Abstract
Histone deacetylase inhibitors (HDACIs) that modulate gene expression by inhibiting HDAC enzymes may contribute to the survival of immature hippocampal neurons. However, it remains unknown how and when HDACIs regulate the survival of newly generated immature hippocampal neurons. In the present study, if the treatment of valproic acid (VPA) and sodium butyrate (SBt) in the specific time window during the development of newly generated neurons resulted in the increased survival of bromodeoxyuridine (BrdU)(+) neurons in the dentate gyrus (DG) of hippocampus in mice was investigated. It was found that the number of BrdU(+) cells, the expressions of anti-apoptotic Bcl-2 family members and pCREB were increased by HDACIs when HDACIs were treated no later than 2-3 weeks after BrdU labeling. This suggests that epigenetic modification within a specific time window is critical for the survival of newborn hippocampal neurons by inhibiting the apoptotic pathway.
Keywords: HDAC, neurogenesis, survival, valproate
INTRODUCTION
Adult hippocampal neurogenesis is a multi-step process: newborn cells are proliferating, determining their fate, maturing and surviving by way of various regulatory factors. The implications of what adult hippocampal neurogenesis means regarding brain function has yet to be answered. It has been reported that mental activities enhance adult hippocampal neurogenesis, especially by the increased survival of newborn neurons (Sisti et al., 2007). Survival is one of the key issues in a non-pathological condition like adult hippocampal neurogenesis. Interesting findings regarding the survival of newborn neurons were demonstrated: First, the survival of newborn hippocampal neurons is related to their age or maturity. More than half of newborn neurons die in the course of maturating to become functional granule cells (Kempermann et al., 2003). Second, the survival of immature newborn neurons is increased by neuronal activity (Kempermann et al., 1998) and third, such activity-dependent survival is more predominant within a critical time window; during maturation to functional neurons (Tashiro et al., 2007). Accordingly, the ‘use it or lose it’ principle proposed previously (Swaab, 1991) may apply to newly generated hippocampal neurons.
Histone deacetylase inhibitors (HDACIs) facilitate gene expression by inhibiting the action of HDAC enzymes that deacetylate lysine and arginine-rich N-terminal domains of histones so that the acetylation by histone acetyltrans-ferase (HAT) is favorable (Thiagalingam et al., 2003). Recently, HDACIs were given attention as a neuroprotective agent regarding neuropathological dysfunction. For example, sodium phenylbutyrate, one of the histone deacetylase inhibitors, significantly extended survival times and improved both the clinical and neuropathological phenotypes in animal models (Ryu et al., 2005). Another histone deacetylase inhibitor, SBt, ameliorated the phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy (Minamiyama et al., 2004). Although the mechanism underlying the neuroprotective effect remains to be elucidated, those findings suggest that HDACIs might be a target not only for brain tumor therapy but also in various neuropathological settings such as neuronal excitotoxi-city and encephalomyelitis (Kanai et al., 2004; Langley et al., 2008).
HDACIs have been studied for potential beneficial agents which promote brain function like learning and memory, as chromatin remodeling like histone acetylation is involved in long-term memory formation (Vecsey et al., 2007). Furthermore, the activation of NMDA receptors in area CA1 in vitro increased the acetylation of histone H3, and SBt or TSA enhanced the induction of long-term potentiation at Schaffer-collateral synapses in area CA1 of the hippocampus (Levenson et al., 2004).
In the present study, it is hypothesized that HDACIs might facilitate the survival of immature hippocampal neurons within a specific time window during differentiation, consequently augmenting the number of neurons integrated into functioning networks. Anti-apoptotic Bcl-2 family members might be involved in promoting the survival of newborn neurons and thus it is explored whether bcl-2 members are affected by HDACIs.
MATERIALS AND METHODS
Animals and drug treatment
Nine to ten-week-old male C57BL/6 mice were purchased from a local animal distributor (Koatech, Korea). They were divided into 3 groups: saline, VPA, and SBt groups. Six mice per each group were housed with 2-3 mice per cage, then bred and maintained under pathogen-free conditions. Experiments were performed in accordance with the institutional guidelines of the Hanyang University Veterinary Committee. To evaluate the in vivo survival of newborn neurons in the subgranular zone (SGZ) of adult hippocampi, BrdU (150 mg/kg body weight, Sigma-Aldrich, USA) were admini-stered intraperitoneally twice a day for 2 days to mice from each group. Five days later, VPA (300 mg/kg bodyweight, Calbiochem) or SBt (1.2 g/kg body weight, Sigma-Aldrich) was injected daily for 7 consecutive days, with dosages of VPA and SBt based on previous reports (Jessberger et al., 2007; Leven-son et al., 2004). Animals were sacrificed on the day following the last injection
Hippocampal neural progenitor cell cultures
Embryonic day 14.5 (E14.5) embryos were dissected out of C57BL/6 adult pregnant female mice. The hippocampal regions of embryonic brains were isolated in calcium/magnesium free HBSS. Cells were plated at 2.5 × 104 cells/cm2 on 10-cm-diameter dishes coated with 15 μg/ml poly-L-ornithine and 1 μg/ml fibronectin (Invitrogen). Cells were placed in an N2 media supplemented B27 (Invitrogen) at 37℃ in 95% air/5% CO2 gas incubator. Basic fibroblast growth factor (bFGF, 20 ng/ml, R&D Systems) and EGF (20 ng/ml, R&D Systems) were required daily in order to expand the population of neural precursors and the medium was changed every other day. Cells on 80% confluency were passaged and maintained in B27-supplemented N2 media containing bFGF and EGF at 6 × 104 cells/cm2. These subcultured progenitors were induced to differentiate by the withdrawal of bFGF and EGF, and kept in differentiation media (Neurobasal medium supplemented with B27) for 3-5 days. The medium was changed once every 3 days. VPA (1 mM) or SBt (1 mM) was treated 3-5 days after the induction of differentiation.
Histone extraction from hippocampi
Histone proteins were extracted from hippocampi by the modified method of a previous report (Chwang et al., 2007). Briefly, samples prepared with a Dounce homogenizer were centri-fuged at 6,800 × g for 10 min. The pellet containing the nuclear fraction was resuspended in 1 ml of 0.4 N H2SO4 for 30 min and centrifuged at 14,000 × g for 10 min. The supernatant was mixed with 250 μl of cold 100% trichloroacetic acid to precipitate proteins. The precipitate was placed on ice at least for 30 min and centrifuged at 14,000 × g for 30 min. The pellet was washed with 1 ml of acidified acetone (0.1% HCl) and 1 ml of pure acetone for 5 min each. After being air-dried for 15 min at room temperature, the pellet was resuspended by 10 mM Tris (pH 8) and stored at -80℃ for further studies.
Western blotting
The same amount of protein extracts were mixed with SDS sample buffer and boiled for denaturation. The samples were then electrophoresed on 10 or 15% polyacrylamide gel and transferred onto nitrocellulose membrane filters (Amersham Pharmacia Biotech). Blots were blocked with 5% non-fat milk in TTBS (0.05% Tween-20 in 1X TBS) for 1 h and incubated overnight at 4℃ with primary antibodies. The primary antibodies used are as follows; anti-p-CREB (Ser133) (1:500, Abcam Inc), anti-CREB (1:500, Cell Signaling Technology), anti-Bcl-2 (1:1000, Sigma-Aldrich), anti-Bcl-xL (1:1000, Cell Signaling).
Immunohistochemistry
After the brains were sliced into 30-μm-thick serial sections using a freezing microtome, a series of one-in-eight sections starting from the emerging point of hippocampal tissue was used for BrdU immunostaining (six sections per brain, 180 μm apart). Immunoperoxidase staining was performed using DAB (3,3′-Diaminobenzidine). Rabbit anti-phospho (ser133)-CREB antibody (1:300, Santa Cruz Biotechnology) was used, detected by the biotinylated anti-rabbit IgG antibody (Vector Laboratories). For double immunofluorescent staining for BrdU and Dcx, sections were incubated in 50% formamide/50% 2X SSC buffer (0.3 M NaCl and 0.03 M sodium citrate) at 65℃ for 2 h and rinsed in 2X SSC buffer for 10 min. They were then incubated in 2 N HCl for 30 min at 37℃ and rinsed in 0.1 M boric acid (pH 8.5) for 15 min. After washing with PBS, the sections were in-cubated in a blocking buffer for 1 h at room temperature. Sections were then incubated with rat anti-BrdU antibody (1:200, Abcam) and goat anti-Dcx antibody (1:200, Santa Cruz Biotechnology) in a blocking buffer overnight at 4℃. The sections were then further incubated in PBS containing such secondary antibodies for 1 h at room temperature as Alexa Fluor 546-conjugated anti-rat IgG and Alexa Fluor 488-conjugated anti-goat IgG (Molecular Probes, USA). The sections were mounted on glass slides with Vectashield (Vector Laboratories).
Statistical analysis
All experiments were performed at least three times in triplicate. Optical densities were measured using Image J software. Quantified data is presented as mean ± SEM. The significance of differences was assessed by an unpaired Student’s t-test (*p < 0.05).
RESULTS
VPA and SBt promote the survival of newborn neurons in the mouse hippocampal SGZ
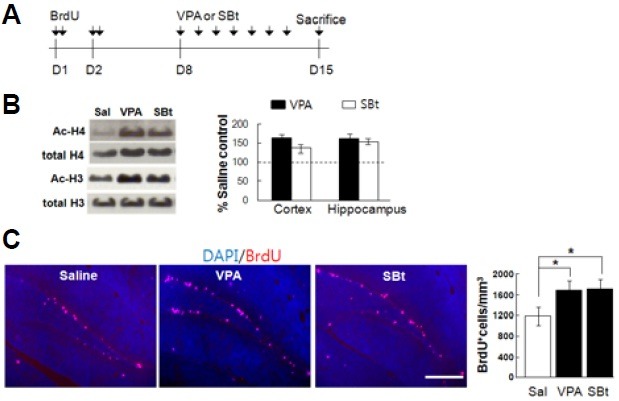
To trace the newborn neurons, BrdU (150 mg/kg body weight/ pulse) was intraperitoneally administrated at 2 pulses per day for 2 days to nine-to-ten-week-old male C57BL/6 mice (n = 6 per group). As previous studies reported that newborn neurons of 7-14 days of birth are most susceptible to neuronal activity (Ambrogini et al., 2000; Tashiro et al., 2007), mice of each group were given normal saline, VPA (300 mg/kg body weight), or SBt (1.2 g/kg body weight) daily for 7 consecutive days, 5 days after the last BrdU injection and then sacrificed for BrdU(+) cell counting. Figure 1A shows schematics of the experimental protocol employed for BrdU(+) cell counting. The single pulse injection of HDACIs substantially increased the acetylation of histone 3 (H3) and histone 4 (H4) proteins in the hippocampus as well as in the cortex (Fig. 1B; Cortex: VPA; acetylated H3 = 120.1%, acetylated H4 = 203.7%, SBt; acetylated H3 = 116.8%, acetylated H4 = 228.8%, Hippocampus: VPA; acetylated H3 = 160.5%, acetylated H4 = 162.8%, SBt; acetylated H3 = 152.1%, acetylated H4 = 136.0%; values were normalized to salinetreated control, n = 2 per group). The number of BrdU(+) cells was also significantly increased in the VPA and SBt groups (Fig. 1C).
Fig. 1. VPA and SBt enhance the survival of newborn neurons in the mouse hippocampal SGZ. (A) The paradigm setup employed for studying the survival effect of VPA and SBt. A detailed procedure is described in the material and method. (B) Representative immunoblots for actylated H3 and H4 and normalized values. Single pulse treatment of VPA (300 mg/kg) or SBt (1.2 g/kg) significantly enhanced acetylation of H3 and H4 histones in both the cortex and the hippocampus compared to those of saline treatment. Values are expressed as percentage of saline treatment group (Sal) and represent mean ± SEM (n = 2 per group). (C) Representatives are immunohistograms of BrdU (red) and DAPI (blue). (Graph) The number of BrdU(+) cells is increased by VPA and SBt in hippocampal SGZ. Normalized graph indicates BrdU(+) cells in a unit mm3. Scale bar = 100 μm.
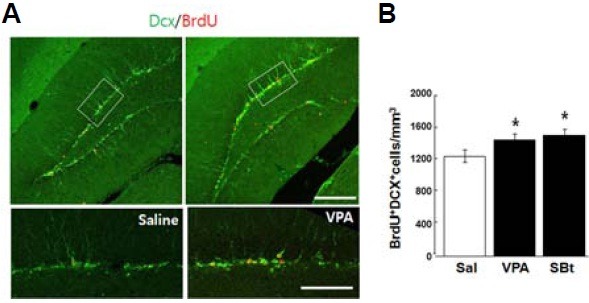
Doublecortin (Dcx) is an immature neuronal marker. To see the degree to which BrdU(+) cells are destined to become neu-rons , BrdU and Dcx double-labeled cells were counted in the granular layer (Fig. 2A). Interestingly, the HDACIs groups have a higher total number of cells co-labeled for BrdU and Dcx (BrdU+Dcx+) compared to a saline group (Fig. 2B; mean ± SEM; saline,1231 ± 46 /mm3; VPA, 1427 ± 59/mm3; SBT, 1487 ± 76 /mm3, n = 6 per group, *p < 0.05, Student’s t- test), suggesting that increased acetylation of histones by HDACIs treatment might be related to the promoted survival of newborn neurons in mouse hippocampal SGZ.
Fig. 2. Increased survival of new born neurons by HDACIs. (A) Representative double immunostaining images for BrdU and Dcx. Cells co-labeled [H6] for BrdU and Dcx (BrdU+Dcx+) are more frequently detected in VPA or SBt treated groups. Insets are magnified in bottom panel. (B) Values are average BrdU+Dcx+ cells/mm3 granule layer ± SEM. *p < 0.05, Student’s t test. Scale bar = 100 μm.
HDAC increases the activation of CREB and anti-apoptotic molecules, Bcl-2 and Bcl-xL
The immunoreactivity for pCREB (Fig. 3A) was remarkably increased in DG in HDACIs treated groups compared to the saline group, which is consistent with the results of Western blotting (Figs. 3B and 3C). Notably, the CA1 region showed a similar pattern of pCREB immunoreactivity in both saline and HDACIs groups (Fig. 3A). pCREB(+) cells were mainly detect-ed within the inner half of the granular layer where immature neurons locate, which is in accordance with a previous report (Nakagawa et al., 2002) (Fig. 3A).
Fig. 3. HDACIs increase the activation of CREB and anti-apoptotic molecules, Bcl-2 and Bcl-xL, in cultured immature neurons. (A) Each hippocampal slice was prepared by sacrificing mice 6 h after systemic injection with saline, VPA, and SBt. The expression level of phosphorylated CREB (pCREB) was visualized by immunoperoxidase staining. All three groups show a similar immunoreactivity for pCREB in CA1 subregion, but VPA or SBt groups display a significant increase compared to the saline group at the inner half layer of DG. Scale bar = 100 μm. VPA (B) or SBt (C) treatments on cultured immature hippocampal neurons increased the expressions of Bcl-2 and Bcl-xL in a time dependent manner. Immature cells to be committed to neurons were prepared by withdrawing bFGF and EGF for 3-5 days from N2 supplemented B27 media. (Graphs) Densitometric analyses of three independent Western blot experiments. pCREB, Bcl-2 and Bcl-xL levels were normalized to the amount of CREB and β-actin respectively, and are shown relative to expression in non-treated control cells. CREB activation was not significantly detected rela-tive to that of Bcl-2 or Bcl-xL. Note that the activation of CREB occurs after the expression of Bcl-2 or Bcl-xL increases. *p < 0.05, **p < 0.01.
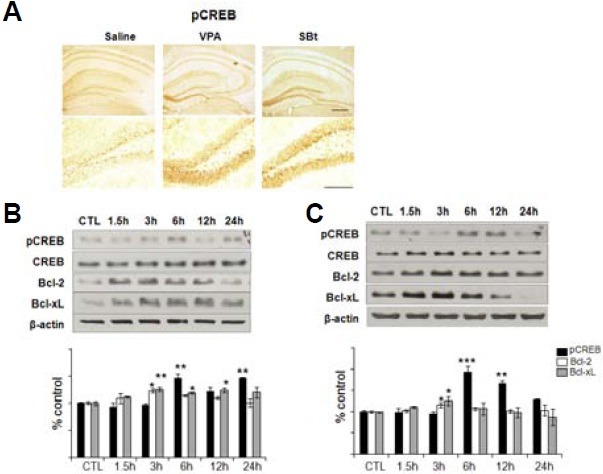
Bcl-2 plays a protective role against apoptotic and necrotic cell death in response to various stimuli including ischemia (Sasaki et al., 2006; Zhang et al., 2006). It is known that HDACIs increase the expression of Bcl-2 (Chen et al., 1999; Faraco et al., 2006). To see the increase of Bcl-2 expression in immature neurons by VPA and SBt, Western blotting was performed (Figs. 3B and 3C). As shown in Figs. 3B and 3C, VPA and SBt significantly increased the expression of Bcl-2 in immature neurons of 3-5 days after induction of differentiation in a time dependent manner [H5] (Figs. 3B and 3C; Bcl-2: VPA 3 h, 123.3 ± 3.2%; SBt 3 h, 115.8 ± 5.0%; Bcl-xL: VPA 3 h, 125.4 ± 3.6%, VPA 6 h, 118.8 ± 1.7%, VPA 12 h, 124.2 ± 4.5%; SBt 3 h, 125.4 ± 9.6, n = 3 per group). Bcl-2 is one of the genes targeted by CREB (Kitagawa, 2007; Meller et al., 2005), and CREB plays a crucial role in the survival of immature hippocampal neurons during adult hippocampal neurogenesis. To ascertain whether CREB is activated by HDACI treatment, CREB expression was examined by Western blotting after VPA treatment. The phosphorylation of CREB (pCREB) was significantly increased by VPA compared to phosphorylation by control (Figs. 3B and 3C; VPA 6 h, 146.9 ± 6.7%, VPA 24 h, 146.9 ± 1.6%; SBt 6 h, 193.4.8 ± 13.6%, SBt 12 h, 167.3 ± 5.5%, n = 3 per group). Although Bcl-2 may play a major role in promoting the survival of immature neurons, Bcl-xL, another anti-apoptotic Bcl-2 family member, may also be involved (Dietz et al., 2007; Hansen et al., 2007; Le et al., 2008). The presented results show that the expression of Bcl-xL increased by VPA and SBt (Figs. 3B and 3C).
The extended survival effect by HDACIs on the newborn neurons is a time-specific event on development
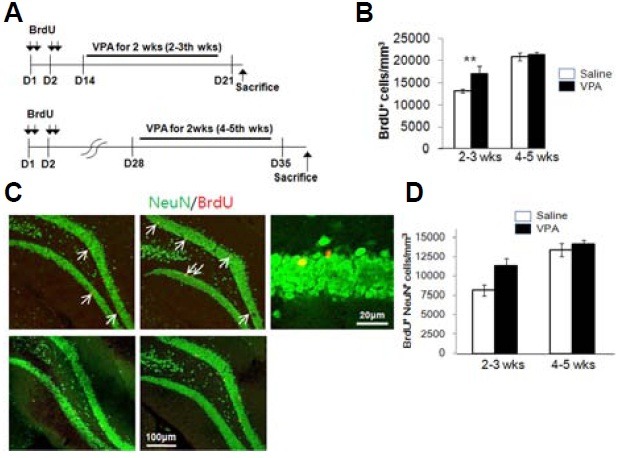
To investigate whether a specific time window during development is critical for the survival of newly generated hippocampal neurons, HDACI was treated in two different time windows: at 2-3 weeks and at 4-5 weeks after neurons were generated. To test whether the production and the survival of the newborn neurons in the subgranular zone of the hippocampi are affected by HDACIs in the limited time window, VPA were treated for 2 weeks; 2-3 weeks or 4-5 weeks after the newborn neurons were generated (Fig. 4A). The number of BrdU(+) cells was significantly increased compared to that of the control by VPA treatment 2 weeks after the BrdU injection, whereas the number of BrdU(+) cells was not increased by VPA treatment 4 weeks after BrdU injection (Fig. 4B). To determine the extent of differention of the BrdU(+) cells into neuronal lineage, double immunolabeling for BrdU and NeuN, a neuronal marker, was performed. Figure 4C shows that the number of BrdU(+) and NeuN(+) cells (BrdU+NeuN+) was significantly increased by VPA treatment during the 2-3 week period, but not at 4-5 weeks after the BrdU injection (Fig. 4D). The result suggests that it might be important for the proliferation and the survival of newborn neurons how they intereact the microenvironment and what kind of signal they receive during the critical period of development.
Fig. 4. The effect of HDACIs on the survival of newborn neurons is limited in a specific time window. (A) A schematic paradigm for the time-specific effect of VPA and SBt on the survival of newborn neurons. VPA was injected for 14 days, either 2 or 4 weeks after BrdU injection. (B) The number of BrdU(+) cells was increased by VPA treatment when treated 2 weeks after the BrdU injection. VPA treatment 4 weeks after the BrdU injection was not effective on the survival of newborn neurons. (C) Double-labeled images for BrdU (red) and NeuN (green). The increased number of BrdU and NeuN labeled cells (BrdU+NeuN+; arrows) in VPA treated group is significantly distinct 2-3 week treatment after BrdU injection, not in 4-5 week time point. (D) Values are shown as an averaged number of BrdU+NeuN+ cells/mm3 ± SEM. *p < 0.05, Student’s t-test.
DISCUSSION
Whether HDACIs alone can enhance the survival of newborn hippocampal neurons in a specific time window, thus enhancing adult hippocampal neurogenesis was investigated. If so, what molecules mediate such a survival-promoting effect of HDACIs on newborn hippocampal neurons? In the present study, we demonstrate that HDACIs increase the number of BrdU(+) cells committed to neurons in the DG when HDACs are administered within 3 weeks after the neural progenitor cells were born. In addition, increased expressions of anti-apoptotic Bcl-2 and Bcl-xL proteins might be, at least in part, responsible for the survival-promoting effect of HDACIs.
Previous studies show that the neural plasticity correlated with the Morris water maze or contextual fear conditioning involves histone acetylation (Vecsey et al., 2007). However, no study has thus far investigated whether HDACIs can directly exert such a beneficial effect without relation to adult hippocampal neurogenesis. The presented results, for the first time, pose a possibility that pharmacological modulation of histone acetylation status may engender the same effect as neural plasticity resulting from mental activities within a critical time window.
These results show that HDACIs could increase expressions of Bcl-2 and Bcl-xL (Figs. 2A and 2B). Interestingly, just 1.5 h of incubation with VPA or SBt increased the expressions of Bcl-2 and Bcl-xL, suggesting that the expressions of Bcl-2 and Bcl-xL by HDACIs might be regulated not by newly generated transcription factors, but by activation of intracellular signaling molecules. It is well known that CREB is a common target of many intracellular signaling cascades activated by neuronal activities and Bcl-2 is one of a variety of target genes controlled by antivation of CREB. Therefore, it was investigated whether the activation of CREB is correlated with increased expression of Bcl-2 by VPA or SBt. While the immunoblotting results showed that CREB could be activated by HDACIs (Fig. 2), the causality between CREB activation and Bcl-2 expression by HDACIs needs further evidence; the increased expression of Bcl-2 preceded the activation of CREB, suggesting that the expression of Bcl-2 by HDACIs might involve other mediators to be activated much earlier. Moreover, direct evidence is needed that Bcl-2 or Bcl-xL increases at the dentate gyrus of the hippo-campus treated with HDACIs and that inhibition of activated CREB attenuates the increase of Bcl-2 or Bcl-xL expression in the hippocampus treated with HDACIs. However, despite lack of necessary and sufficient evidence to explain the increased survival of newborn neurons by HDACIs, these findings at least indicate that increased expressions of Bcl-2 and Bcl-xL by VPA or SBt treatment might underlie the enhanced survival of immature hippocampal neurons. For complete evidence, whether expressions of Bcl-2 or Bcl-xL can directly increase at the DG of the hippocampus when treated with VPA or SBt is currently under investigation. At the same time, experiments are to be conducted to identify the upstream molecules mediating the increased expressions of Bcl-2 and Bcl-xL by HDACIs.
Although the current results support the role of HDACIs as an enhancer of adult hippocampal neurogenesis, the regulation of neurogenesis by HDACIs does not always lean toward increasing neurogenesis. It has been shown that learning has different influences on neural progenitor cells at different developmental stages. The survival of 3-day-old, but not 8-day-old newborn cells is inhibited by training in the Morris water maze through increased apoptosis. Interestingly, blocking apoptosis of neural progenitor cells during the late phase of Morris water maze training led to impaired performance in rats, implicating a requirement for selective integration of newborn neurons in spatial learning and memory (Dupret et al., 2007). Therefore, the regulation of hippocampal neurogenesis by histone modifications can be complicated and warrants further investigations to clarify regulators involved.
Acknowledgments
This research was supported by a grant No. (R01-2005-000-10596-0) from the Basic Research Program of Korea Science & Engineering Foundation awarded to CH Lee and a grant (M103KV010008-06K2201-00810) awarded to H. Son from the Brain Research Center (BRC) of the 21st Century Frontier Research Program funded by the Ministry of Science and Tech-nology, Republic of Korea awarded to H. Son.
References
- 1.Ambrogini P., Cuppini R., Cuppini C., Ciaroni S., Cecchini T., Ferri P., Sartini S., Del Grande P. Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci. Lett. (2000);286:21–24. doi: 10.1016/s0304-3940(00)01074-0. [DOI] [PubMed] [Google Scholar]
- 2.Chen G., Zeng W.Z., Yuan P.X., Huang L.D., Jiang Y.M., Zhao Z.H., Manji H.K. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J. Neurochem. (1999);72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- 3.Chwang W.B., Arthur J.S., Schumacher A., Sweatt J.D. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J. Neurosci. (2007);27:12732–12742. doi: 10.1523/JNEUROSCI.2522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietz G.P., Dietz B., Bahr M. Bcl-xL protects cerebellar granule neurons against the late phase, but not against the early phase of glutamate-induced cell death. Brain Res. (2007);1164:136–141. doi: 10.1016/j.brainres.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Dupret D., Fabre A., Dobrossy M.D., Panatier A., Rodriguez J.J., Lamarque S., Lemaire V., Oliet S.H., Piazza P.V., Abrous D.N. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. (2007);5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faraco G., Pancani T., Formentini L., Mascagni P., Fossati G., Leoni F., Moroni F., Chiarugi A. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol. Pharmacol. (2006);70:1876–1884. doi: 10.1124/mol.106.027912. [DOI] [PubMed] [Google Scholar]
- 7.Hansen M.R., Roehm P.C., Xu N., Green S.H. Overexpression of Bcl-2 or Bcl-xL prevents spiral ganglion neuron death and inhibits neurite growth. Dev. Neurobiol. (2007);67:316–325. doi: 10.1002/dneu.20346. [DOI] [PubMed] [Google Scholar]
- 8.Jessberger S., Nakashima K., Clemenson G.D. Jr., Mejia E., Mathews E., Ure K., Ogawa S., Sinton C.M., Gage F.H., Hsieh J. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J. Neurosci. (2007);27:5967–5975. doi: 10.1523/JNEUROSCI.0110-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanai H., Sawa A., Chen R.W., Leeds P., Chuang D.M. Valproic acid inhibits histone deacetylase activity and suppresses excitotoxicity-induced GAPDH nuclear accumulation and apoptotic death in neurons. Pharmacogenomics J. (2004);4:336–344. doi: 10.1038/sj.tpj.6500269. [DOI] [PubMed] [Google Scholar]
- 10.Kempermann G., Kuhn H.G., Gage F.H. Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. (1998);18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempermann G., Gast D., Kronenberg G., Yamaguchi M., Gage F.H. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. (2003);130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 12.Kitagawa K. CREB and cAMP response element-mediated gene expression in the ischemic brain. FEBS J. (2007);274:3210–3217. doi: 10.1111/j.1742-4658.2007.05890.x. [DOI] [PubMed] [Google Scholar]
- 13.Langley B., D’Annibale M.A., Suh K., Ayoub I., Tolhurst A., Bastan B., Yang L., Ko B., Fisher M., Cho S., et al. Pulse inhibition of histone deacetylases induces complete resistance to oxidative death in cortical neurons without toxicity and reveals a role for cytoplasmic p21(waf1/cip1) in cell cycle-independent neuroprotection. J. Neurosci. (2008);28:163–176. doi: 10.1523/JNEUROSCI.3200-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Y.Z., Zheng L., Le Y., Rucker E.B. 3rd, Anderson R.E. Role of BCL-XL in photoreceptor survival. Adv. Exp. Med. Biol. (2008);613:69–74. doi: 10.1007/978-0-387-74904-4_7. [DOI] [PubMed] [Google Scholar]
- 15.Levenson J.M., O’Riordan K.J., Brown K.D., Trinh M.A., Molfese D.L., Sweatt J.D. Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. (2004);279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 16.Meller R., Minami M., Cameron J.A., Impey S., Chen D., Lan J. Q., Henshall D.C., Simon R.P. CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J. Cereb. Blood Flow Metab. (2005);25:234–246. doi: 10.1038/sj.jcbfm.9600024. [DOI] [PubMed] [Google Scholar]
- 17.Minamiyama M., Katsuno M., Adachi H., Waza M., Sang C., Kobayashi Y., Tanaka F., Doyu M., Inukai A., Sobue G. Sodium butyrate ameliorates phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Hum. Mol. Genet. (2004);13:1183–1192. doi: 10.1093/hmg/ddh131. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa S., Kim J.E., Lee R., Chen J., Fujioka T., Malberg J., Tsuji S., Duman R.S. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J. Neurosci. (2002);22:9868–9876. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu H., Smith K., Camelo S.I., Carreras I., Lee J., Iglesias A.H., Dangond F., Cormier K.A., Cudkowicz M.E., Brown R.H. Jr., et al. Sodium phenylbutyrate prolongs survival and regulates expression of anti-apoptotic genes in transgenic amyotrophic lateral sclerosis mice. J. Neurochem. (2005);93:1087–1098. doi: 10.1111/j.1471-4159.2005.03077.x. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki T., Kitagawa K., Yagita Y., Sugiura S., Omura-Matsuoka E., Tanaka S., Matsushita K., Okano H., Tsujimoto Y., Hori M. Bcl2 enhances survival of newborn neurons in the normal and ischemic hippocampus. J. Neurosci. Res. (2006);84:1187–1196. doi: 10.1002/jnr.21036. [DOI] [PubMed] [Google Scholar]
- 21.Sisti H.M., Glass A.L., Shors T.J. Neurogenesis and the spacing effect: learning over time enhances memory and the survival of new neurons. Learn. Mem. (2007);14:368–375. doi: 10.1101/lm.488707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swaab D.F. Brain aging and Alzheimer’s disease, “wear and tear” versus “use it or lose it”. Neurobiol. Aging. (1991);12:317–324. doi: 10.1016/0197-4580(91)90008-8. [DOI] [PubMed] [Google Scholar]
- 23.Tashiro A., Makino H., Gage F.H. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J. Neurosci. (2007);27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiagalingam S., Cheng K.H., Lee H.J., Mineva N., Thiagalingam A., Ponte J.F. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann. N Y Acad. Sci. (2003);983:84–100. doi: 10.1111/j.1749-6632.2003.tb05964.x. [DOI] [PubMed] [Google Scholar]
- 25.Vecsey C.G., Hawk J.D., Lattal K.M., Stein J.M., Fabian S.A., Attner M.A., Cabrera S.M., McDonough C.B., Brindle P.K., Abel T., et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J. Neurosci. (2007);27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang R., Xue Y.Y., Lu S.D., Wang Y., Zhang L.M., Huang Y.L., Signore A.P., Chen J., Sun F.Y. Bcl-2 enhances neurogenesis and inhibits apoptosis of newborn neurons in adult rat brain following a transient middle cerebral artery occlusion. Neurobiol. Dis. (2006);24:345–356. doi: 10.1016/j.nbd.2006.07.012. [DOI] [PubMed] [Google Scholar]


