Abstract
Objective
The treatment of FIGO stage IB2 cervical cancer is controversial. Our aim was to assess treatment patterns, outcomes, and complications in patients with stage IB2 cervical cancer.
Methods
A retrospective study of patients with stage IB2 cervical carcinoma at a single institution between January 1982 and September 2006 was performed. To adequately control treatment variables, we only included patients who underwent their entire treatment at our institution. Toxicity was assessed using NCI Common Toxicity Criteria (CTC).
Results
We identified 82 patients, of whom 47 met the strict inclusion criteria. Of these, 27 patients (57%) underwent primary radical hysterectomy (RH) and 20 (43%) were treated with definitive radiation/chemoradiation therapy (RT/CRT). Patients selected for RT/CRT had a higher American Society of Anesthesiologist (ASA) score than those selected for surgery (P=0.037). The 3-year progression free survival rate was 52% for the RH group and 55% for the RT/CRT group (P=0.977). The 3-year overall survival rates were 72% and 55%, respectively (P=0.161). Overall, 52% of patients in the RH group received postoperative radiation therapy as part of their adjuvant treatment. CTC grade 3, 4, and 5 complications affected 5 patients (19%) in the RH group and 3 (15%) in the RT/CRT group.
Conclusion
Both RH and definitive RT/CRT are adequate management strategies for patients with FIGO stage IB2 cervical cancer. However, there was a subset of patients in whom RH as monotherapy was appropriate. Further studies are needed to evaluate the role of new preoperative models that will accurately identify these patients.
Keywords: FIGO stage IB2 cervical carcinoma, radical hysterectomy, chemoradiation therapy
INTRODUCTION
Cervical cancer is the second most common cancer and the fifth leading cause of cancer-related deaths in women worldwide [1]. In 2008, there will be an estimated 11,070 newly diagnosed cervical cancers and 3,870 deaths from this disease in the United States [2]. International Federation of Gynecology and Obstetrics (FIGO) stage IB cervical cancer is subdivided upon a clinical tumor diameter of 4 cm into stage IB1 and IB2. Patients with large (> 4 cm) tumors have worse local control and survival rates than patients with smaller stage IB cancers, independent of treatment modality [3–6]. This division by tumor size delineates the wide range of recurrence rates and outcomes in this particular group of patients with early-stage disease. Overall survival rates range from 80–95% for patients with stage IB1 cancer to 65–80% for patients with IB2 cervical cancer [7–9].
The heterogeneity of this patient population has led to the assessment of a wide variety of treatment modalities ranging from radical hysterectomy with or without individualized postoperative treatment, definitive radiation therapy or concurrent chemoradiation therapy as well as neoadjuvant chemotherapy [5, 10–12]. With respect to the treatment of stage IB2 cervical cancer, there is controversy as to what the most appropriate treatment modality for these patients should be [10, 11, 13]. Both radical hysterectomy with lymphadenectomy and primary radiation therapy with or without concurrent chemotherapy have been used as primary therapy. Comparison studies suggest that both approaches are acceptable initial treatment modalities, with similar effectiveness in patients with early-stage cervical cancer [5, 14, 15]. The associated morbidity, however, may differ between different approaches. The choice of treatment usually depends on the institutional practice, gynecologic and radiation oncologists involved, and the age and general health of the patient [16, 17]. Furthermore, cost effectiveness analyses have shown that primary radical hysterectomy compares favorably to definitive radiation/chemoradiation therapy [18, 19].
The aim of this study was to assess the associations between clinical variables and the primary mode of treatment for patients with FIGO stage IB2 cervical cancer at a single institution over a 17-year period. We also sought to determine the 3-year progression free survival (PFS) and 3-year overall survival (OS), as well as the pattern of recurrence and complications of patients managed with each treatment modality.
METHODS
After obtaining Institutional Review Board approval, we used the prospectively maintained Virginia K. Pierce database to identify all patients with FIGO stage IB2 cervical carcinoma managed by the gynecologic oncology service as inpatients at our institution from January 1982 to September 2006. During this time period, 82 patients with stage IB2 cervical cancer were identified. We excluded 35 patients who received any part of their primary treatment at other institutions and patients who presented with unusual histology (sarcoma, choriocarcinoma).
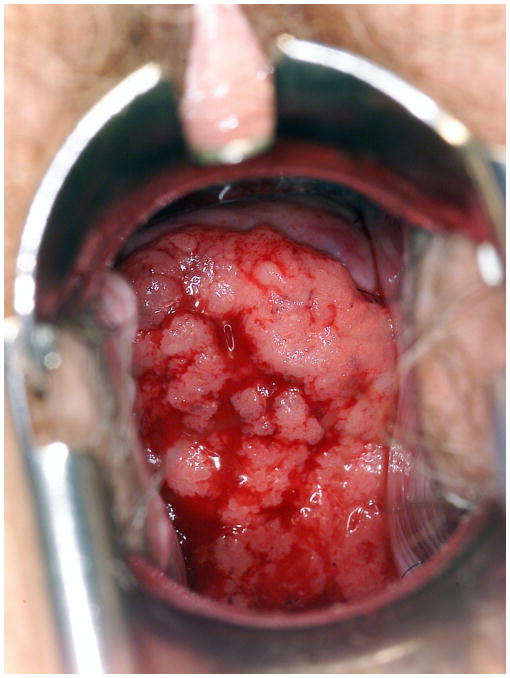
Bulky cervical lesions (Figure 1) were defined as follows: exophytic lesions with an external tumor diameter of 4 cm or larger, as measured by calipers; or a cervix expanded to 4 cm or larger, and presumed by the clinician to be principally involved with cancer; or large or barrel-shaped lesions of 4 cm or larger. Data extracted from medical records included patient age at the time of diagnosis, American Society of Anesthesiologist (ASA) score, histologic subtype, tumor grade, tumor diameter, treatment modality, date and site of first recurrence, and date of last follow-up. All patients treated with primary surgery underwent a Type III radical hysterectomy with or without bilateral salpingo-oophorectomy [20]. In all cases, a bilateral pelvic lymph node dissection with or without a paraaortic lymph node dissection was performed. Postoperative individualized treatment varied over the study period and was usually recommended to patients who had risk factors such as nodal metastases or lymph-vascular space invasion with deep stromal invasion of the outer third of the cervix. Postoperative treatment consisted of external radiation therapy, concurrent chemoradiation therapy, or chemotherapy alone.
Figure 1.
A cervical lesion classified as FIGO Stage IB2 cervical cancer
Postoperative radiation therapy was administered as whole pelvic external-beam radiation with or without additional vaginal cylinder brachytherapy, with a total median pelvic dose of 5040 cGy (range, 3780–6000 cGy) over 5 weeks. Chemotherapy was administered in the form of weekly cisplatin (40mg/m2) or cisplatin (75mg/m2) and bleomycin (20 U/m2/day) according to the high-risk cervical cancer protocol as previously described by Curtin et al. [21].
Definitive primary radiation therapy was administered as external-beam pelvic radiation followed by additional intracavitary brachytherapy, with a total median dose to point A (external beam plus brachytherapy) of 7770 cGy (range, 400–10000 cGy). Concurrent chemotherapy was administered as weekly cisplatin (40mg/m2) in the latter part of the study. Toxicity was assessed using the NCI Common Toxicity Criteria for Adverse Events (CTCAE, Version 3.0).
The association test was performed using the Fisher Exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. For survival analysis, the starting time of both OS and PFS was defined as either surgery date or first radiation/chemoradiation therapy. Death was considered an event in the OS analysis, while first recurrence and progression of disease were considered as events in the PFS analysis. The 3-year OS and PFS rates were estimated by the Kaplan-Meier method. The univariate P value in survival analysis was obtained by using the log-rank test for categorical predictors and score test based on the Cox regression model for continuous variables. The Cox regression model was also used in estimating the hazard ratio.
RESULTS
Forty-seven patients were included in this analysis, of whom 27 (57%) underwent primary radical hysterectomy with or without individualized adjuvant treatment and 20 (43%) were treated with radiation therapy or chemoradiation therapy. A lymph node dissection was performed in 32 patients. All patients selected to undergo primary surgery underwent a lymph node dissection, and 5 patients treated with primary radiation or chemoradiation therapy had a pretreatment lymph node dissection. Three patients had laparoscopic extraperitoneal lymph node samplings, and the remaining 2 patients underwent a laparotomy with the intent to perform a radical hysterectomy. In both patients, the radical hysterectomy was aborted because of positive lymph nodes on frozen section. In total, 3 (60%) of 5 patients were found to have positive nodes on final pathology.
Clinical characteristics and associations between treatment modalities are listed in Table 1. When comparing both treatment groups, there were no statistically significant differences in age, grade, histology, and tumor diameter. There was, however, a significant association between the general health status and the use of primary radiation/chemoradiation therapy. Patients selected to undergo primary radiation therapy or chemoradiation therapy had a significantly higher ASA score (75% ASA 2/3) compared to those patients selected to undergo radical hysterectomy (41% ASA 2/3; P=0.037).
Table 1.
Patient characteristics and associations between treatment modalities
| Variable N | All patients 47 | Surgery 27 (57%) | Radiation/chemoradiation 20 (43%) | P value |
|---|---|---|---|---|
| Age | ||||
| Mean, years (median) | 42.4 (39) | 41.9 (37) | 43.2 (39) | 0.613* |
| Range | 21–78 | 27–75 | 21–78 | |
|
| ||||
| Tumor grade | 1.000 | |||
| G1/G2 | 16 (38%) | 10 (40%) | 6 (35%) | |
| G3 | 26 (62%) | 15 (60%) | 11 (65%) | |
| Histology | 1.000 | |||
| Squamous | 27 (57%) | 16 (59%) | 11 (55%) | |
| Others | 20 (43%) | 11 (41%) | 9 (45%) | |
| ASA score | ||||
| 1 | 21 (45%) | 16 (59%) | 5 (25%) | 0.037 |
| 2, 3 | 26 (55%) | 11 (41%) | 15 (75%) | |
| Tumor size | ||||
| Mean, cm (median) | 5.6 | 5.1 (5.0) | 6.1 (6.0) | 0.073 |
| Range | 4.1–9.0 | 4.2–7.0 | 4.1–9.0 | |
Note: The P value with * was obtained using the Wilcoxon rank sum test. All the other P values were obtained using the Fisher exact test.
ASA, American Society of Anesthesiologist
Histologic variables and postoperative treatment modalities of all patients treated with primary surgery are listed in Table 2. Seventeen patients (63%) in the surgery group received individualized postoperative adjuvant treatment. Fourteen patients (52%) received radiation therapy as part of their adjuvant treatment.
Table 2.
Pathological risk factors and postoperative treatment in the surgery group
| Variable N | 27 |
|---|---|
|
| |
| Histology | |
| Squamous | 16 (59%) |
| Adenocarcinoma | 5 (19%) |
| Adeno-squamous | 3 (11%) |
| Others | 3 (11%) |
| Depth of invasion (mm) | |
| Mean (Median) | 15.2 (13) |
| Range | 3–40 |
| Cervical stroma invasion | |
| Inner third | 4 (15%) |
| Middle third | 5 (19%) |
| Outer third | 18 (67%) |
| Histological parametrial invasion | 0 (0%) |
| LVSI | 13 (48%) |
| Lymph node metastases | 2 (7%) |
| Adjuvant treatment | |
| Yes | 17 (63%) |
| No | 10 (37%) |
| Postoperative radiation | 6 (22%) |
| Postoperative chemoradiation | 8 (30%) |
| Postoperative chemotherapy | 3 (11%) |
Of the 20 patients selected to undergo primary radiation or chemoradiation therapy, 6 (30%) received radiation therapy alone and 13 (65%) were treated with definitive chemoradiation. Two patients underwent adjuvant simple hysterectomy after primary radiation therapy.
The median follow-up time for the surviving patients was 3.6 years (range, 1–25 years). The median follow-up time for the surviving patients without recurrence was 2.95 years (range, 0.025–16 years). During the study period, 17 patients (36%) died—12 patients died of intercurrent or progressive disease, 2 patients died from fatal complications during the treatment of the disease, 2 died of other causes (leukemia and metastatic colon cancer), and 1 patient died of an unknown cause.
The 3-year PFS and OS rates for all patients were 54% and 65%, respectively (Tables 3 and 4). When comparing both treatment groups there was no statistically significant difference in PFS and OS. The 3-year PFS rate for patients in the surgery group was 52% compared to 55% in the primary radiation or chemoradiation therapy group (P=0.977). The 3-year OS rate for patients selected to undergo primary surgery was 72% compared to 55% for patients in the radiation or chemoradiation therapy group (P=0.161). On univariate analysis, age, treatment modality, histology (squamous versus other), grade, ASA score, and tumor size were not significantly associated with recurrence and survival.
Table 3.
Univariate analysis of 3-year PFS
| N | 3-Year PFS Rate (95%CI) | P value | Hazard ratio | |
|---|---|---|---|---|
|
| ||||
| ALL | 46 | 54% (30–61%) | ||
|
| ||||
| Age at diagnosis | 0.85* | 1.00 (0.96–1.03) | ||
|
| ||||
| Tumor Diameter (cm) | 0.625* | 0.98 (0.95–1.02) | ||
|
| ||||
| Treatment group | ||||
| Surgery | 26 | 52% (28–72%) | 0.977 | Ref. Level |
| Non-Surgery | 20 | 55% (30–74%) | 1.01 (0.4–2.57) | |
|
| ||||
| Histology | ||||
| Squamous | 27 | 41% (21–61%) | 0.479 | Ref. Level |
| Others | 19 | 53% (27–74%) | 0.73 (0.31–1.73) | |
|
| ||||
| Grade | ||||
| G1/G2 | 16 | 56% (26–78%) | 0.264 | Ref. Level |
| G3 | 25 | 35% (16–55%) | 1.68 (0.67–4.2) | |
|
| ||||
| ASA | ||||
| ASA 1 | 21 | 50% (26–69%) | 0.726 | Ref. Level |
| ASA 2/3 | 25 | 42% (19–63%) | 1.16 (0.5–2.69) | |
Note: The P values with * were obtained using score test based on Cox regression model; while the other P values were obtained using the log-rank test.
ASA, American Society of Anesthesiologist
Table 4.
Univariate analysis of 3-year OS
| N | 3-year survival rate (95%CI) | P value | Hazard ratio | |
|---|---|---|---|---|
|
| ||||
| ALL | 47 | 65% (47–78%) | ||
|
| ||||
| Age at diagnosis | 0.188* | 1.03 (0.99–1.06) | ||
|
| ||||
| Tumor Diameter (cm) | 0.98* | 0.98 (0.94–1.02) | ||
|
| ||||
| Treatment group | ||||
| Surgery | 27 | 72% (48–87%) | 0.161 | Ref. Level |
| Non-Surgery | 20 | 55% (28–75%) | 1.98 (0.75–5.24) | |
|
| ||||
| Histology | ||||
| Squamous | 27 | 57% (35–75%) | 0.075 | Ref. Level |
| Others | 20 | 76% (46–90%) | 0.37 (0.12–1.15) | |
|
| ||||
| Grade | ||||
| G1/G2 | 16 | 59% (28–81%) | 0.711 | Ref. Level |
| G3 | 26 | 66% (43–82%) | 1.21 (0.44–3.35) | |
|
| ||||
| ASA | ||||
| ASA 1 | 21 | 58% (34–77%) | 0.513 | Ref. Level |
| ASA 2/3 | 26 | 69% (42–85%) | 0.73 (0.28–1.9) | |
Note: The P values with * were obtained using score test based on the Cox regression model; while the other P values were obtained using the log-rank test.
ASA, American Society of Anesthesiologist
Overall, 18 patients (38%) developed recurrent disease (Table 5)—10 (37%) of the 27 patients in the surgery group and 8 (40%) of the 20 patients in the radiation/chemoradiation therapy group. Of these 18 patients who recurred, 12 (67%) died of disease. Six patients are still alive after the diagnosis of relapse. Two patients are alive with disease, and 4 patients are alive without evidence of disease after the treatment of the recurrence. The percentage of synchronous local and distant metastases was more than two-fold higher in the radiation or chemoradiation therapy group when compared to patients in the primary surgery group (20% vs 7%, respectively). The rate of local recurrence only was 29% in the surgery group compared to 20% in the radiation or chemoradiation therapy group.
Table 5.
Recurrence rate and pattern
| Surgery
|
Non-Surgery | |||
|---|---|---|---|---|
| Surgery | Surgery + adjuvant therapy | Surgery Total | ||
|
| ||||
| Number of patients | 10 | 17 | 27 | 20 |
|
| ||||
| Local, only | 4 (40%) | 4 (24%) | 8 (30%) | 4 (20%) |
| Distant, only | 0 | 0 | 0 | 0 |
| Local and distal | 1 (10%) | 1 (6%) | 2 (7%) | 4 (20%) |
|
| ||||
| Total number | 5 (50%) | 5 (29%) | 10 (37%) | 8 (40%) |
Complications were classified according to the CTCAE Version 3.0 and are listed in Table 6. Overall, there were 8 (17%) severe adverse events (grade 3–5). In the surgery group, 5 patients (19%) developed severe adverse events (grade 3–5) that required medical or surgical treatment compared to 3 (15%) in the radiation or chemoradiation therapy group. Among patients who were treated with surgery followed by adjuvant therapy the rate of severe adverse events was documented in 4 (24%) of 17 patients.
Table 6.
Adverse events
| Surgery
|
Non-Surgery | |||
|---|---|---|---|---|
| Surgery | Surgery + adjuvant therapy | Surgery Total | ||
|
| ||||
| Number of patients | 10 | 17 | 27 | 20 |
|
| ||||
| CTCAE | ||||
| 3–5 | 1 (10%) | 4 (24%) | 5 (19%) | 3 (15%) |
| *Adverse event | Number of patients | |||
| Hematologic | 1 | 5 | 6 | 4 |
| Gastrointestinal | 1 | 8 | 9 | 12 |
| Genitourinary | 6 | 2 | 8 | 3 |
| Neurologic | 0 | 2 | 2 | 4 |
| Cutaneous | 0 | 2 | 2 | 0 |
| Others | 5 | 9 | 14 | 9 |
CTCAE, Common Toxicity Criteria for Adverse Events
Some patients had more than one adverse event. Other adverse events were as follows: lymphocele, 2; pulmonary emboli, 2; deep venous thrombosis, 2; cardiac ischemia, 1; cerebral hemorrhage, 1; electrolyte wasting syndrome, 1; lost percutaneous drain, 1; vaginal stenosis, 2; venotomy, 2; wound infection, 2; lower extremity edema, 1; fatigue, 3; and fever, 3.
There were 2 deaths during primary treatment, both in the radiation/chemoradiation therapy group—myocardial infarction (10 days after initiation of pelvic irradiation) and massive cerebral hemorrhage (55 days after initiation of chemoradiation therapy). Among the patients selected to undergo surgery, 1 patient (10%) developed severe morbidity (grade 3) after radical surgery alone compared to 4 (24%) patients who received postoperative adjuvant treatment. Mild and moderate adverse events (grade 1–2) were recorded in 22 patients (81%) in the surgery group compared to 17 (85%) patients in the primary radiation/chemoradiation group.
Genitourinary symptoms including transient neurologic bladder symptoms, cystitis, and stress incontinence were observed in 8 patients (30%) in the surgery group and 3 patients (15%) in the primary radiation/chemoradiation group. Gastrointestinal symptoms including colitis, proctitis, diarrhea, nausea, rectal bleeding, and abdominal pain were observed in 9 patients (33%) in the surgery group compared to 12 (60%) in the primary radiation/chemoradiation therapy group. The majority of these symptoms were mild (grade 1–2). One severe proctitis associated with temporary stool incontinence was observed in a patient treated with surgery followed by chemoradiation therapy. No intestinal perforation was recorded. No treatment-related fistula formation was recorded in either treatment arm. No treatment-related small- or large-bowel obstruction was recorded.
DISCUSSION
After radical hysterectomy and pelvic lymphadenectomy certain pathological risk factors have been identified in patients with stage I disease. Independent clinicopathological risk factors reported for this patient population include lymph node metastases, large tumor diameter, deep stromal invasion, and lymph-vascular space invasion [3, 14, 15, 22]. According to Gynecologic Oncology Group (GOG) data, 25% of lymph node negative patients with stage IB cervical cancer meet high-risk criteria, and for these patients, adjunctive radiation therapy has been shown to reduce the number of local recurrences and may improve survival [3, 23, 24]. However, this additional treatment is associated with an increased risk of adverse events, possibly due to reduced blood supply to the bladder and distal ureters after surgery as well as to the postoperative adhesions of the gastrointestinal tract [24, 25].
Some authors suggest primary radiation/chemoradiation therapy in order to minimize the risk of adverse events. Landoni et al. compared both treatment approaches in a prospective randomized trial in 343 eligible patients with newly diagnosed stage IB and IIA cervical carcinoma. The 5-year OS and PFS rates were identical in the surgery and radiation therapy groups, but treatment-related morbidity was higher in the surgery group, especially in patients treated with the combination of surgery followed by radiation therapy [5]. Eighty-four percent of patients with large tumors (≥ 4 cm) received postoperative adjuvant treatment. This was significantly more than in patients with smaller tumors (54%). The authors concluded that the appropriate candidates for primary radical surgery should be patients with normal ovarian function and cervical diameters of 4 cm or smaller.
In our study, we assessed the management of FIGO stage IB2 cervical cancer over a relatively long time period at a single institution. We tested for associations between different clinical variables in the two treatment groups in order to compare baseline characteristics. We found that patients selected to undergo surgery had significantly lower ASA scores at the time of initial diagnosis. Patients with impaired general health status (higher ASA scores) and trend towards greater tumor diameter were more likely to be treated with definitive radiation/chemoradiation.
When analyzing histologic risk factors in all patients who had undergone surgery, one can appreciate the distinctly favorable (low) rate of lymph node metastases, parametrial invasion, positive margin status and lymph-vascular space invasion, suggesting a strong confounding factor in this study. Five patients in the radiation/chemoradiation therapy group had a lymphadenectomy prior to initiating treatment. In all 5 cases, a computed tomography (CT) scan of the abdomen and pelvis revealed suspect enlarged lymph nodes. Three (60%) out of these 5 patients had positive lymph node metastases. This is distinctly higher than in the surgery group, in which only 2 (7%) out of 27 patients were found to have positive lymph nodes. These data and the low incidence of lymph node metastases indicate that the patients in the surgery group describe a favorable group of patients with stage IB2 cervical cancer. When comparing the patients in the surgery group to the patients with bulky cervical cancer from the study by Landoni et al. we can appreciate that lymph-vascular space invasion is less frequent in the present study. This may explain why in the present study only 63% of patients underwent postoperative adjuvant treatment and only 52% received radiation therapy as part of their postoperative patient compared to 84% in the randomized study by Landoni et al [5]. Unfortunately, we cannot include the pre-treatment imaging findings as a variable in our study because CT scans, MRIs, and other imaging studies had been used inconsistently throughout the study period or were not available for review.
Despite the differences in general health and the presumed selection bias with regards to clinical risk factors such as lymph node involvement and lymph-vascular space invasion, there was no statistically significant difference in the 3-year PFS and OS rates of patients with bulky stage IB2 cervical carcinoma in each treatment group. Due to the presumed confounding factors, a true comparison of outcomes between both treatment groups is not feasible. However, we can discuss the outcomes in the context of other reports in the literature. The 3-year OS rate was 65% for the entire group, which is slightly lower when compared to other reports in the literature. The patients selected to undergo surgery had a 72% 3-year OS rate, which is comparable to other reports in the literature [5, 23]. In our study, however, the patients selected to undergo primary radiation therapy or chemoradiation therapy had a 3-year OS rate of 55%, which is lower than previously reported by Keys et al. [26], who reported a 74% 3-year OS rate for patients with bulky stage IB cervical cancer undergoing primary radiation therapy and 83% for the patients undergoing cisplatin-based chemoradiation therapy. One explanation is that the patients selected to undergo primary radiation therapy in our study had a significantly higher rate of co-morbidities, as evinced by the ASA status. Two patients died during treatment and 2 patients died of other causes. Although there is only limited histologic data on the patients treated with primary radiation/chemoradiation, one has to assume a relatively high rate of lymph node involvement and lymph-vascular space invasion. A potential indicator is the more than two-fold higher rate of distant metastases in patients treated with definitive radiation/chemoradiation.
Adverse events are associated with both treatment modalities. Genitourinary symptoms were more commonly seen in the surgery group, whereas gastrointestinal symptoms were more common and compromising in patients selected to undergo radiation therapy or chemoradiation therapy. As expected, patients who underwent surgery followed by postoperative therapy were more likely to experience severe adverse events when compared to the patients who were treated with surgery alone.
Limitations of our study include the retrospective nature of our database, the small sample size, and the long study period, which may not permit the generalization of these findings to contemporary patients. The heterogeneity of treatment strategies throughout the long study period makes it difficult to account for differences in treatment regimens used during therapy. During this time period, both radiation therapy and surgical techniques have improved significantly. Finally, a comparison of both treatment strategies is not applicable due to the strong confounding factors present in the study. Another important limitation of our study is that a meaningful subgroup analysis of patients who received radiation therapy and combined chemoradiation therapy as part of their primary treatment is not applicable due to the small sample size present in this study.
In summary, this single-institution analysis demonstrates the treatment pattern of patients with FIGO stage IB2 cervical cancer over a long study period. At our institution, definitive radiation therapy was, for a significant part, delivered to patients with co-morbidities and a trend towards greater tumor diameter. Furthermore, additional clinical information such as suspected lymph node metastases on preoperative imaging or laparoscopic evaluation seemed to have an influence on treatment modality selection. The selection of patients to surgical management possibly led to a decreased rate of postoperative adjuvant treatment when compared to other reports. Only 63% of the patients undergoing radical surgery needed additional postoperative treatment, and only 52% received radiation therapy as part of their postoperative treatment, which explains the lower incidence of complications in the surgery group when compared to other reports. Therefore, we conclude that radical hysterectomy remains a reasonable option for selected patients with stage IB2 cervical cancer and may avoid the untoward effects of ovarian ablation, especially for premenopausal women who may not require postoperative radiation therapy.
Further studies are needed to evaluate the role of new prognostic models such as PET/CT scan, MRI, or laparoscopic evaluation that will be able to predict with accuracy and consistency whether a patient with bulky stage IB2 cervical cancer will need postoperative treatment or not.
Footnotes
- Oliver Zivanovic, MD: no conflicts of interest to declare
- Kaled Alektiar, MD: no conflicts of interest to declare
- Yukio Sonoda, MD: Plasma Surgical – research support; Covidien – Consultant; Genzyme – Speaker
- Qin Zhou, PhD: no conflicts of interest to declare
- Alexia Iasonos, PhD: no conflicts of interest to declare
- William P. Tew, MD: no conflicts of interest to declare
- John P. Diaz, MD: no conflicts of interest to declare
- Dennis S. Chi, MD: no conflicts of interest to declare
- Richard R. Barakat, MD: no conflicts of interest to declare
- Nadeem R. Abu-Rustum, MD: no conflicts of interest to declare
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Delgado G, Bundy BN, Fowler WC, Jr, Stehman FB, Sevin B, Creasman WT, et al. A prospective surgical pathological study of stage I squamous carcinoma of the cervix: a Gynecologic Oncology Group Study. Gynecol Oncol. 1989;35:314–20. doi: 10.1016/0090-8258(89)90070-x. [DOI] [PubMed] [Google Scholar]
- 4.Perez CA, Grigsby PW, Nene SM, Camel HM, Galakatos A, Kao MS, et al. Effect of tumor size on the prognosis of carcinoma of the uterine cervix treated with irradiation alone. Cancer. 1992;69:2796–806. doi: 10.1002/1097-0142(19920601)69:11<2796::aid-cncr2820691127>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–40. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 6.Homesley HD, Raben M, Blake DD, Ferree CR, Bullock MS, Linton EB, et al. Relationship of lesion size to survival in patients with stage IB squamous cell carcinoma of the cervix uteri treated by radiation therapy. Surg Gynecol Obstet. 1980;150:529–31. [PubMed] [Google Scholar]
- 7.Eifel PJ, Morris M, Wharton JT, Oswald MJ. The influence of tumor size and morphology on the outcome of patients with FIGO stage IB squamous cell carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1994;29:9–16. doi: 10.1016/0360-3016(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 8.Piver MS, Chung WS. Prognostic significance of cervical lesion size and pelvic node metastases in cervical carcinoma. Obstet Gynecol. 1975;46:507–10. [PubMed] [Google Scholar]
- 9.Rettenmaier MA, Casanova DM, Micha JP, Moran MF, Ramsanghani NS, Syed NA, et al. Radical hysterectomy and tailored postoperative radiation therapy in the management of bulky stage 1B cervical cancer. Cancer. 1989;63:2220–3. doi: 10.1002/1097-0142(19890601)63:11<2220::aid-cncr2820631127>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Keys HM, Bundy BN, Stehman FB, Okagaki T, Gallup DG, Burnett AF, et al. Gynecologic Oncology Group. Radiation therapy with and without extrafascial hysterectomy for bulky stage IB cervical carcinoma: a randomized trial of the Gynecologic Oncology Group. Gynecol Oncol. 2003;89:343–53. doi: 10.1016/s0090-8258(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 11.Ryu HS, Kang SB, Kim KT, Chang KH, Kim JW, Kim JH. Efficacy of different types of treatment in FIGO stage IB2 cervical cancer in Korea: results of a multicenter retrospective Korean study (KGOG-1005) Int J Gynecol Cancer. 2007;17:132–6. doi: 10.1111/j.1525-1438.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 12.Chang TC, Lai CH, Hong JH, Hsueh S, Huang KG, Chou HH, et al. Randomized trial of neoadjuvant cisplatin, vincristine, bleomycin, and radical hysterectomy versus radiation therapy for bulky stage IB and IIA cervical cancer. J Clin Oncol. 2000;18:1740–7. doi: 10.1200/JCO.2000.18.8.1740. [DOI] [PubMed] [Google Scholar]
- 13.Micha JP, Goldstein BH, Rettenmaier MA, Brown JV, 3rd, John CR, Markman M. Surgery alone or surgery with a combination radiation or chemoradiation for management of patients with bulky-stage IB2 cervical carcinoma. Int J Gynecol Cancer. 2006;16:1147–51. doi: 10.1111/j.1525-1438.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- 14.Morley GW, Seski JC. Radical pelvic surgery versus radiation therapy for stage I carcinoma of the cervix (exclusive of microinvasion) Am J Obstet Gynecol. 1976;126:785–98. doi: 10.1016/0002-9378(76)90668-2. [DOI] [PubMed] [Google Scholar]
- 15.Hopkins MP, Morley GW. Radical hysterectomy versus radiation therapy for stage IB squamous cell cancer of the cervix. Cancer. 1991;68:272–7. doi: 10.1002/1097-0142(19910715)68:2<272::aid-cncr2820680210>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Shingleton HM, Jones WB, Russell A, Fremgen A, Chmiel JS, Ocwieja K, et al. Hysterectomy in invasive cervical cancer: a national patterns of care study of the American College of Surgeons. J Am Coll Surg. 1996;183:393–400. [PubMed] [Google Scholar]
- 17.Russell AH, Shingleton HM, Jones WB, Stewart AK, Fremgen A, Winchester DP, et al. Trends in the use of radiation and chemotherapy in the initial management of patients with carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1998;40:605–13. doi: 10.1016/s0360-3016(97)00858-4. [DOI] [PubMed] [Google Scholar]
- 18.Rocconi RP, Chiang S, Richter HE, Straughn JM., Jr Management strategies for abnormal early pregnancy: a cost-effectiveness analysis. J Reprod Med. 2005;50:486–90. [PubMed] [Google Scholar]
- 19.Jewell EL, Kulasingam S, Myers ER, Alvarez Secord A, Havrilesky LJ. Primary surgery versus chemoradiation in the treatment of IB2 cervical carcinoma: a cost effectiveness analysis. Gynecol Oncol. 2007;107:532–40. doi: 10.1016/j.ygyno.2007.08.056. [DOI] [PubMed] [Google Scholar]
- 20.Piver MS, Rutledge F, Smith JP. Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol. 1974;44:265–72. [PubMed] [Google Scholar]
- 21.Curtin JP, Hoskins WJ, Venkatraman ES, Almadrones L, Podratz KC, Long H, et al. Adjuvant chemotherapy versus chemotherapy plus pelvic irradiation for high-risk cervical cancer patients after radical hysterectomy and pelvic lymphadenectomy (RH-PLND): a randomized phase III trial. Gynecol Oncol. 1996;61:3–10. doi: 10.1006/gyno.1996.0087. [DOI] [PubMed] [Google Scholar]
- 22.Fuller AF, Jr, Elliott N, Kosloff C, Hoskins WJ, Lewis JL., Jr Determinants of increased risk for recurrence in patients undergoing radical hysterectomy for stage IB and IIA carcinoma of the cervix. Gynecol Oncol. 1989;33:34–9. doi: 10.1016/0090-8258(89)90598-2. [DOI] [PubMed] [Google Scholar]
- 23.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–13. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 24.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol. 1999;73:177–83. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- 25.Kjorstad KE, Martimbeau PW, Iversen T. Stage IB carcinoma of the cervix, the Norwegian Radium Hospital: results and complications. III. Urinary and gastrointestinal complications. Gynecol Oncol. 1983;15:42–7. doi: 10.1016/0090-8258(83)90115-4. [DOI] [PubMed] [Google Scholar]
- 26.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–61. doi: 10.1056/NEJM199904153401503. Erratum in: N Engl J Med 1999, 341, 708. [DOI] [PubMed] [Google Scholar]