjust.-R | Adjusted-A | Adjusted-D | Adjusted-R | |
|---|---|---|---|---|---|---|
| P Value | P Value | P Value | P Value | P Value | P Value | |
| SNP ID | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| 1 | 0.309 | 0.699 | 0.038 | 0.133 | 0.102 | 0.415 |
| rs10116277* | 0.88 (0.94–1.58) | 1.08 (0.73–1.61) | 0.65 (0.43–0.98) | 1.26 (0.93–1.70) | 1.50 (0.92–2.45) | 1.22 (0.75–1.99) |
| 2 | 0.806 | 0.895 | 0.628 | 0.907 | 0.802 | 0.954 |
| rs1547705 | 1.05 (0.71–1.54) | 1.03 (0.67–1.57) | 1.45 (0.32–6.54) | 0.97 (0.60–1.56) | 0.783 (0.12–5.27) | 0.984 (0.59–1.65) |
| 3 | 0.104 | 0.032 | 0.796 | 0.116 | 0.409 | 0.104 |
| rs1333040* | 1.23 (0.96–1.59) | 1.49 (1.03–2.15) | 1.06 (0.66–1.71) | 0.782 (0.58–1.06) | 0.781 (0.43–.140) | 0.695 (0.45–1.08) |
| 4 | 0.247 | 0.042 | 0.872 | 0.077 | 0.386 | 0.046 |
| rs1333042 | 1.15 (0.91–1.47) | 1.51 (1.01–2.25) | 0.97 (0.65–1.45) | 0.765 (0.57–1.03) | 0.803 (0.49–1.32) | 0.615 (0.38–0.99) |
| 5 | 0.153 | 0.042 | 0.806 | 0.079 | 0.323 | 0.065 |
| rs2383207* | 1.19 (0.94–1.52) | 1.50 (1.01–2.21) | 1.05 (0.70–1.59) | 0.766 (0.57–1.03) | 0.775 (0.47–1.28) | 0.643 (0.40–1.03) |
A indicates additive model; D, dominant model; R, recessive model; Unadjust., P value before adjusted for known stroke risk factors.
SNPs previously associated with MI.
Table 2.
Association Between SNP Haplotypes at 9p21 and Risk for IS Focusing on Haplotypes That Contain SNPs Previously Associated With MI (numbers 1, 3 and 5)
| Data | SNP Range | Model | Calls | P Values | Odds Ratios | 95% CI |
|---|---|---|---|---|---|---|
| Unadjusted | 3–4–5 | additive | CAA | 0.0177 | 1.36 | (1.06–1.76) |
| Unadjusted | 1–2–3–4–5 | additive | GACAA | 0.0196 | 1.36 | (1.05–1.75) |
| Unadjusted | 3–4–5 | dominant | CAA | 0.0088 | 1.61 | (1.13–2.29) |
| Unadjusted | 1–2–3–4–5 | dominant | GACAA | 0.0102 | 1.59 | (1.12–2.27) |
| Adjusted | 3–4–5 | additive | CAA | 0.0207 | 1.44 | (1.06–1.96) |
| Adjusted | 1–2–3–4–5 | additive | GACAA | 0.0229 | 1.43 | (1.05–1.95) |
| Adjusted | 3–4–5 | dominant | CAA | 0.0131 | 1.72 | (1.12–2.65) |
| Adjusted | 1–2–3–4–5 | dominant | GACAA | 0.0152 | 1.70 | (1.11–2.62) |
1=rs10116277, 2=rs1547705, 3=rs1333040, 4=rs1333042, 5=rs2383207.
Discussion
A significant association was seen between IS and SNPs initially associated with MI or located within the same LD block. This association was most striking at the haplotype level.
Our dataset is currently too small for us to determine whether one particular subtype of ischemic stroke contributes to this association or whether it is a general association with IS. It would be of interest to investigate this in a larger cohort, and such a study would also clarify if these variants may confer risk for IS independent of conventional risk factors. We are aware of the possibility of false-positive association; however, it is encouraging that independent studies find common genetic risk variants for different but related diseases.
CDKN2A and CDKN2B, the two characterized genes closest to the risk loci, are well established as tumor suppressor genes, and recent data suggest that their expression levels increase markedly with aging in primate skin, human vasculature and rodent and human kidney12; although clearly the proximity of these genes to the risk locus makes them strong candidates for functional analysis, it should be noted that genetic variability can affect distal gene expression, and thus the observed association in these diseases may not reflect a biological effect on CDKN2A or CDKN2B.
These data are the first step in a comprehensive association study, and studies with larger sample sizes are necessary in order to define a consistent association. However, these data are important for 3 reasons: first, they suggest a genetic association for stroke; second, they suggest that this association shares pathogenic mechanisms with heart disease and diabetes; third, they illustrate that public release of data can facilitate rapid risk locus discovery.
Acknowledgments
We thank the participants and the submitters for depositing samples at the NINDS neurogenetics repository.
Sources of Funding This study was supported by grants from the NINDS (R01 NS42733), the intramural programmes of the National Institute on Aging and NINDS and by an extramural NINDS contract funding the Coriell Repository.
Footnotes
Disclosures None.
References
- 1.Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, Sigurdsson A, Baker A, Palsson A, Masson G, Gudbjartsson D, Magnusson KP, Andersen K, Levey AI, Backman VM, Matthiasdottir S, Jonsdottir T, Palsson S, Einarsdottir H, Gunnarsdottir S, Gylfason A, Vaccarino V, Hooper WC, Reilly MP, Granger CB, Austin H, Rader DJ, Shah SH, Quyyumi AA, Gulcher JR, Thorgeirsson G, Thorsteinsdottir U, Kong A, Stefansson K. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1488–1491. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 2.McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, Hinds DA, Pennacchio LA, Tybjaerg-Hansen A, Folsom AR, Boer-winkle E, Hobbs HH, Cohen JC. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1491–1493. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wellcome Trust Case Control C Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, Barrett JH, Konig IR, Stevens SE, Szymczak S, Tregouet DA, Iles MM, Pahlke F, Pollard H, Lieb W, Cambien F, Fischer M, Ouwehand W, Blankenberg S, Balmforth AJ, Baessler A, Ball SG, Strom TM, Braenne I, Gieger C, Deloukas P, Tobin MD, Ziegler A, Thompson JR, Schunkert H. Consortium WatC. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–453. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 6.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, Burton PR, Clayton DG, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, Ouwehand WH, Samani NJ, Todd JA, Donnelly P, Davison D, Easton D, Evans D, Leung HT, Spencer CC, Tobin MD, Attwood AP, Boorman JP, Cant B, Everson U, Hussey JM, Jolley JD, Knight AS, Koch K, Meech E, Nutland S, Prowse CV, Stevens HE, Taylor NC, Walters GR, Walker NM, Watkins NA, Winzer T, Jones RW, McArdle WL, Ring SM, Strachan DP, Pembrey M, Breen G, St Clair D, Caesar S, Gordon-Smith K, Jones L, Fraser C, Green EK, Grozeva D, Hamshere ML, Holmans PA, Jones IR, Kirov G, Moskvina V, Nikolov I, O'Donovan MC, Owen MJ, Collier DA, Elkin A, Farmer A, Williamson R, McGuffin P, Young AH, Ferrier IN, Ball SG, Balmforth AJ, Barrett JH, Bishop DT, Iles MM, Maqbool A, Yuldasheva N, Hall AS, Braund PS, Dixon RJ, Mangino M, Stevens S, Thompson JR, Bredin F, Tremelling M, Parkes M, Drummond H, Lees CW, Nimmo ER, Satsangi J, Fisher SA, Forbes A, Lewis CM, Onnie CM, Prescott NJ, Sanderson J, Mathew CG, Barbour J, Mohiuddin MK, Todhunter CE, Mansfield JC, Ahmad T, Cummings FR, Jewell DP, Webster J, Brown MJ, Lathrop GM, Connell J, Dominiczak A, Braga Marcano CA, Burke B, Dobson R, Gungadoo J, Lee KL, Munroe PB, Newhouse SJ, Onipinla A, Wallace C, Xue M, Caulfield M, Farrall M, Barton A, Bruce IN, Donovan H, Eyre S, Gilbert PD, Hider SL, Hinks AM, John SL, Potter C, Silman AJ, Symmons DP, Thomson W, Worthington J, Dunger DB, Widmer B, Newport M, Sirugo G, Lyons E, Vannberg F, Hill AV, Bradbury LA, Farrar C, Pointon JJ, Wordsworth P, Brown MA, Franklyn JA, Heward JM, Simmonds MJ, Gough SC, Seal S, Stratton MR, Rahman N, Ban M, Goris A, Sawcer SJ, Compston A, Conway D, Jallow M, Rockett KA, Bumpstead SJ, Chaney A, Downes K, Ghori MJ, Gwilliam R, Hunt SE, Inouye M, Keniry A, King E, McGinnis R, Potter S, Ravindrarajah R, Whittaker P, Widden C, Withers D, Cardin NJ, Ferreira T, Pereira-Gale J, Hallgrimsdottir IB, Howie BN, Su Z, Teo YY, Vukcevic D, Bentley D, Ouwehand NJ, Samani MR, Isaacs JD, Morgan AW, Wilson GD, Ardern-Jones A, Berg J, Brady A, Bradshaw N, Brewer C, Brice G, Bullman B, Campbell J, Castle B, Cetnarsryj R, Chapman C, Chu C, Coates N, Cole T, Davidson R, Donaldson A, Dorkins H, Douglas F, Eccles D, Eeles R, Elmslie F, Evans DG, Goff S, Goodman S, Goudie D, Gray J, Greenhalgh L, Gregory H, Hodgson SV, Homfray T, Houlston RS, Izatt L, Jackson L, Jeffers L, Johnson-Roffey V, Kavalier F, Kirk C, Lalloo F, Langman C, Locke I, Longmuir M, Mackay J, Magee A, Mansour S, Miedzybrodzka Z, Miller J, Morrison P, Murday V, Paterson J, Pichert G, Porteous M, Rogers M, Rowe S, Shanley S, Saggar A, Scott G, Side L, Snadden L, Steel M, Thomas M, Thomas S, McCarthy MI, Hattersley AT. Replication of genome-wide association signals in uk samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zee RY, Ridker PM. Two common gene variants on chromosome 9 and risk of atherothrombosis. Stroke. 2007;38:e111. doi: 10.1161/STROKEAHA.107.497669. [DOI] [PubMed] [Google Scholar]
- 9.Witt BJ, Brown RD, Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med. 2005;143:785–792. doi: 10.7326/0003-4819-143-11-200512060-00006. [DOI] [PubMed] [Google Scholar]
- 10.Matarin M, Brown WM, Scholz S, Simon-Sanchez J, Fung HC, Hernandez D, Gibbs JR, De Vrieze FW, Crews C, Britton A, Langefeld CD, Brott TG, Brown RD, Jr, Worrall BB, Frankel M, Silliman S, Case LD, Singleton A, Hardy JA, Rich SS, Meschia JF. A genome-wide genotyping study in patients with ischaemic stroke: Initial analysis and data release. Lancet Neurol. 2007;6:414–420. doi: 10.1016/S1474-4422(07)70081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meschia JF, Brott TG, Brown RD, Jr, Crook RJ, Frankel M, Hardy J, Merino JG, Rich SS, Silliman S, Worrall BB. The ischemic stroke genetics study (ISGS) protocol. BMC Neurol. 2003;3:4. doi: 10.1186/1471-2377-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim WY, Sharpless NE. The regulation of ink4/arf in cancer and aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]