Abstract
Ginsenosides, active ingredients of Panax ginseng, are known to exhibit neuroprotective effects. Large-conductance Ca2+-activated K+ (BKCa) channels are key modulators of cellular excitability of neurons and vascular smooth muscle cells. In the present study, we examined the effects of ginsenosides on rat brain BKCa (rSlo) channel activity heterologously expressed in Xenopus oocytes to elucidate the molecular mechanisms how ginsenoside regulates the BKCa channel activity. Ginsenoside Rg3 (Rg3) enhanced outward BKCa channel currents. The Rg3-enhancement of outward BKCa channel currents was concentration-dependent, voltage-dependent, and reversible. The EC50 was 15.1 ± 3.1 μM. Rg3 actions were not desensitized by repeated treatment. Tetraetylammonium (TEA), a K+ channel blocker, inhibited BKCa channel currents. We examined whether extracellular TEA treatment could alter the Rg3 action and vice versa. TEA caused a rightward shift of the Rg3 concentration-response curve (i.e., much higher concentration of Rg3 is required for the activation of BKCa channel compared to the absence of TEA), while Rg3 caused a rightward shift of the TEA concentration-response curve in wild-type channels. Mutation of the extracellular TEA binding site Y360 to Y360I caused a rightward shift of the TEA concentration-response curve and almost abolished both the Rg3 action and Rg3-induced rightward shift of TEA concentration-response curve. These results indicate that Tyr360 residue of BKCa channel plays an important role in the Rg3-enhancement of BKCa channel currents.
Keywords: BKca channel, Ginsenoside Rg3, interaction site, Panax ginseng
INTRODUCTION
Large-conductance Ca2+-activated K+ (BKCa) channels are a family of potassium-selective ion channels activated in response to elevation of intracellular free Ca2+ level following mem-brane depolarization (Hille, 2001). BKCa channels are composed of two subunits: the α (also called Slo) subunit, which forms the channel pore (Ghatta et al., 2006; Salkoff et al., 2006), and the β subunit (Wanner et al., 1999), which modifies the voltage and calcium sensitivity of the pore-forming subunit (Qian et al., 2002). The α subunit has large cytoplasmic C terminus, is responsible for the calcium-dependent activation of the channel (Fig. 1B) (Schreiber and Salkoff, 1997; Schreiber et al., 1999; Wei et al., 1994), and is activated by increased intracellular Ca2+ and/or Ca2+-dependent kinases including CaM kinases, PKA, and PKC (Toro et al., 1998; Weiger et al., 2002). BKCa channels play key roles in a variety of neuronal and non-neuronal cell functions. For example, in neurons, BKCa channels regulate frequency of firing, action potential afterhyperpolarization and neurotransmitter release. BKCa channels are also one of the main ion channels that contribute action potential repolarization and afterhyperpolarization during excitation-contraction coupling in vascular smooth muscle cells (Ohi et al., 2001).
Fig. 1. Chemical structures of ginsenosides and the primary amino acid sequence of mutated BKCa subunit. (A) Structures of ginsenosides. Abbreviations for carbohydrates are as follows: Glc, glucopyranoside; Ara (pyr), arabinopyranoside; Rha, rhamnopyrano-side. The subscript indicates the carbon in the glu-cose ring that links the two carbohydrates. (B) The brief sequence alignment of BKCa channel and the mutated amino acid residues (underlined) in the pore helix. Amino acid residues in BKCa, KCNQ1, and Kv1.4 channels that were proposed to interact with 20(S)-ginsenoside Rg3 (Rg3) are marked (*) amino acid.
Ginseng, the root of Panax ginseng C.A. Meyer, has been used as a representative tonic or an adaptogen to promote longevity and enhance bodily functions against hypertension and various ailments for several hundred years in Far Eastern countries. Recent reports have shown that ginsenosides, one of the active ingredients in ginseng, inhibit ion channels that are involved in neuronal excitabilities such as voltage-dependent Ca2+ and Na+ channels (Fig. 1A) (Choi et al., 2009; Lee et al., 2005). BKCa channels are usually co-localized with voltage-dependent Ca2+ channels (Berkefeld et al., 2006). Little is known about ginsenoside effects on BKCa channel activities.
In the present study, we examined ginsenoside effects on rat brain BKCa (rSlo) channel activities expressed in Xenopus oocytes using the two-electrode voltage clamp and outside-out patch clamp techniques (Ha et al., 2006; Lu et al., 1990). We demonstrated that among various ginsenosides Rg3 most potently enhanced BKCa channel currents and that this action was concentration- and voltage-dependent and reversible. However, site-directed mutagenesis of the channel pore entry site greatly attenuated Rg3 action. In this communication, we present results suggesting that the Y360 residue in the channel pore entry site is involved in Rg3-mediated enhancement of the BKCa channel currents.
MATERIALS AND METHODS
Materials
Ginsenosides were provided by AMBO Institute (Korea) (Fig. 1A). We used the cDNAs for rat brain BKCa channel (GenBank No. NM_000218) that were previously reported (Ha et al., 2006). All other reagents were purchased from Sigma-Aldrich (USA).
Preparation of Xenopus oocytes and microinjection
Xenopus laevis frogs were purchased from Xenopus I (Ann Arbor, USA). Their care and handling were in accordance with the highest standards of institutional guidelines of Konkuk University. For isolation of oocytes, frogs were anesthetized with an aerated solution of 3-amino benzoic acid ethyl ester followed by removal of ovarian follicles. The oocytes were treated with col-lagenase and then agitated for 2 h in Ca2+-free OR2 medium containing 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, 2.5 mM sodium pyruvate, 100 units/ml penicillin and 100 μg/ml streptomycin. Stage V-VI oocytes were collected and stored in ND96 medium (in mM: 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES, pH 7.5) supplemented with 50 μg/ml gentamicin. The oocyte-containing solution was maintained at 18℃ with continuous gentle shaking and renewed daily. Electrophysiological experiments were performed within 5-6 days of oocyte isolation, with ginsenoside applied to the bath. For BKCa channel experiments, BKCa channel-encoding cRNAs (40 nl) were injected into the animal or vegetal pole of each oocyte one day after isolation, using a 10 μl microdispenser (VWR Scien-tific, USA) fitted with a tapered glass pipette tip (15-20 μm in diameter) (Lee et al., 2008).
Site-directed mutagenesis of the BKCa α and in vitro transcription of BKCa channel cDNAs
Single amino acid substitutions of BKCa channel (Fig. 1B) were made using a QuikChange™ XL Site-Directed Mutagenesis Kit (Stratagene, USA), along with Pfu DNA polymerase, in addition to sense and antisense primers encoding the desired mutations. Overlap extension of the target domain by sequential polymerase chain reaction (PCR) was carried out according to the manufacturer’s protocol. The final PCR products were trans-formed into E. coli strain DH5α, screened by PCR and confirmed by sequencing of the target regions. The mutant DNA constructs were linearized at their 3′ ends by digestion with NotI, and run-off transcripts were prepared using the methylated cap analog, m7G(5′)ppp(5′)G. The cRNAs were pre-pared using a mMessage mMachine transcription kit (Ambion, USA) with T7 RNA polymerase. The absence of degraded RNA was con-firmed by denaturing agarose gel electrophoresis followed by ethidium bromide staining. Similarly, recombinant plasmids containing rat BKCa channel cDNA inserts were linearized by digestion with the appropriate restriction enzymes, and cRNAs were obtained using the mMessage mMachine in vitro transcription kit (Ambion, USA) with SP6 RNA polymerase or T7 polymerase. The final cRNA products were resuspended at a concentration of 1 μg/μl in RNase-free water, and stored at -80℃ (Lee et al., 2008).
Data recording
Data recording for BKCa channel currents were performed as described by Lu et al. (1990). A custom-made Plexiglas net chamber was used for two-electrode voltage-clamp recordings as previously reported. The oocytes were impaled with two microelectrodes filled with 3 M KCl (0.2-0.7 MΩ), and electro-physiological experiments were carried out at room temperature using an Oocyte Clamp (OC-725C, Warner Instruments, USA). Stimulation and data acquisition were controlled with a pClamp 8 (Axon Instruments, USA). For most electrophysio-logical experiments, oocytes were perfused initially with Cl-- and Ca2+-free solution (in mM: 96 NaOH, 2 KOH, 8 Mg-gluconate, 5 HEPES, 5 EGTA, pH 7.4 with methanesulfonic acid) in the presence of Cl- channel blocker (500 μM anthracene-9-carboxylic acid) (Lu et al., 1990) to inhibit endogenous Cl- channels. The oocytes were then clamped at a holding potential of -80 mV, membrane potential was depolarized to +40 mV for 400 ms at 10 s intervals, and currents were recorded as otherwise indicated.
All macroscopic current recordings were performed using the gigaohm-seal patch-clamp method in an outside-out configuration (Hamil et al., 1981). Patch pipettes were fabricated from borosilicate glass (WPI, USA) and were fire-polished to a resistance of 5-7 MΩ. The channel currents were amplified using an Axopatch 200B amplifier (Axon Instruments, USA), low-pass filtered at 1 or 2 kHz using a four-pole Bessel filter, and digitized at a rate of 10 or 20 points/ms using a Digidata 1200A apparatus (Axon Instruments). The macroscopic currents of expressed channels were activated by voltage-clamp pulses delivered from a holding potential of -100 mV to membrane poten-tials ranging from -80 to 200 mV in 10 mV increments. The intracellular and extracellular solutions contained the following components, unless otherwise specified: 116 mM KOH, 4 mM KCl, 10 mM HEPES, and 5 mM EGTA at pH 7.2.
To provide the precise free concentration of intracellular Ca2+ ([Ca2+]i), the appropriate amount of total Ca2+ to be added to the intracellular solution was calculated using the MaxChelator software (Patton et al., 2004; http://www.standford.edu/~cpatton/maxc.html). The pH was adjusted to 7.0 with 2-[N-morpholino]ethanesulfonic acid. To compare the channel characteri-stics accurately, an identical set of intracellular solutions was used throughout the experiments. Commercial software packages, which included Clampex 8.0 or 8.1 (Axon Instruments) and Origin 6.1 (OriginLab Corp., USA), were used for the acquisition and analysis of macroscopic recording data.
Data analysis
To obtain the concentration-response curve of the effect of Rg3 or TEA on the K+ currents from the BKCa channel, the peak amplitudes at different concentrations of Rg3 or TEA were plot-ted. The current enhancements or inhibitions evoked by drug treatment were analyzed after subtraction of currents elicited by H2O injection. Origin software (Origin, USA) was used to fit the plot to the Hill equation: y/ymax = [A]nH/([A]nH + [EC50]nH), where y represented the peak current at a given concentration of Rg3, ymax was the maximal peak current, EC50 was the concentration of Rg3 producing a half-maximal effect, [A] was the concentra-tion of Rg3, and nH was the Hill coefficient. All values were pre-sented as mean ± S.E.M. The significance of differences be-tween mean control and treatment values was determined using Student’s t-test, where P < 0.05 was considered statisti-cally significant.
RESULTS
Treatment of Rg3 enhances BKCa channel currents in Xenopus oocytes expressing rat brain BKCa channels
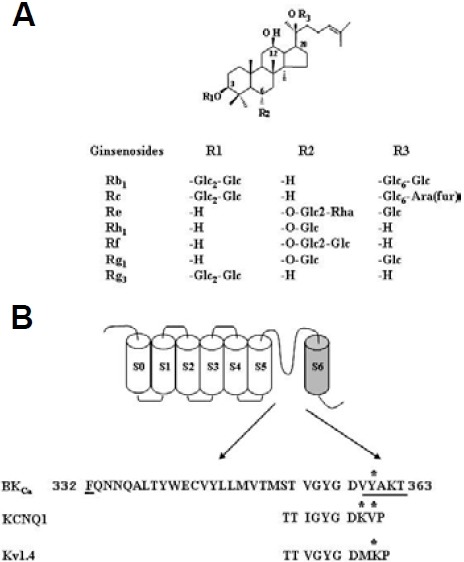
Application of a voltage step from -80 to +40 mV with duration of 400 ms at 10-s intervals to oocytes injected with cRNA encoding the BK α subunit gene elicited a large outward current in the absence of Rg3 (Fig. 2A, inset). Charybdotoxin and Iberio-toxin, highly specific peptidyl inhibitors of maxi-K channels (Candia et al., 1992; Gao et al., 2003), blocked the outward currents (98.2 ± 1.2 and 95.5 ± 2.1% inhibition by 100 nM charybdotoxin and iberiotoxin, respectively, n = 5), indicating that the BKCa channel is functionally working (Kaczorowski et al., 1996). Next, we examined the effects of various individual gin-senosides on the BKCa channel currents in Xenopus oocytes expressing BKCa channel α subunits (Fig. 1A). Rg3 (100 μM) activated the BKCa channel currents by an average of 340%, and other ginsenosides (Rb1, Rc, Rf, Rh1, and Rg1) were much less effective (Fig. 2A). As shown in Fig. 2B, application of Rg3 to oocytes injected with rSlo cRNAs resulted in a rapid stimula-tion of outward BKCa channel currents in a concentration-dependent manner. However, Rg3 had no effect on H2O-injected control oocytes that were not injected with cRNA en-coding the BK α subunit gene. Thus, the outward currents in H2O-injected control oocytes were 130 ± 25 and 131 ± 10.2 nA in the absence and presence of Rg3, respectively (n = 4). In addition, in oocytes expressing α subunit alone, Rg3 (100 μM) enhanced BKCa channel currents to a degree similar to that observed in oocytes co-expressing α and β subunits (350 ± 80.6% for α subunit alone; 367 ± 83.1% for α + β subunits, n = 4), indicating that Rg3-mediated enhancement of BKCa channel currents is independent of β subunit. These results indicated that Rg3 is most potent among various ginsenosides in enhancing BKCa channel currents.
Fig. 2. Effects of Rg3 on wild-type BKCa channel activities. (A) Effects of various ginsenosides (100 μM each) on wild-type BKCa channel currents. Inset, the representative traces elicited by ginse-noside Rb1, Rg1 and Rg3. (B) The representative traces on BKCa channel current enhancements by different concentrations of Rg3 (0, 10, 30 or 100 μM). Rg3 enhanced BKCa channel current in a con-centration-dependent manner. Currents were in response to 400 ms voltage steps to +40 mV from a holding potential of -80 mV. Data represented the mean ± S.E.M. (n = 6-7).
Rg3-enhancement of BKCa channel currents was not desensitized by repeated application and was independent of intracellular Ca2+
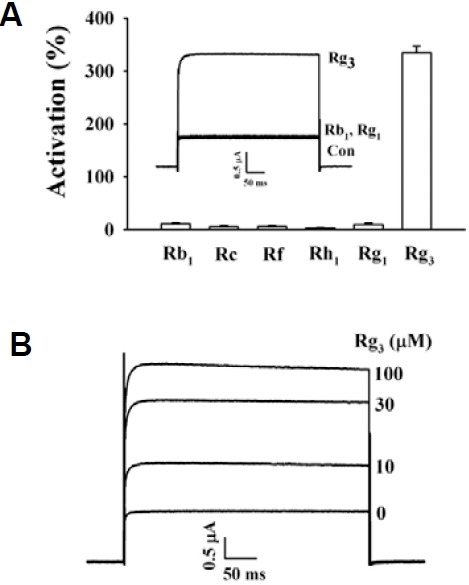
We next examined the possible changes of Rg3 effect on BKCa channel currents by repeated application of Rg3 to oocytes expressing BKCa channel. As shown in Fig. 3A, treatment with Rg3 induced an enhancement of BKCa channel currents. Oocytes stimulated with Rg3 were first washed with recording buffer for 3 min until the basal current was recovered, and then restimulated with Rg3. The second, third, or fourth BKCa current responses for Rg3 were not significantly diminished and the magnitudes of BKCa currents were 94.8 ± 10.3, 78.7 ± 15.4 and 81.0 ± 9.3 % respectively, of the first responses of Rg3 (n = 15, oocytes from three different batches of donors) (Fig. 3B). In addition, we also examined the effect of BAPTA-AM, a cell permeable intracellular Ca2+ chelator, on Rg3 enhancement of BKCa channel currents to investigate whether Rg3 enhancement of BKCa channel currents was due to the increase of intracellular free Ca2+ levels. As shown in Figs. 3C and 3D, pretreatment of BAPTA-AM (10 μM, 3 h) to oocytes had no affect Rg3-mediated BKCa channel currents. Rather, Rg3 enhanced BKCa channel currents with concentration-dependent manner, indicating that Rg3-mediated enhancement of BKCa channel currents is not desensitized and independent of intracellular Ca2+.
Fig. 3. Rg3-mediated BKCa channel current enhancements were not desensitized and were independent of intracellular Ca2+. (A) The representative peak outward current amplitude at +40 mV from a holding potential of -80 mV was measured and plotted against time before and after repeated applications of Rg3 (100 μM) for 30 s. (B) The normalized currents after repeated Rg3 treatment. BKCa channel currents were not desensitized after repeated treatment of Rg3. Data represented the mean ± S.E.M. (n = 6). (C) Oocytes were first treated with BAPTA-AM (10 μM, 3 h). The representative peak outward current amplitude at +40 mV from a holding potential of -80 mV was measured and plotted against time before and after repeated applications of the indicated concentration of Rg3. (D) The normalized currents after treatment of various concentrations of Rg3 in oocytes pre-treated with BAPTA-AM. Data represented the mean ± S.E.M. (n = 6).
The presence of Rg3 caused a rightward shift of TEA- mediated concentration curve on BKCa channel currents and vice versa
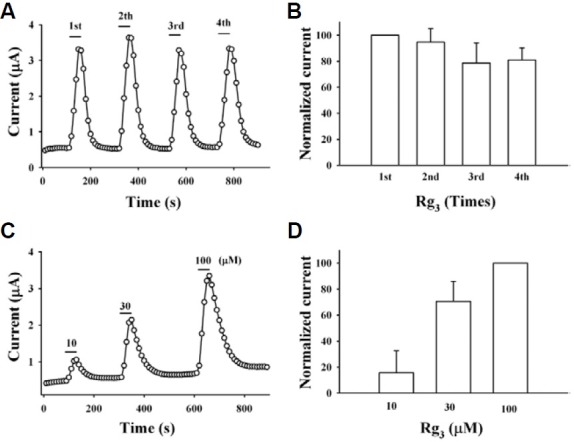
Since we demonstrated in the previous report that the regulatory effects of Rg3 on human Kv1.4 channel currents ex-pressed in Xenopus oocytes was affected by extracellular application of TEA (Lee et al., 2008), we first examined the effects of TEA on BKCa channel currents. As shown in Fig. 4A, in wild-type chan-nels, TEA inhibited BKCa channel currents in a con-centration-dependent manner. We next examined Rg3 effects on TEA-mediated inhibition of BKCa channel currents. Interest-ingly, the presence of Rg3 (30 μM) caused a rightward shift of the TEA concentration curve on BKCa channel current inhibitions from 0.8 ± 0.5 to 20.1 ± 2.3 mM (p < 0.001, compared to the absence of Rg3) (Fig. 4A). Second, we examined the effects of TEA on Rg3-mediated enhancement of BKCa channel cur-rents. The presence of TEA (300 μM) caused a rightward shift of the Rg3-mediated concentration curve on BKCa channel cur-rents from 16.0 ± 1.8 to 224.2 ± 13.8 μM (p < 0.001, compared to the absence of TEA) (Fig. 4B). In experiments using TEA, our results indicated that Rg3 enhancement of BKCa channel currents is achieved through interaction from the outside of the cell.
Fig. 4. Effect of TEA on Rg3 action and vice versa in wild-type channel and Y360I mutant effects on TEA and Rg3 action. (A) Concentration-response curves for TEA in the absence or presence of Rg3 (30 μM) in wild-type BKCa channels. The presence of Rg3 caused a significant rightward shift of TEAconcentration-response curves from 0.9 ± 0.1 to 20.1 ± 2.3 mM (p < 0.001, compared to the absence of Rg3). In addition, mutation of Y360 to Y360I in the absence of Rg3 shifted TEA concentration-response curve rightward from 0.9 ± 0.1 to 34.2 ± 2.6 mM (p < 0.001, compared to wild-type). (B) Concentration-response curves for Rg3 in the presence of TEA (300 μM) in wild-type BKCa channels. The presence of TEA caused a significant rightward shift of Rg3 concentration-response curves from 16.0 ± 1.8 to 224.2 ± 13.9 μM (p < 0.001, compared to the absence of TEA). (C) Effect of Rg3 on TEA concentration-response curve in Y360I mutant channel. Rg3 almost had no effect on TEA concentration-response curve in Y360I mutant channels. Data represented the mean ± S.E.M. (n = 8).
The pore entryway of channel is involved in Rg3-mediated enhancement of BKCa channel currents
Since Rg3 regulates human Kv1.4 and KCNQ1 plus KCNE1 channels through interaction with amino acids at the channel pore entryway (Choi et al., 2010; Lee et al., 2008), we subsequently examined the possibility of whether Rg3-induced regulations of BKCa channel activity were also mediated through interactions with the analogous amino acids from Tyr360 to The363 in the pore entryway (Fig. 1B). The mutants constructed are listed in Table 1. Since mutation Y360A attenuated Rg3-induced enhancement of BKCa channel currents (Table 1), we further substituted Y360 with other amino acid residues, including glutamine, isoleucine, lysine, serine, and valine (generating Y360Q, Y360I, Y360K, Y360S and Y360V, respectively), and examined the effect of Rg3 on BKCa channel currents in oocytes expressing these mutants. Our results revealed that mutation Y360I greatly attenuated Rg3-induced enhancement of BKCa channel currents (Figs. 5A and 5B) and was followed by order of Y360S > Y360E > Y360A. These results indicate that substitutions of Y360 with amino acid residues such as alanine, isoleucine, serine, or glutamate attenuate the Rg3-induced enhancement of BKCa channel currents compared to wild-type. However, substi-tution of other amino acid residues in other positions such as charybdotoxin binding site (F332A) had no discernable effects (Gao et al., 2003, Table 1).
Table 1.
Effect of Rg3 on wild-type and various mutant BKCa channels expressed in Xenopus laevis oocytes. Currents were elicited by single-step voltage pulses from -80 to +40 mV.
| Imax | EC50 | nH | |
|---|---|---|---|
| Wild-type | 370.4 ± 35.1 | 15.1 ± 3.1 | 1.7 ± 0.5 |
| F332A | 371.4 ± 52.4 | 41.6 ± 13.7 | 2.7 ± 1.9 |
| V359A | 641.3 ± 67.9 | 26.8 ± 7.4 | 1.4 ± 0.5 |
| Y360A | 287.2 ± 12.8# | 43.8 ± 4.5* | 2.5 ± 0.4 |
| Y360I | 93.4 ± 0.1* | 46.8 ± 0.1* | 1.1 ± 0.0 |
| Y360K | 409.6 ± 29.9 | 15.5 ± 2.6 | 1.4 ± 0.2 |
| Y360Q | 440.0 ± 36.4 | 16.4 ± 3.2 | 1.3 ± 0.2 |
| Y360S | 181.8 ± 27.5* | 34.4 ± 10.7# | 1.3 ± 0.3 |
| Y360V | 598.0 ± 97.5 | 43.9 ± 17.2* | 1.5 ± 0.6 |
| Y360E | 214.2 ± 25.9* | 13.6 ± 3.4 | 2.5 ± 1.3 |
| A361T | 373.9 ± 43.4 | 14.7 ± 3.3 | 1.2 ± 0.3 |
| K362A | 341.1 ± 57.6 | 12.5 ± 3.0 | 1.1 ± 0.2 |
| T363A | 311.1 ± 45.9 | 15.0 ± 1.0 | 2.2 ± 0.2 |
Imax (%), maximal activation by Rg3. Rg3 activation was determined as the % difference of currents in the presence and absence of Rg3. EC50, Values were mean ± S.E.M (n = 8-10/group). nH, Hill coefficient. #p < 0.05, *p < 0.01 compared with the wild-type BKCa channel.
Fig. 5. Effects of Rg3 on mutant BKCa channel activities. (A) The representative traces on Rg3-mediated BKCa channel current enhancement in Y360I mutant BKCa channels. Rg3 slightly enhanced Y360I channel currents. (B) Concentration-response curves of Rg3 on wild-type and various mutant BKCa channel currents at Y360 residue. The representative outward current was measured from an oocyte injected with cRNAs that encoded BKCa channel wild-type or mutant α subunit. Solid lines have been fitted to the Hill equation. Currents were in response to 400 ms voltage steps to +40 mV from a holding potential of -80 mV. Data represented the mean ± S.E.M. (n = 6-7).
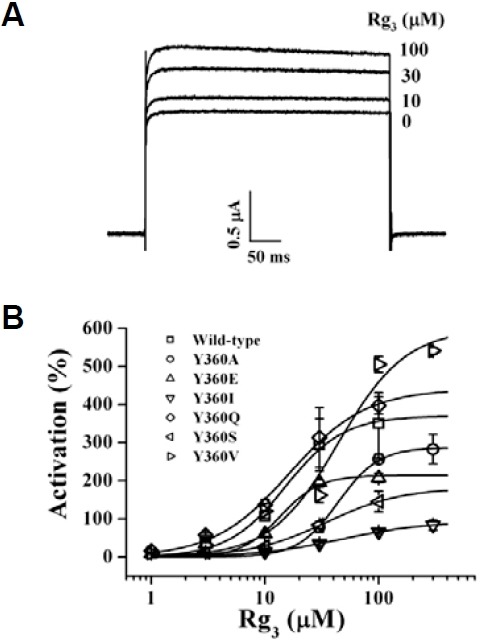
Mutation of Y360 to Y360I caused TEA concentration-response curve to rightward shift and abolished Rg3 effects on TEA concentration-response curve observed in wild-type channel
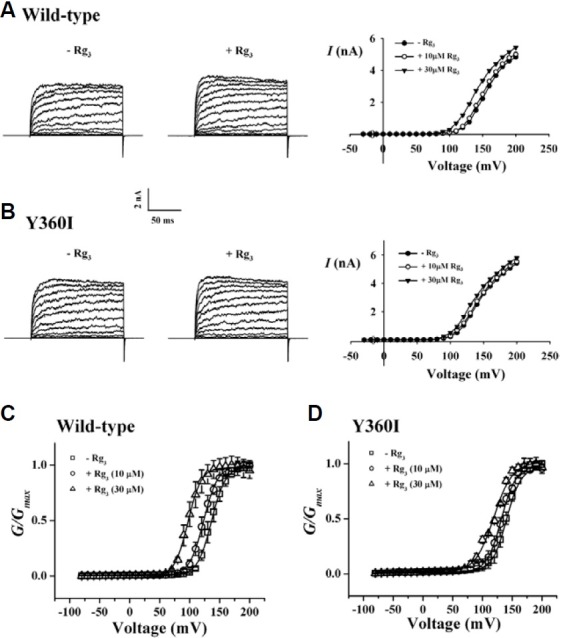
Interestingly, the previous report showed that Y308 residue of Drosophila BKCa (dSlo) channel is the extracellular TEA binding site and that mutation of Y308 to Y308V almost abolished TEA sensitivity (Shen et al., 1994). Similarly, mutation of Y360, which is the analogous amino acid of Y308 residue of Drosophila BKCa (dSlo) channel, to Y360I also caused a shift of the TEA concentration response curve towards the right and we meas-ured IC50 values of 0.9 ± 0.1 and 34.2 ± 2.5 mM (p < 0.001, compared to wild-type) in wild-type and Y360I mutant, respec-tively (Fig. 4A). In addition, we examined Rg3 effects on TEA-mediated BKCa channel regulation using Y360I mutant channel. As shown in Fig. 4C, Rg3 had almost no effect on TEA-mediated inhibition of mutant BKCa channel currents. These results indicated that mutation of Y360 to Y360I caused a loss of Rg3 effects on TEA-mediated BKCa channel regulations (Lagrutta et al., 1998), again showing a possibility that Rg3 enhanced BKCa channel currents by sharing the extracellular TEA binding site of the channel.
Rg3 activated wild-type but not mutant Y360I BKCa channels in outside-out configuration
Since Rg3 enhanced BKCa channel currents, we next examined Rg3 effects on BKCa channel currents using outside-out patch clamp configuration in wild-type and Y360I mutant channels. In current-voltage (I-V) relationships the application of 10 μM Rg3 slightly enhanced wild-type BKCa channel currents but the application of 30 μM Rg3 caused a large enhancement of wild-type BKCa channel currents (Fig. 6A, right panel). However, in mutant Y360I channels, the enhancing effect of Rg3 (30 μM) was greatly attenuated (Fig. 6B, right panel). The G-V relationship was also constructed for Rg3 action in wild-type and Y360I mutant channels. In the absence of Rg3 the half-activation volt-age (V1/2) was 136.4 ± 0.4 mV. The presence of 10 and 30 μM Rg3 caused a shift of V1/2 to 123.3 ± 0.3 and 96.30 ± 0.4 mV in wild-type, respectively (n = 5, p < 0.05, compared to the presence of 30 μM Rg3). In Y360I mutant channels, V1/2 was 140.4 ± 0.4 mV in the absence of Rg3. The presence of 10 and 30 μM Rg3 did not cause a significant shift of V1/2 to 131.8 ± 0.5 and 120.0 ± 1.0 mV, respectively (n = 5) (Figs. 6C and 6D). These results again indicate that Rg3 activates BKCa channels by inducing more negative shift of voltage-activation profile and that mutation of Y360 to Y360I attenuates Rg3 action.
Fig. 6. Effects of Rg3 on wild-type and Y360I mutant BKCa channel currents in outside-out patch clamp configuration. (A and B) Effects of 30 μM Rg3 on the macroscopic I-V relationship of wild-type and Y360I mutant BKCa channel in the presence of 1 μM Ca2+. Representative current traces of macroscopic wild-type (A) or Y360I mutant (B) BKCa channel currents. Their respective concentrantion-dependent I-V relationships are shown in right panel for wild-type and Y360I mutant channels. Ionic currents were elicited with 200-ms step-pulses of different voltage protocols: -30 to 200 mV in 10-mV in-crements from holding potentials of -50 mV. Each data point in I-V relationships represents the mean current value measured over 198 ± 1.5 ms of test pulses. (C and D) Effects of Rg3 on the G-V relationships of wild-type (C) or Y360I mutant (D) BKCa channel currents. Conductance values were obtained from peak tail currents and normalized to the maximum conductance observed at 10 and 30 μM Rg3. V½ values were obtained by fitting independent data sets with Boltzmann function. Each symbol represents the conduc-tance values obtained from two different concentration of Rg3. Each data point represents the mean value ± S.E.M. (n = 5-6 each).
DISCUSSION
In the nervous system, stimulations above threshold by various stimuli cause activation of voltage-dependent Ca2+ channels that induce an elevation of [Ca2+]i and its resulting elevation initiates signaling pathways for the release of a variety of neuro-transmitters from presynaptic sites and Ca2+-dependent cellular events (Hille, 2001). In addition to the role as a second messenger, the elevation of [Ca2+]i in presynaptic sites is also coupled to activation of BKCa channels, which are usually co-localized with voltage-dependent Ca2+ channels in presynaptic sites for repolarization or return to resting membrane potential (Berkefeld et al., 2006). Thus, since BKCa channels play a negative feedback control with [Ca2+]i, BKCa channel activators or openers could be utilized as neuroprotective agents against excessive Ca2+ influx through depolarization or excitatory neurotransmitters (Lawson, 2000). Although BKCa channels are involved in synaptic regulation in the nervous system, relatively little has been studied about the molecular mechanisms by which ginsenosides regulate BKCa channel activity.
In the present study, we made three major findings. First, we observed that Rg3 among various ginsenosides enhanced BKCa channel currents in concentration- and voltage-dependent man-ners, while Rg3-enhancement of BKCa channel currents was independent of intracellular Ca2+. Second, the presence of TEA significantly shifted the concentration response curve of Rg3 towards the right and vice versa in wild-type channel. And third, mutation of the channel pore entry site (Y360 to Y360I), which is also known as extracellular TEA binding site, greatly attenu-ated Rg3 enhancement of BKCa channel currents and caused a rightward shift of the TEA concentration-response curve similar to Rg3, indicating that Rg3 may interact with the external TEA biding site via residue Tyr360.
BKCa channels consist of α and β subunits. The four α subunits assemble to form the channel pore and the auxiliary β subunits function to provide the biophysical and pharmacological properties of channels, including Ca2+ and voltage sensitivity, and gating kinetics (Ha et al., 2006; Qian et al., 2002; Valverde et al., 1999; Wallner et al., 1999; Xia et al., 1999). Some BKCa channel openers such as NS-1619, BMS-204352 and TIBC act independently from the β subunit (Coghlan et al., 2001; Dick et al., 2002; Ha et al., 2006; Imaizumi et al., 2002; Valverde et al., 1999; Vergara et al., 1998), whereas other openers such as dehydrosoyasaponin-I and 17-β-estradiol require β subunit for their action (Giangiacomo et al., 1998; Valverde et al., 1999). In the present study, we found that the Rg3-enhancement of BKCa channel current was independent of β subunit (Fig. 2), suggesting that Rg3 effects on BKCa channel are achieved through interaction with α subunit.
Therefore, we next sought to examine the possible mecha-nisms by which Rg3 achieved the enhancement of BKCa chan-nel currents through interaction with α subunit. As shown in Fig. 1A, Rg3 is a type of glycoside and consists of a carbohydrate portion, a steroid backbone and an alkene side chain (Lee et al., 2008). In a previous report we demonstrated using homology modeling methods that carbohydrate portions of Rg3 form hy-drogen bonds and maintain stable interactions with amino acids of channel proteins (Lee et al., 2008). In addition, we also demonstrated that Rg3 inhibits Kv1.4 channel currents through interaction with K531 residue, which is located at the channel pore entryway and is also one of the extracellular TEA binding and K+ activation sites. In contrast, Rg3 activates KCNQ + KCNE channel through interaction with Lys and Val residues at channel pore entryway (Choi et al., 2010). Based on previous experimental data (Choi et al., 2010; Lee et al., 2008), we first constructed mutant channels at the channel pore entryway of α subunit and found that Rg3 effects on BKCa channel current enhancements were also greatly attenuated in Y360I mutant channels.
We observed some consistent results in Rg3-mediated K+ channel regulation experiments. As shown in Fig. 1B, K+ channels exhibit a common feature in that they all have pore-lining P-loop with a consensus amino acid sequence -TXGYGD- which is called the K+ channel “signature sequence” (Heginbotham et al., 1992; 1994). These residues, repeated in each of the four α subunits, form the K+ selectivity filter. In previous studies, mutations of first or second amino acid after -TXGYGD- abolished Rg3-mediated inhibition (i.e., Kv1.4 channel) (Lee et al., 2008) or Rg3-mediated activation (i.e., KCNQ + KCNE) (Choi et al., 2010). These results indicate that Rg3 interacts with amino acid residues in common interaction regions near the “signature sequence” in subsets of K+ channels examined.
However, it could not be excluded that the Rg3 might regulate BKCa channel activity through allosteric mechanisms. In this case, the mutations introduced in Y360 residue might induce the conformational changes and interfere the interaction of Rg3 with channel proteins, thus attenuating Rg3-enhancement of BKCa channel currents. Taken together, although Y360 residue plays an important role in Rg3-mediated BKCa channel activation, further studies will be required to elucidate the detailed mecha-nisms how Rg3 regulates BKCa channels.
In summary, we found that Rg3 enhances BKCa channel currents without desensitization after repeated treatment and with a Ca2+-independent manner. We have used site-directed muta-genesis to further characterize the Rg3 interaction site with BKCa channel. We found that the Y360 residue at the channel pore entryway of brain BKCa channel is involved in Rg3-media-ted BKCa channel regulations. These novel findings provide insight into the molecular basis of the pharmacological effects of ginseng in nervous systems.
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea Funded by the Ministry of Education, Science and Technology (R01-2008-000-10448-0), Priority Research Centers Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2009-0093824), and Brain Korea 21 To S. Y. Nah.
References
- 1.Berkefeld H., Sailer C.A., Bildl W., Rohde V., Thumfart J.O., Eble S., Klugbauer N., Reisinger E., Bischofberger J., Oliver D., et al. BKCa-Cav channel complexes mediate rapid and lo-calized Ca2+-activated K+ signaling. Science. (2006);314:615–620. doi: 10.1126/science.1132915. [DOI] [PubMed] [Google Scholar]
- 2.Candia S., Garcia M.L., Latorre R. Mode of action of iberiotoxin, a potent blocker of the large conductance Ca2+-activated K+ channel. Biophys. J. (1992);63:583–590. doi: 10.1016/S0006-3495(92)81630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi S.H., Lee J.H., Pyo M.K., Lee B.H., Shin T.J., Hwang S.H., Kim B.R., Lee S.M., Oh J.W., Kim H.C., et al. Mutations Leu427, Asn428, and Leu431 residues within transmembrane domain-I-segment 6 attenuate ginsenoside-mediated L-type Ca2+ channel current inhibitions. Biol. Pharm. Bull. (2009);32:1224–1230. doi: 10.1248/bpb.32.1224. [DOI] [PubMed] [Google Scholar]
- 4.Choi S.H., Shin T.J., Lee B.H., Chu D.H., Choe H., Pyo M.K., Hwang S.H., Kim B.R., Lee S.M., Lee J.H., et al. Ginsenoside Rg3 activates human KCNQ1 K+ channel currents through interacting with the K318 and V319 residues: a role of KCNE1 subunit. Eur. J. Pharmacol. (2010);63:138–147. doi: 10.1016/j.ejphar.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Coghlan M.J., Carroll W.A., Gopalakrishnan M. Recent developments in the biology and medicinal chemistry of potassium channel modulators: update from a decade of progress. J. Med. Chem. (2001);44:1627–1653. doi: 10.1021/jm000484+. [DOI] [PubMed] [Google Scholar]
- 6.Dick G.M., Hunter A.C., Sanders K.M. Ethylbromide tamoxifen, a membrane-impermeant antiestrogen, activates smooth muscle calcium-activated large-conductance potassium channels from the extracellular side. Mol. Pharmacol. (2002);61:1105–1113. doi: 10.1124/mol.61.5.1105. [DOI] [PubMed] [Google Scholar]
- 7.Gao Y.D., Garcia M.L. Interaction of agitoxin2, charybdotoxin, and iberiotoxin with potassium channels: selectivity between voltage-gated and Maxi-K channels. Proteins. (2003);52:146–54. doi: 10.1002/prot.10341. [DOI] [PubMed] [Google Scholar]
- 8.Ghatta S., Nimmagadda D., Xu X., O’Rourke S.T. Large-conductance, calcium-activated potassium channels: structural and functional implications. Pharmacol. Ther. (2006);110:103–116. doi: 10.1016/j.pharmthera.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Giangiacomo K.M., Kamassah A., Harris G., McManus O.B. Mechanism of maxi-K channel activation by dehydro-soyasaponin-I. J. Gen. Physiol. (1998);112:485–501. doi: 10.1085/jgp.112.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ha T.S., Lim H.H., Lee G.E., Kim Y.C., Park C.S. Electrophysiological characterization of benzofuroindole-induced potentiation of large-conductance Ca2+-activated K+ channels. Mol. Pharmacol. (2006);69:1007–1114. doi: 10.1124/mol.105.016170. [DOI] [PubMed] [Google Scholar]
- 11.Hamill O.P., Marty A., Neher E., Sakmann B., Sigworth F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. (1981);391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 12.Heginbotham L., Abramson T., Mackinnon R. A functional conncetion between the pores of distantly related ion channels as revealed by mutant K+ channels. Science. (1992);258:1152–1155. doi: 10.1126/science.1279807. [DOI] [PubMed] [Google Scholar]
- 13.Heginbotham L., Lu Z., Abramson T., Mackinnon R. Mutations in the K+ channel signature sequence. Biophys. J. (1994);66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hille B. Ion channels of excitable membranes. Sinauer Associates, Inc.; (Sunderland, MA, USA): (2001). [Google Scholar]
- 15.Imaizumi Y., Sakamoto K., Yamada A., Hotta A., Ohya S., Muraki K., Uchiyama M., Ohwada T. Molecular basis of pimarane compounds as novel activators of large-conductance Ca2+-activated K+ channel α-subunit. Mol. Phar-macol. (2002);62:836–846. doi: 10.1124/mol.62.4.836. [DOI] [PubMed] [Google Scholar]
- 16.Kaczorowski G.J., Knaus H.G., Leonard R.J., McManus O.B., Garcia M.L. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. J. Bioenerg. Biomembr. (1996);28:255–267. doi: 10.1007/BF02110699. [DOI] [PubMed] [Google Scholar]
- 17.Lagrutta A.A., Shen K.Z., Rivard A., North R.A., Adelman J.P. Aromatic residues affecting permeation and gating in dSlo BK channels. Pflugers Arch. (1998);435:731–739. doi: 10.1007/s004240050575. [DOI] [PubMed] [Google Scholar]
- 18.Lawson K. Is there a role for potassium channel openers in neuronal ion channel disorders? Expert. Opin. Investig. Drugs. (2000);9:2269–2280. doi: 10.1517/13543784.9.10.2269. [DOI] [PubMed] [Google Scholar]
- 19.Lee J.H., Jeong S.M., Kim J.H., Lee B.H., Yoon I.S., Lee J.H., Choi S.H., Kim D.H., Rhim H., Kim S.S., et al. Characteristics of ginsenoside Rg3-mediated brain Na+ current inhibition. Mol. Pharmacol. (2005);68:1114–1126. doi: 10.1124/mol.105.015115. [DOI] [PubMed] [Google Scholar]
- 20.Lee J.H., Lee B.H., Choi S.H., Yoon I.S., Pyo M.K., Shin T.J., Choi W.S., Lim Y., Rhim H., Won K.H., et al. Ginsenoside Rg3 inhibits human Kv1.4 channel currents by interacting with the Lys531 residue. Mol. Pharmacol. (2008);73:619–626. doi: 10.1124/mol.107.040360. [DOI] [PubMed] [Google Scholar]
- 21.Lu L., Montrose-Rafizadeh M., Guggino W.B. Ca2+-activated K+ channels from rabbit kidney medullary thick ascending limb cells expressed in Xenopus oocytes. J. Biol. Chem. (1990);265:16190–16194. [PubMed] [Google Scholar]
- 22.Ohi Y., Yamamura H., Nagano N., Ohya S., Muraki K., Wata-nabe M., Imaizumi Y. Local Ca2+ transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder. J. Physiol. (2001);534:313–326. doi: 10.1111/j.1469-7793.2001.t01-3-00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patton C., Thomson S., Epei D. Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium. (2004);35:427–431. doi: 10.1016/j.ceca.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Qian X., Nimigean C.M., Niu X., Moss B.L., Magleby K.L. Slo1 tail domains, but not the Ca2+ bowl, are required for the beta 1 subunit to increase the apparent Ca2+ sensitivity of BK channels. J. Gen. Physiol. (2002);120:829–843. doi: 10.1085/jgp.20028692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salkoff L., Butler A., Ferreira G., Santi C., Wei A. High-conductance potassium channels of the SLO family. Nat. Rev. Neurosci. (2006);7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber M., Salkoff L. A novel calcium-sensing domain in the BK channel. Biophys. J. (1997);73:1355–1363. doi: 10.1016/S0006-3495(97)78168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schreiber M., Yuan A., Salkoff L. Transplantable sites confer calcium sensitivity to BK channels. Nat. Neurosci. (1999);2:416–421. doi: 10.1038/8077. [DOI] [PubMed] [Google Scholar]
- 28.Shen K.Z., Lagrutta A., Davies N.W., Standen N.B., Adelman J.P., North R.A. Tetraethylammonium block of slowpoke calcium-activated potassium channels expressed in Xenopus oocytes. Pflugers Arch. (1994);426:440–445. doi: 10.1007/BF00388308. [DOI] [PubMed] [Google Scholar]
- 29.Toro L., Wallner M., Meera P., Tanaka Y. Maxi-KCa, a unique member of the voltage-gated K+ channel superfamily. News Physiol. Sci. (1998);13:112–117. doi: 10.1152/physiologyonline.1998.13.3.112. [DOI] [PubMed] [Google Scholar]
- 30.Valverde M.A., Rojas P., Amigo J., Cosmelli D., Orio P., Bahamonde M.I., Mann G.E., Vergara C., Latorre R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. (1999);285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 31.Vergara C., Latorre R., Marrion N.V., Adelman J.P. Calcium-activated potassium channels. Curr. Opin. Neurobiol. (1998);8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- 32.Wallner M., Meera P., Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a trans-membrane β-subunit homolog. Proc. Natl. Acad. Sci. USA. (1999);96:4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanner S.G., Koch R.O., Koschak A., Trieb M., Garcia M.L., Kaczorowski G.J., Knaus H.G. High-conductance calcium-activated potassium channels in rat brain: pharmacology, distribution, and subunit composition. Biochemistry. (1999);38:5392–400. doi: 10.1021/bi983040c. [DOI] [PubMed] [Google Scholar]
- 34.Wei A., Solaro C., Lingle C., Salkoff L. Calcium sensitivity of BK-type KCa channels determined by a separable domain. Neuron. (1994);13:671–681. doi: 10.1016/0896-6273(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 35.Weiger T.M., Hermann A., Levitan I.B. Modulation of calcium-activated potassium channels. J. Comp. Physiol. A. Neuroethol. Sens. Neural. Behav. Physiol. (2002);188:79–87. doi: 10.1007/s00359-002-0281-2. [DOI] [PubMed] [Google Scholar]
- 36.Xia X.M., Ding J.P., Lingle C.J. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J. Neurosci. (1999);19:5255–5264. doi: 10.1523/JNEUROSCI.19-13-05255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


