Abstract
The mammalian reproductive tract is known to contain 1.5-5.3% oxygen (O2), but human embryonic stem cells (hESCs) derived from preimplantation embryos are typically cultured under 21% O2 tension. The aim of this study was to investigate the effects of O2 tension on the long-term cul-ture of hESCs and on cell-fate determination during early differentiation. hESCs and embryoid bodies (EBs) were grown under different O2 tensions (3, 12, and 21% O2). The expression of markers associated with pluripotency, em-bryonic germ layers, and hypoxia was analyzed using RT-PCR, immunostaining, and Western blotting. Proliferation, apoptosis, and chromosomal aberrations were examined using BrdU incorporation, caspase-3 immunostaining, and karyotype analysis, respectively. Structural and morpho-logical changes of EBs under different O2 tensions were comparatively examined using azan- and hematoxylineosin staining, and scanning and transmis-sion electron microscopy. Mild hypoxia (12% O2) increased the number of cells expressing Oct4/Nanog and reduced BrdU incorporation and aneuploidy. The percentage of cells positive for active caspase-3, which was high during normoxia (21% O2), gradually decreased when hESCs were continuously cultured under mild hypoxia. EBs subjected to hypoxia (3% O2) exhibited well-differentiated microvilli on their surface, secreted high levels of collagen, and showed enhanced differentiation into primitive endoderm. These changes were associated with increased expression of Foxa2, Sox17, AFP, and GATA4 on the EB periphery. Our data suggest that mild hypoxia facilitates the slow mitotic division of hESCs in long-term culture and reduces the frequency of chromosomal abnormalities and apoptsis. In addition, hypoxia promotes the differentiation of EBs into extraembryonic endoderm.
Keywords: differentiation, embryoid body, germ-layer, human embryo stem cell, hypoxia
INTRODUCTION
Human embryonic stem cells (hESCs) are pluripotent cells capable of both indefinite proliferation and differentiation into a wide spectrum of cell types (Reubinoff et al., 2000; Thomson et al., 1998). The unlimited self-renewal capacity and pluripotency of hESCs suggests that they could be used as a potential source for cell-replacement therapies. Thus, ongoing efforts have focused on establishing defined culture conditions that do not require serum or feeder cells for future clinical applications (Amit et al., 2003; Pick et al., 2007; Richards et al., 2008). However, few studies have evaluated the physicochemical factors that could affect the growth and differentiation of the early embryo.
Oxygen (O2) is essential for the survival of all aerobic organisms and its concentration regulates virtually all cellular processes. Nonetheless, O2 tension is rarely considered when performing in vitro culture protocols, including those used to grow and induce the differentiation of stem cells. Most traditional cell culture incubators utilize room air containing atmospheric levels (21%) of O2, termed “normoxia”. However, studies report that tissues are exposed to significantly lower O2 concentrations in vivo depending on their location (Csete, 2005; Gassmann et al., 1996). For example, arterial blood contains approximately 12% O2, whereas the mean level of O2 in other tissues is approximately 3% and varies both regionally and locally (Csete, 2005).
Under normal physiological conditions, the mammalian reproductive tract is exposed to relatively low O2 tension (1.5-5.3%) (Aplin, 2000; Fischer and Bavister, 1993). Therefore, it has been speculated that naturally conceived embryos at an undifferentiated stage or during the three-germ-layer stage are exposed to low O2 tension. Based on this observation, the relatively high O2 tension used in traditional cell culture may not recapitulate the physiological environment of the reproductive tract where early-stage embryos develop. A previous study assessing the proliferation of hESCs under hypoxia (3% and 5%) determined that there was no difference in the growth rates between these cells and those grown under normoxia (Ezashi et al., 2005). Furthermore, hypoxia is better for maintaining the undifferentiated state and increasing embryoid body (EB) formation (Ezashi et al., 2005). However, few studies have addressed the effects of hypoxia on the biochemical and morphological alterations that occur during hESC growth and germlayer development in differentiating EBs.
To further our understanding of how hypoxic culture conditions affect EB ultrastructure and germ-layer development, this study sought to: 1) investigate the effects of different O2 tensions on the differentiation of hESCs in long-term culture, including assessment of glycogen and collagen accumulation; and 2) evaluate the early differentiation of the three embryonic germ layers (endoderm, mesoderm and ectoderm) and extraembryonic tissue (primitive endoderm) under different O2 tensions.
MATERIALS AND METHODS
hESC culture and EB formation
The hESC lines HSF6 (NIH code: UC06) and H9 (NIH code: WA09) were provided by the Wisconsin International Stem Cell Bank (http://www.wicell.org). The Miz-hES4 line (Korean Stem Cell Band code: Kor-hES12, http://koreastemcellbank.org) was obtained from the Miz medi Hospital (Korea). HSF6 was used for all experiments, and Miz4 and H9 were used as comparisons. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM)/F12 (Gibco-BRL, USA) supplemented with 20% KnockOut serum replacement (Gibco-BRL), 1 mM nonessential amino acids (Invitrogen, USA), 0.1 mM β-mercaptoethanol (Sigma, USA), 100 U/ml penicillin G (Gibco-BRL), 100 μg/ ml streptomycin (Gibco-BRL) and 4 ng/ml human fibroblast growth factor 2 (FGF2; R&D Systems, USA), as previously described (Abeyta et al., 2004; Son et al., 2005). For passaging, hESC colonies were treated with 1 mg/ml collagenase type IV (Invitrogen) and gently triturated. The clumps were transferred onto mitomycin C (Roche, Germany)-treated mouse embryonic fibroblasts (MEFs) and cultured 5-7 days. Prior to maintaining hESCs under different O2 tensions, cells were incubated in a standard gas atmosphere containing humidified 5% CO2 and air (21% O2) at 37℃. To examine the effects of hypoxia on long-term culture, hESC colonies were dissociated into small clumps and seeded onto six-well plates at a low density (10-20 cells/well). The cultures were grown up to 21 days. The six-well plates were placed in chambers (MiniGalaxy 4, RS Biotech Laboratory Equipment Ltd., Scot-land, UK and a Sanyo MCO-175M O2/CO2 incubator, Sanyo Scientific, USA) containing different oxygen tensions with the appropriate humidified gas mixture (3% O2, 5% O2, or 12% O2/5% CO2/balance N2) at 37℃. The O2 content of each gas mixture in the chamber was confirmed using a Fyrite gas analyzer (Bacharach, USA). The culture medium was degassed by preincubation overnight with a standard gas mixture. The medium was changed every day.
For feeder-free cultures, plates were coated with 20 μg/ml laminin (Roche, Switzerland) (Draper et al., 2004), and culture medium was changed daily. For EB formation, hESC colonies cultured under 21% O2 tension were harvested, gently triturated, and transferred into a suspension culture dish (Corning Life Sciences, USA) containing EB medium (DMEM/F12 without FGF2) (Kim et al., 2003). Culture dishes containing hESC clumps were cultured under different O2 tensions for an additional 8 days for EB formation.
Immunostaining
hESCs or EBs were fixed in cold 4% paraformaldehyde in phosphate-buffered saline (PBS) and equilibrated in 20% su-crose overnight at 4℃. The equilibrated cells and EBs were embedded in paraffin or frozen in Optimal Cutting Temperature (O.C.T.) compound (Tissue-Tek, Japan). The specimens were sectioned into 10-μm sections. After blocking and permeabilizing the specimens in 0.3% Triton X-100/PBS and 10% serum in 0.1% BSA/PBS, the sections were incubated at 4℃ overnight with the following antibodies used at their optimal concentrations (Table 1): mouse anti-Oct4 (Santa Cruz Bio-technology, USA), goat anti-Nanog (R&D Systems), mouse anti-Foxa2 (DSHB, USA), goat anti-Sox17 (R&D Systems), rabbit anti-GATA4 (Santa Cruz Biotechnology), and mouse anti-AFP (Sigma), mouse anti-HIF1-α (Santa Cruz Biotechnology), rabbit anti-HIF2-α (Novus Biologicals, USA), mouse anti-α tubulin (Santa Cruz Biotechnology). Sections were then washed and incubated with the appropriate fluorescence-tagged secondary antibody (Jackson Immunoresearch Laboratories, USA) in 0.1% BSA/PBS at room temperature for 1.5 h. Slide-mounted sections were imaged with an Apotome-Axiovert 200 M fluorescence microscope (Carl Zeiss, Germany) after counterstaining with DAPI (4,6-diamidino-2-phenylindole dihydrochloride) to stain nuclei. Expression of germ-layer mark-ers was quantitatively evaluated using Axiovision software (version 2.4, Carl Zeiss). Randomly selected fields (n = 5) from a minimum of three independent experiments were analyzed using the 10 × objective of the Zeiss Apotome optical-sectioning microscope. Results for each O2 condition are expressed as a percentage of cells expressing markers in hESC colonies or EBs containing 1,000-2,000 DAPI-positive cells.
Table 1.
Antibodies used for immunostaining
| Marker | Company | Dilution factor |
|---|---|---|
| Oct4 | Santa Cruz Biotechnology | 1:500 |
| Nanog | R&D Systems | 1:100 |
| Sox17 | R&D Systems | 1:100 |
| Foxa2 | DSHB | 1:1000 |
| Brachyury | R&D Systems | 1:200 |
| Nestin | R&D Systems | 1:200 |
| Pax6 | Chemicon | 1:2000 |
| AFP | Sigma | 1:500 |
| GATA4 | Santa Cruz Biotechnology | 1:200 |
| BrdU | Accurate | 1:200 |
| PECAM | BD Biosciences | 1:500 |
| Tuj1 | R&D Systems | 1:200 |
| MAP2 | Sigma | 1:200 |
| HIF1α | Santa Cruz Biotechnology | 1: 100 |
| HIF2α | Novus Biology | 1:100 |
| α-tubulin | Santa Cruz Biotechnology | 1:1000 |
5-Bromo-2-deoxy-uridine (BrdU) incorporation by phase-contrast microscopy
To examine the effects of different O2 tensions on cell proliferation, we incubated 5-day-cultured hESCs grown under 21% or 12% O2 tension with 10 μg/ml BrdU (Roche) for 2 h at 37℃. After incubation with BrdU, cells were washed three times with washing buffer (Roche) and fixed with ethanol for 20 min at -21℃. Fixed cells were subsequently incubated with 10 μl mouse anti-BrdU antibody (1:100; Roche) for 30 min at room temperature and washed three times with PBS. The cells were then incubated with an anti-mouse-Ig-akaline phosphatase solution for 30 min at 37℃. Cells were covered with freshly prepared color-substrate solution (Roche) and incubated for 30 min at 20℃. Methylene green was used as a nuclear counterstain. The growth rate of colonies cultured under different O2 tensions was quantified by measuring the number of colonies with diameters of approximately 2,000-6,000 μm on days 7, 14, and 21 of culture using ProgRess Capture Pro 2.7 (Jenoptik, Germany). Cell proliferation was quantified by measuring the density of BrdU staining in randomly selected fields (n = 5) with the aid of Scion Image software (version 2.4, Scion, USA).
Evaluation of apoptosis by caspase-3 immunostaining
hESCs grown for 6 days at an O2 tension of 12% or 21% were assessed for apoptosis. The cells were fixed with 4% para-formaldehyde, incubated with mouse anti-Caspase-3 (Santa Cruz Biotechnology) and labeled with the appropriate fluorescence-tagged secondary antibody (Jackson Immuno Research Laboratories). After staining, the cells were observed under an Apotome-Axiovert 200 M Fluorescence/Live-Cell Imaging microscope (Carl Zeiss). Axiovision software (version 2.4, Carl Zeiss) was used to quantitatively evaluate apoptosis in randomly selected fields (n = 5) from a minimum of three independent experiments. A total of 500-800 colonies were counted for each O2 condition. Results are expressed as the percentage of DAPI- positive cells that were Caspase 3-positive.
Periodic acid-Schiff (PAS) staining
hESCs cultured for 6 days under 12% or 21% O2 tension were fixed with a solution of 10% formalin in 95% cold ethanol. Fixed cells were incubated with periodic acid solution (Sigma) for 5 min at room temperature. After rinsing several times with distilled water, cells were treated with Schiff’s reagent (Sigma) for 15 min, washed with tap water for 5 min, and stained with hematoxylin (Sigma) for 90 s at room temperature. Glycogen granules (purple) were detected by light microscopy (Carl Zeiss).
Karyotyping
hESCs were incubated with 20 μg/ml colcemid (Gibco-BRL) for 30 min under 12% or 21% O2 tension. hESCs were then incubated with 0.7% sodium citrate for 40 min at 37℃ and fixed with a freshly prepared methanol:acetic acid (3:1) solution. GTG banding was performed by incubating the glass slides in a 0.05% trypsin solution (Gibco-BRL), followed by rinsing in PBS and staining with a 5% Giemsa stain solution (Sigma) for 5 min. The slides were rinsed with water and air-dried to detect chromosomal abnormalities. The presence of a normal karyotype was confirmed by GTG band staining (CHIPs analysis, Korea).
Effect of O2 tension on EB morphology
The effect of O2 tension on the early-stage differentiation of hESCs was tested by culturing colonies under normoxia, and then the hESC clumps were transferred into different O2 tensions (3%, 5%, 12%, or 21%). hESC clumps were then cultured for an additional 8 days to allow them to form EBs, which were used to study whether there were morphological and ultrastructural changes. The 8-day differentiated EBs cultured under 3% or 21% O2 tension were analyzed by light microscopy (phase contrast), scanning electron microscopy (S4700, Hitachi, Japan), and transmission electron microscopy (TEM; H7600, Hitachi).
Azan staining
EBs cultured under 3% or 21% O2 tension for 8 days were embedded in paraffin or O.C.T. compound. The specimens were sectioned into 10-μm sections and fixed by incubating them with 4% paraformaldehyde for 1 h. They were washed twice with 1X PBS. Randomly selected slides were stained with 0.2% azocamine G solution (Sigma) for 1 h at 50℃. After staining, the slides were rinsed with distilled water for 5 min, followed by 0.1% aniline in ethanol (Fluka, Switzerland). The specimens were then dipped a few times in acid alcohol. The slides were treated with phosphomolybdic acid (Sigma) for 2 h and rinsed again in distilled water for 5 min prior to staining with an aniline blue-orange G solution (Sigma) for 10-15 min at 60℃. The slides were rinsed in distilled water for 5 min, dipped a few times in 95% ethanol, coverslipped, and mounted.
RNA extraction and reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was isolated from hESCs using Stat 60 (Tel-Test, Inc, USA), and cDNA was synthesized from 2 μg of total RNA using the Revert Aid H Minus First Strand cDNA Synthesis Kit (Fermentas, Canada), following the manufacturer’s instructions. PCR was carried out using AccuPowerⓇ PCR-Premix (Bioneer, Korea) with primers designed to amplify the following human genes: HNF3-β (Foxa2), Sox1, Sox17, Nestin, α-fetoprotein (AFP), Tuj1, hypoxia inducible factor-1α (HIF1α), hypoxia inducible factor-2α (HIF2α), erythropoietin (EPO), glucose transporter-1 (Glut1), vascular endothelial growth factor (VEGF), and glyceraldehyde phosphate dehydrogenase (GAPDH). Primer sequences and reaction conditions are provided in Table 2. PCR products were confirmed by ethidium-bromide staining following 1.5% agarose gel electrophoresis.
Table 2.
RT-PCR primers for differentiation markers
| Target mRNA | Sequence (5′-3′) | Product size (bp) | Annealing temp (℃) |
|---|---|---|---|
| OCT4 | cgtgaagctggagaaggagaagctg | 250 | 60 |
| aagggccgcagcttacacatgttc | |||
| Nanog | ccaaaggcaaacaacccact | 398 | 60 |
| tgaattgttccaggtctggttg | |||
| Tuj1 | catggacagtgtccgctcag | 175 | 60 |
| caggcagtcgcagtttccac | |||
| Nestin | cagctggcgcacctcaagatg | 208 | 55 |
| agggaagttgggctcaggactgg | |||
| AFP | tgaaaaccctcttgaatgcc | 492 | 55 |
| tcttgcttcatcgtttgcag | |||
| GATA4 | ctcccctggcaaaacaagag | 422 | 60 |
| tgccgtgtcttagcagtcgt | |||
| Sox1 | acgccgagctcagcaagat | 73 | 53 |
| tccacgtacggcctcttctg | |||
| Foxa2 | atgaacggcatgaacacgta | 482 | 63 |
| cggtagaaggggaagaggtc | |||
| Sox17 | agcgcccttcacgtgtacta | 246 | 60 |
| cttgcacacgaagtgcagat | |||
| HIF1α | ctccatctcctacccacata | 240 | 50 |
| gagcattctgcaaagctagt | |||
| HIF2α | tcatcatgtgtgaaccaatc | 256 | 50 |
| cggtactggccacttactac | |||
| EPO | tcccagacaccaaagttaat | 294 | 45 |
| ggaaagtgtcagcagtagtt | |||
| VEGF | gtggacatcttccaggagta | 219 | 50 |
| tctgcattcacatttgttgt | |||
| Glut1 | taccctggatgtcctatctg | 174 | 50 |
| ccacaatgaaatttgaggtc | |||
| GAPDH | agccacatcgctcagacacc | 302 | 60 |
| gtactcagcggccagcatcg | |||
Western blot analysis
Expression of marker proteins at different developmental stages was examined in EBs cultured under 3% or 21% O2 tension for 8 days. Protein lysates were made using RIPA lysis buffer containing protease inhibitors (Upstate, USA). Equal amounts of protein (50 μg/lane) were separated by SDS-PAGE and transferred to nitrocellulose membranes (Schleicher & Schull, Germany). Membranes were incubated with mouse anti-HIF1α, anti-HIF2α and mouse anti-α-tubulin (Santa Cruz Biotechnology) antibodies overnight at 4℃. The membranes were rinsed with Tris-Buffered Saline Tween-20 (TBS-T) and incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) for 1.5 h at room temperature. Immunoreactive proteins were detected using enhanced chemiluminesence reagents (Santa Cruz Biotechnology), following the manufacturer’s instructions.
Statistical analysis
Numerical values were expressed as the mean ± standard error of the mean (SEM) of three independent experiments performed in triplicate. Statistical significance was determined using a one-way analysis of variance (ANOVA). Paired Student’s t-tests were performed to compare the means when ANOVAs indicated a significant difference. P-values less than 0.05 were determined to be significant.
RESULTS
Effect of O2 tension on the maintenance of the undifferentiated state of hESCs in long-term culture
To examine the effect of O2 tension on the maintenance of pluripotency in hESCs, cells were grown under 3%, 12%, or 21% O2 tension. These values were selected to reflect the O2 levels encountered in the reproductive tract, arterial blood and atmosphere, respectively. They are referred to as hypoxia (3%), mild hypoxia (12%), and normoxia (21%) for the remainder of this study. Previous studies report that O2 tensions of 4-5% effectively reduce the spontaneous differentiation of hESCs when they are cultured for 12 days (Ezashi et al., 2005; Westfall et al., 2008). Based on these findings, we examined the effects of 3% and 5% O2 tensions on the undifferentiated state of hESCs over a prolonged culture period (up to 21 days). A similar level of apoptosis was observed under both hypoxic conditions (3% and 5% O2). Cell death began approximately 14 days after subculture, and there was a substantial level of apoptosis after 21 days in both 3 and 5% O2 tensions (Figs. 1A and 1D, 5% data not shown). hESCs cultured under mild hypoxic (12%) or normoxic (21%) conditions up to 7 days formed relatively flat, compact colonies with well-defined edges, which is typical of hESC colony morphology (Figs. 1B and 1C). These hESCs grown under mild hypoxia (12%) maintained their undifferentiated state up to 21 days (Fig. 1E), while the colonies from hESCs cultured under normoxic conditions began to show signs of overt differentiation in their central region or around their margins by day 21 (Fig. 1F). In comparison to colonies grown under an O2 tension of 21%, in which only a few cells expressed Oct4 and Nanog, most cells cultured under 12% O2 tension retained a normal pattern of expression for both of these markers after 21 days (Figs. 1G-1L). Quantitative analysis clearly showed that the number of cells expressing Oct4 and Nanog was significantly higher under mild hypoxia vs. normoxia (Fig. 1M).
Fig. 1. Effect of O2 tension on maintenance of the undifferentiated state of hESCs. hESCs (passage 44) were cultured on MEF feeder cells under different O2 tensions. (A-F) Phase contrast images of hESC colonies cultured under different O2 tensions. (G-L) Immunostaining to detect expression of the undifferentiated markers Oct4 (red) and Nanog (green). DAPI (blue) was used as a nuclear counterstain. (M) Quantitative analysis of Oct4 and Nanog expression in colonies. Randomly selected fields (n = 5) from a minimum of three independent experiments were analyzed. *P < 0.05. Results for each O2 condition were present as a percentage of the expression of undifferentiated-cell markers in colonies containing 1,000-2,000 DAPI-positive cells. (N) Expression of HIF2α in undifferentiated hESCs was analyzed by Western blotting. (O) Immunostaining to detect the expression of HIF2α (green) and DAPI (blue) in hESC colonies. Magnification, 100×.
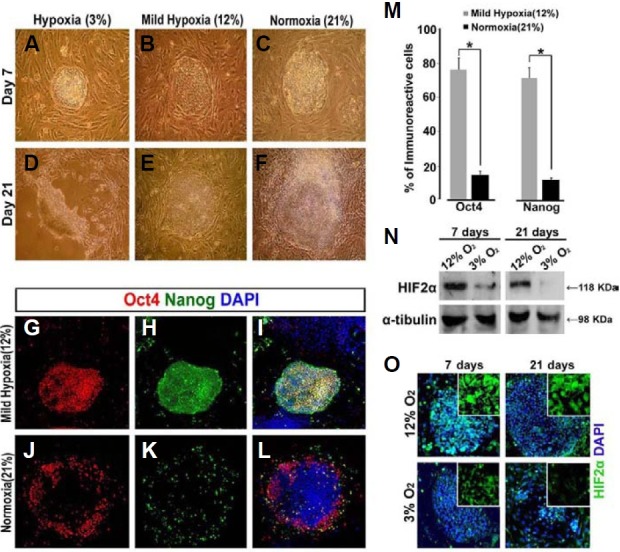
Hypoxia inducible factors (HIFs) are transcription factors known to regulate the cellular response to hypoxia (Loboda et al., 2010; Semenza et al., 2000) Among the oxygen-dependent α subunits of HIFs, HIF2α has been reported to affect Oct4 expression by directly binding to the Oct4 promoter region under hypoxic conditions (Covello et al., 2006), suggesting a potential role for HIF2α in maintaining the pluripotency of hESCs. Based on these data, we examined the protein expression of HIF2α under two different hypoxic conditions (3% and 12%). Western blot and immunocytochemistry analyses showed that the expression of HIF2α was much higher in hESCs cultured under mild hypoxia (12%) than in cells cultured under the stronger hypoxic condition (3%) for both short-term (7 days) and long-term (21 days) culture (Figs. 1N and 1O). Results obtained from hESCs cultured in the absence of feeder cells were similar to those obtained for hESCs cultured on feeder cells (data not shown). Taken together, our data suggest that mild hypoxia (12% O2) may be optimal for preventing the spontaneous differentiation of hESCs when they must be cultured long-term.
Effect of O2 tension on the growth and apoptosis of hESCs
The size of colonies and BrdU-incorporation indices were compared in hESC cultures maintained under different O2 tensions for up to 21 days. After 14 days of culture, the average diameters of hESC colonies (n = 50) under normoxia were significantly higher than those for colonies maintained under mild hypoxia (Figs. 2A, 2D, and 2G). Consistent with this finding, BrdU-incorporation assays demonstrated that hESCs were more proliferative under normoxia than those cultured under mild hypoxia in colonies of similar size (Figs. 2B and 2E). Quantification of BrdU staining showed that the number of proliferative cells was significantly higher under normoxia than under mild hypoxia (P < 0.05; Fig. 2H). It was previously reported that glycogen accumulation in the cytoplasm might be an indicator of the growth stage of hESC colonies (Johkura et al., 2004). Our PAS staining data showed that hESCs cultured under normoxia exhibited very strong PAS staining, suggesting that these cells have active energy metabolism, which is consistent with their higher proliferative index. In contrast, hESCs grown under mild hypoxia showed weak and very limited PAS staining (Figs. 2C and 2F).
Fig. 2. Effects of O2 tension on the proliferation and apoptosis of hESCs. hESCs from passages 44 (P1) to 48 (P5) were cultured on MEF feeder cells for 21 days. (A, D) Phase contrast images of hESC colonies grown under two different O2 tensions. (B, E) BrdU (black) incorporation assays showing reduced proliferation of hESCs under mild hypoxia, as compared to normoxia. Methylene green was used as a nuclear counterstain. (C, F) PAS staining (purple) of hESC colonies. Note a higher glycogen accumulation in cells under normoxia, as compared to mild hypoxia. (G) Size of hESC colonies under two different O2 tensions on days 7, 14, and 21 of culture. Diameters of colonies (approximately 2,000-6,000 μm) were presented as mean ± SEM. *P < 0.05. (H) The percentages of BrdU-positive pixels/total area in five randomly selected fields analyzed using Scion software. *P < 0.05. (I-L) Detection of apoptotic cells under indicated O2 tensions at P1, P3 and P5 using an antibody against active Caspase-3. The boxed area of the left panel in I is shown in the right panel as a black-and-white image at high magnification. Red arrows indicate nuclear fragmentation. (M) The apoptotic rates under different O2 ten-sions are shown as the average percentage of active Caspase-3-positive cells ± SEM over DAPI-positive cells in randomly selected fields (n = 5) from a minimum of three independent experiments. *P < 0.05; **P < 0.01. Magnification: 100× in A, B, C, E, F, G, I (left panel), J, K and L; 200× in I (right panel).
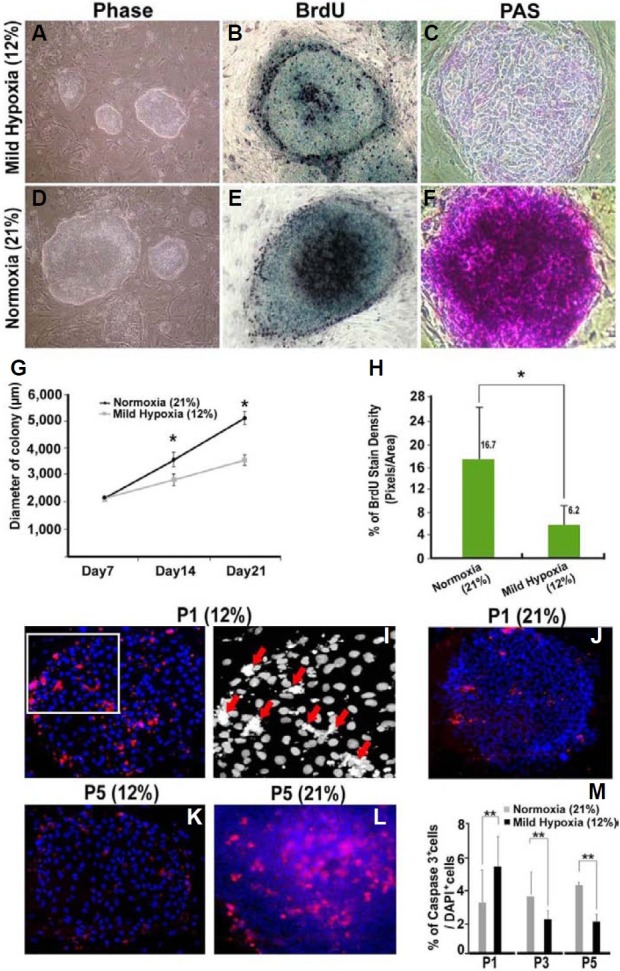
We next examined the effect of O2 tension on the apoptosis of undifferentiated hESCs. hESCs grown under normoxia were transferred into mild hypoxic conditions (P1) and cultured for five passages (~P5). Apoptotic cells were identified by staining for activated Caspase-3, a major apoptotic signaling effector. Transferring undifferentiated hESCs from normoxia to mild hypoxia increased the rate of apoptosis at P1 (Fig. 2I). Apopto-sis was further confirmed in black-and-white images showing that the immunoreactive signals for active Caspase-3 colocalized with cells exhibiting nuclear fragmentation (Fig. 2I, red arrows). However, interestingly, the rate of apoptosis was significantly decreased in cells continuously grown under mild hypoxia at P3 and P5 (Figs. 2K and 2M), whereas those cells continuously grown under normoxia showed a trend towards increased apoptosis (Figs. 2J, 2L, and 2M). Thus, our data suggest that although hESCs are highly proliferative under normoxia, mild hypoxic conditions may be more effective at preventing apoptosis of hESCs in long-term cultures.
Effect of O2 tension on genetic stability during the mainte-nance of hESCs
A previous study showed that the frequency of chromosomal aberrations detected under normoxia (21% O2) could be reduced when hESC lines were cultured under 2% hypoxia (Forsyth et al., 2006). To investigate whether our 12% mild hypoxic conditions can reduce the extent of chromosomal aberrations, we performed cytogenetic analyses at every fifth sub-culture using conventional karyotyping. We observed an increased trend towards aneuploidy of chromosomes 5 and 11 in hESCs cultured under normoxia, as compared to cells exposed to mild hypoxia (Figs. 3A and 3B). The overall rate of aneuploidy was higher in cells grown under normoxia than in cells cultured under mild O2 conditions (25% vs. 8.3%, P < 0.001; Table 3). These data for hESCs cultured under mild hypoxia (12%) are comparable to those reported by Forsyth et al., who reported that 2% hypoxia significantly reduces the extent of chromosomal aberrations (chromosomal gaps, breaks, and exchanges) in hESCs (H1 and H9 cell lines), as compared to normoxia (38% vs. 15%, respectively). Our data suggest that culturing hESCs under mild hypoxia reduces the incidence of chromosomal abnormalities.
Fig. 3. Effect of O2 tension on genetic stability during hESC proliferation. (A, B) Representative karyotypes of hESCs grown under normoxia at passage 63 (A) and hESCs grown under hypoxia (B) A total of 20 consecutive metaphase spreads (passages 44 to 64) were analyzed to identify chromosomal abnormalities (both numerical and structural). Arrows denote chromosome 5 and 11 abnormalities.
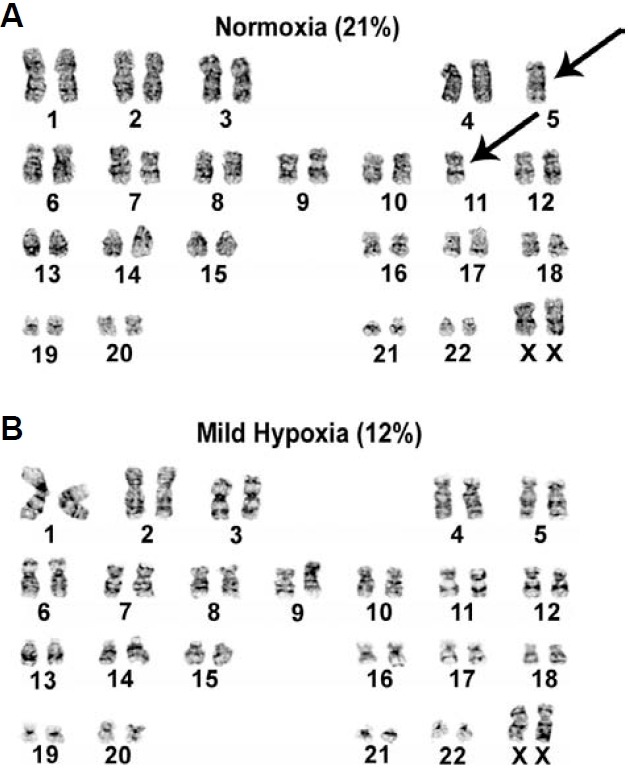
Table 3.
Chromosomal abnormalities in HSF6 cells. A comparison of chromosome abnormalities in hESCs at passages 49, 54, 59, and 64 (presented in the text as P5, P10, P15, and P20)
| Passage No. | Number of studied meta-phase plates | Chromosome abnormality | ||||
|---|---|---|---|---|---|---|
| Structure | Aneuploidy | |||||
| NO | MH | NO (%) | MH (%) | NO (%) | MH (%) | |
| P5 | 50 | 38 | 2 (4.0) | 1 (2.6) | 5 (10.0) | 1 (2.0) |
| P10 | 38 | 32 | 3 (7.8) | 1 (2.6) | 6 (15.8) | 1 (2.6) |
| P15 | 30 | 18 | 3 (10.0) | 2 (6.7) | 5 (16.7) | 2 (6.7) |
| P20 | 24 | 20 | 5 (20.8) | 2 (8.3)* | 6 (25.0) | 2 (8.3)* |
NO, normoxia (21%); MH, mild hypoxia (12% O2). *P < 0.001.
Effect of O2 tension on EB morphology and histology
Although previous studies have focused on the effect of hypoxia on the self-renewal capacity of hESCs, only a few studies have examined the morphological and histological changes in EBs grown under hypoxia (Ezashi et al., 2005; Ramirez-Bergeron and Simon, 2001). Therefore, we examined the effects of O2 tension on EB formation, as well as EB morphology and histology. hESC clumps grown under normoxia were transferred and maintained under low O2 tensions (3%, 5%, or 12%) or 21% O2 for an additional 8 days to allow the hESCs to form EBs. The significant cell death, which was observed in undifferentiated hESCs transferred to 3 or 5% O2 tensions, was not found in EBs grown under 3% O2 tension during the 8 days. EBs of similar size grown under 3% or 21% O2 conditions were comparatively analyzed using phase-contrast and scanning electron microscopy to determine whether there were any morphological and ultrastructural differences (Figs. 4A-4F). In general, EBs formed under hypoxia were smaller and less variable in size than those formed under normoxia (300 ± 50 μm vs. 500 ± 100 μm, respectively). Morphological study using scanning electron microscopy showed that the peripheral cells exposed to hypoxia retained a large number of well-differentiated microvilli (Figs. 4E and 4F), which were not observed in those grown under normoxia (Figs. 4B and 4C).
Fig. 4. Effects of O2 tension on the morphology and histology of differentiating EBs. (A, D) Phase contrast images of the EB surface under two different O2 tensions. (B, C, E, and F) Ultrastructural images of EB surfaces obtained by scanning electron microscopy (L, low magnification; H, high magnification). Note a large number of well-developed microvilli on the surface of EBs grown under hypoxia (E, F). (G, J) Representative TEM images of a EB showing apoptotic bodies containing condensed nuclear material under normoxia (white arrows in G), and normal nuclei with prominent nucleoli and no condensed chromatin under hypoxia. (H, K) Hematoxylin and eosin staining of EB sections (black: nuclei, red: cytoplasm). (I, L) Azan and Masson’s trichrome staining of EB sections for detection of collagen accumulation (blue) in EBs grown under normoxia or hypoxia. Magnification: 100× in (A), (D), (H), (I), (K) and (L); 700× in (B) and (E); 2,000× in (C) and (F); 10,000× in (G) and (J).
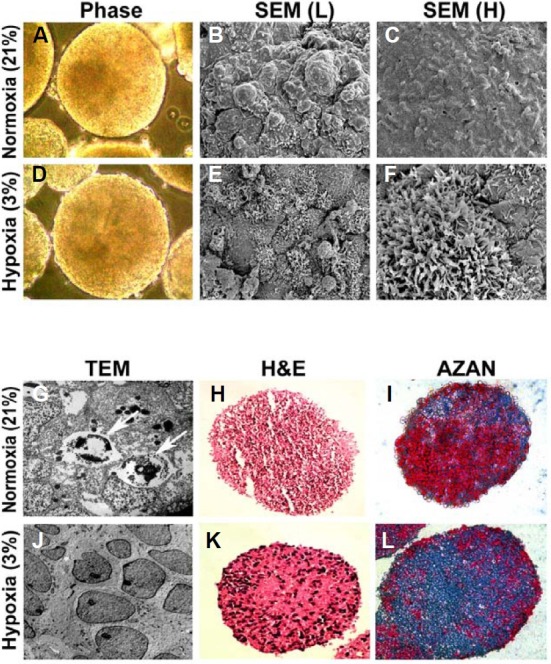
Consistent with our Caspase-3 data for undifferentiated hESCs, apoptotic bodies (Fig. 4G, white arrows) were frequently observed in differentiating EBs exposed to normoxia, as compared to EBs grown under hypoxia (Figs. 4G and 4J). Hematoxylin and eosin staining also showed that EBs exposed to normoxia contained a higher number of pyknotic cells displaying condensed and fragmented nuclei than EB cells maintained under hypoxia (Figs. 4H and 4K). Previous studies have demonstrated that hypoxia increases collagen synthesis and facilitates the proliferation of adipose-derived stem cells (Lee et al., 2009; Wang et al., 2005). Consistent with these previous studies, we found that the collagen content was higher in the extracellular matrix (ECM) of EBs exposed to hypoxia than in the ECM of those maintained under normoxia, as determined by Azan and Masson’s trichrome staining (Figs. 4I and 4L, blue).
Effect of O2 tension on the formation of the three- germ-layer during early differentiation
We next investigated the effect of O2 tension on the developmental competence of EBs. Western blot analysis of 8-day-old EBs showed that HIF1α protein expression gradually increased as the O2 tension decreased (Fig. 5A). In addition, the expression of hypoxia-related genes, including EPO, VEGF, and Glut-1, all increased in EBs cultured under hypoxia (Fig. 5B). These results suggest that differentiating EBs recognize the hypoxic O2 tension. We next analyzed the expression of genes specific for the three embryonic germ layers (ectoderm, endoderm and mesoderm) and extraembryonic tissue. Since we had previously observed different developmental competencies depending on the hESC line (Kim et al., 2007), we compared the differentiation potential of three different hESC lines under hypoxia. There was a trend towards an increase in the expression of the endodermal markers Foxa2 and Sox17 under hypoxia, while the expression of neuroectodermal markers Sox1 and TuJ1 decreased for all hESC lines (Fig. 5C). Expression of AFP was not considerably changed under different O2 tensions and Nestin expression decreased in two of three cell lines under hypoxia.
Fig. 5. Effects of O2 tension on the differentiation of early cell types during EB formation. (A) Expression of HIF1α at 8 days of EB formation was analyzed by Western blotting. (B) Expression of hypoxia markers (EPO, VEGF, and Glut1) in EBs were examined by RT-PCR. (C) RT-PCR analysis for markers of embryonic cell lineages in EBs grown under three different O2 conditions. (D-G) Representative immunofluorescence images of frozen EB sections stained for endoderm markers. Note the prominent localization of immunoreactive cells at the periphery of EBs grown under hypoxia, as compared to those grown under normoxia. Magnification, 100×.
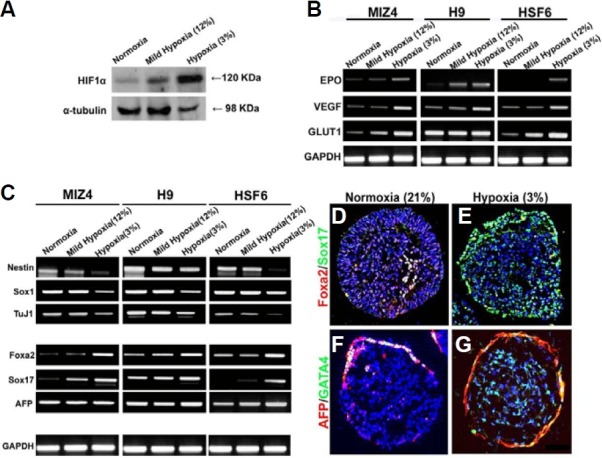
Immunostaining analysis showed that cells expressing Foxa2, Sox17, AFP, and GATA4 were preferentially localized to the periphery of EBs, although a few cells expressing Sox17 and GATA4 were detected inside the EBs (Figs. 5D-5G). Among the EBs containing 1,000-2,000 cells, the percentage of EBs dis-playing the complete outer layer of primitive endoderm (Fig. 5G), which is characterized by AFP and GATA4 expression, was 10% for EBs cultured under normoxia vs. 56% for hypoxia. Thus, our gene expression and immunostaining data suggest that hypoxia may facilitate the differentiation of hESCs into primitive endoderm rather than definitive endoderm.
DISCUSSION
Previous studies report that 4-5% O2 tensions reduce the spontaneous differentiation of hESCs, resulting in the maintenance of the pluripotent stem cell population (Ezashi et al., 2005; Westfall et al., 2008; Yoshida et al., 2009). However, a recent study reported no difference between hypoxia (5% O2) and normoxia (21% O2) with respect to the maintenance of the undifferentiated state of hESCs subcultured at 7-day splitting intervals (Chen et al., 2009). In the present study, we determined that mild hypoxia (12% O2) improves the pluripotency of long-term hESC cultures. These conflicting results could reflect differences in the selected cell lines and cell culture protocols (e.g., intervals between splitting). These results suggest that the O2-tension required for maintaining optimal hESC pluripotency should be considered when planning experiments. We also found that HIF2α protein levels increase under mild hypoxia. Previously, HIF1α and HIF2α were reported to regulate the pluripotency of hESCs (Covello et al., 2006; Hu et al., 2006), and HIF2α protein can directly bind to the Oct4 promoter (Covello et al., 2006; Loboda et al., 2010). In addition, a recent study provided evidence that HIF2α protein expression is confined to undifferentiated cells, while HIF1α protein is expressed in both the undifferentiated and differentiated cells within hESC colonies (Westfall et al., 2008). Thus, our study supports the idea that HIF2α plays an important role in the maintenance of hESC pluripotency, although the mechanism of action for HIF2α needs to be further examined at the molecular level.
The dynamic balance between cell death and proliferation is important and extensively regulated during early embryonic development. We showed that proliferation rate of hESCs was decreased under hypoxia, as compared to normoxia. This finding is consistent with a previous study (Ezashi et al., 2005), although another study performed under 2% O2 hypoxia showed an increased proliferation under hypoxia (Forsyth et al., 2006). In addition, the Caspase-3 assay indicates that although the rate of apoptosis for hESCs initially increases after their transfer from normoxic to hypoxic conditions, hESCs gradually adapted to the cellular stress imposed by the changing O2 concentrations. After serial passages in mild hypoxia, the number of apoptotic hESCs significantly decreased as compared to that for cells continuously grown under normoxia (P < 0.05). Thus, culturing hESCs under low O2 conditions for an extended period of time may significantly reduce apoptosis as compared to that for hESCs cultured under normoxia. Therefore, our study suggests that mild hypoxia reduces the proliferation and apoptosis of hESCs, although the exact mechanisms of hypoxia remain to be elucidated. We also found that hESCs grown under mild hypoxia exhibited less chromosomal abnormalities than those grown under normoxia. Previous studies have reported frequent chromosomal abnormalities in ESCs grown under normoxia (Catalina et al., 2009; Forsyth et al., 2006; Peura et al., 2008). These results, together with our findings, imply that the rapid proliferation of hESCs under normoxia could be responsible for the increased incidence of genetic abnormalities. It is also speculated that the slow mitotic division that occurs under mild hypoxia may allow hESCs to accurately segregate chromosomes, thereby decreasing the number of abnormal cells that need to be eliminated by apoptosis; this was observed in our study. Therefore, our study supports the idea that growing hESCs under normoxia might be detrimental to their function, as it increases the potential risk of genetic instability and argues that the genetic stability of hESC lines used for stem cell research should be systematically investigated.
Establishment of embryonic germ layers is one of the earliest events that control cell fates during embryonic development and occurs in vitro when ESCs differentiate into EBs. We showed that HIF1α, HIF2α, EPO, Glut1 and VEGF expression are all upregulated during hypoxia under 3% O2 tension. These results are consistent with previous reports that the latter three genes are regulated by the activation of HIFs as part of the cellular response to hypoxia (Gassmann et al., 1996). However, in that study, the EBs were exposed to relatively extreme hypoxia (1% O2), and the number of viable EBs decreased after 5 days of differentiation, suggesting that the low O2 tension was toxic. Therefore, further investigation is needed to elucidate the molecular mechanisms responsible for the effects of hypoxia on EB formation and determining the optimal oxygen concentration to generate EBs. We also found that hypoxia stimulated the development of microvilli on the cellular surface of differentiating EBs. This phenomenon has been reported for other cell types, including endothelial cells in the rat, human yolk sac and human uterus (Barberini et al., 2007; Beckman et al., 1991; Pereda and Motta, 1999). In general, the presence of a large number of microvilli is indicative of healthy intracellular metabolism (Oh et al., 2005), suggesting that hypoxia increases the metabolic rate in EBs and might be nontoxic to differentiating EBs. In addition, we observed increased collagen production in the ECM of EBs grown under hypoxia. Thus, these results suggest that hypoxia may provide a more in vivo-like environment for EBs rather than normoxia.
O2 tension is known to have an important effect on specific germ layer determination during normal embryonic development of the mouse and rabbit, suggesting a potential effect for O2 tension on the determination of cell fates in vitro (Li and Foote, 1993; Umaoka et al., 1992). Although there is increasing evidence that the differentiation of mesenchymal and tissue-specific stem cells could be affected by low oxygen tension (Bassett and Herrmann, 1961; Csete et al., 2001; Lennon et al., 2001; Robins et al., 2005), much less attention has been given to the possible influence of O2 tension on the in vitro formation of the embryonic germ layers during EB formation. Moreover, different results have been reported on the effect of hypoxia on germ layer formation in EBs (Bianco et al., 2009; Chen et al., 2010). Nevertheless, it was previously shown that hypoxia stimulates the proliferation of trophoblast giant cells, which form the outer layer of the placenta (Jaffe et al., 1997; Patel et al., 2010; Pringle et al., 2010). During embryogenesis, it is known that many markers, including Foxa2, Sox17, AFP and GATA4, are shared between primitive endoderm, which contributes to placenta, and definitive endoderm, which gives rise to intestinal organs. However, primitive endodermal cells can apparently be distinguished in EBs by immunostaining because of their localization to the outer cell layer (Kim et al., 2007; Ungrin et al., 2008). In the present study, we show that hypoxia may promote the in vitro differentiation of primitive endoderm from hESCs since the number of endodermal markerpositive cells at the periphery of EBs increased. Furthermore, the number of EBs displaying a complete surface layer of primitive endoderm increased under hypoxia. Primitive or extraembyonic endoderm, also known as the hypoblast, is derived from the inner cell mass of blastocysts and contributes to the formation of the primary yolk sac. Hypoblast plays an important role in the formation of the body plan during early embryonic patterning (Beddington and Robertson, 1999). However, relatively little is known about the molecular pathways that control the differentiation of primitive endoderm. Therefore, EB formation under low O2 tension may provide a useful in vitro model to study the differentiation of extraembryonic endoderm.
Acknowledgments
This research was supported by a grant (SC-3130) from the Stem Cell Research Center of the 21st Century Frontier Research Program funded by the Ministry of Education, Science and Technology, Republic of Korea.
References
- 1.Abeyta M.J., Clark A.T., Rodriguez R.T., Bodnar M.S., Pera R.A., Firpo M.T. Unique gene expression signatures of independently-derived human embryonic stem cell lines. Hum. Mol. Genet. (2004);13:601–608. doi: 10.1093/hmg/ddh068. [DOI] [PubMed] [Google Scholar]
- 2.Amit M., Margulets V., Segev H., Shariki K., Laevsky I., Coleman R., Itskovitz-Eldor J. Human feeder layers for human embryonic stem cells. Biol. Reprod. (2003);68:2150–2156. doi: 10.1095/biolreprod.102.012583. [DOI] [PubMed] [Google Scholar]
- 3.Aplin J.D. Hypoxia and human placental development. J. Clin. Invest. (2000);105:559–560. doi: 10.1172/JCI9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barberini F., Makabe S., Franchitto G., Correr S., Relucenti M., Heyn R., Familiari G. Ultrastructural dynamics of the human endometrium from 14 to 22 weeks of gestation. Arch. Histol. Cytol. (2007);70:21–28. doi: 10.1679/aohc.70.21. [DOI] [PubMed] [Google Scholar]
- 5.Bassett C.A., Herrmann I. Influence of oxygen concentration and mechanical factors on differentiation of connective tissues in vitro. Nature. (1961);190:460–461. doi: 10.1038/190460a0. [DOI] [PubMed] [Google Scholar]
- 6.Beckman D.A., Ornoy A., Jensen M., Arnon J., Brent R.L. Ultrastructure and function of the rat yolk sac: damage caused by teratogenic anti-VYS serum and recovery. Teratology. (1991);44:181–192. doi: 10.1002/tera.1420440206. [DOI] [PubMed] [Google Scholar]
- 7.Beddington R.S., Robertson E.J. Axis development and early asymmetry in mammals. Cell. (1999);96:195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- 8.Bianco C., Cotten C., Lonardo E., Strizzi L., Baraty C., Mancino M., Gonzales M., Watanabe K., Nagaoka T., Berry C., et al. Cripto-1 is required for hypoxia to induce cardiac differentiation of mouse embryonic stem cells. Am. J. Pathol. (2009);175:2146–2158. doi: 10.2353/ajpath.2009.090218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catalina P., Bueno C., Montes R., Nieto A., Ligero G., Sanchez L., Jara M., Rasillo A., Orfao A., Cigudosa J., et al. Genetic stability of human embryonic stem cells: A first-step toward the development of potential hESC-based systems for modeling childhood leukemia. Leuk. Res. (2009);33:980–990. doi: 10.1016/j.leukres.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Chen H.F., Kuo H.C., Chen W., Wu F.C., Yang Y.S., Ho H.N. A reduced oxygen tension (5%) is not beneficial for maintaining human embryonic stem cells in the undifferentiated state with short splitting intervals. Hum. Reprod. (2009);24:71–80. doi: 10.1093/humrep/den345. [DOI] [PubMed] [Google Scholar]
- 11.Chen H.F., Kuo H.C., Lin S.P., Chien C.L., Chiang M.S., Ho H.N. Hypoxic culture maintains self-renewal and enhances embryoid body formation of human embryonic stem cells. Tissue Eng. (2010);16:2901–2913. doi: 10.1089/ten.tea.2009.0722. [DOI] [PubMed] [Google Scholar]
- 12.Covello K.L., Kehler J., Yu H., Gordan J.D., Arsham A.M., Hu C.J., Labosky P.A., Simon M.C., Keith B. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. (2006);20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csete M. Oxygen in the cultivation of stem cells. Ann. N Y Acad. Sci. (2005);1049:1–8. doi: 10.1196/annals.1334.001. [DOI] [PubMed] [Google Scholar]
- 14.Csete M., Walikonis J., Slawny N., Wei Y., Korsnes S., Doyle J.C., Wold B. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J. Cell. Physiol. (2001);189:189–196. doi: 10.1002/jcp.10016. [DOI] [PubMed] [Google Scholar]
- 15.Draper J.S., Moore H.D., Ruban L.N., Gokhale P.J., Andrews P.W. Culture and characterization of human embryonic stem cells. Stem Cells Dev. (2004);13:325–336. doi: 10.1089/scd.2004.13.325. [DOI] [PubMed] [Google Scholar]
- 16.Ezashi T., Das P., Roberts R.M. Low O2 tensions and the prevention of differentiation of hES cells. Proc. Natl. Acad. Sci. USA. (2005);102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer B., Bavister B.D. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J. Reprod. Fertil. (1993);99:673–679. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- 18.Forsyth N.R., Musio A., Vezzoni P., Simpson A.H., Noble B.S., McWhir J. Physiologic oxygen enhances human embryonic stem cell clonal recovery and reduces chromosomal abnormalities. Cloning Stem Cells. (2006);8:16–23. doi: 10.1089/clo.2006.8.16. [DOI] [PubMed] [Google Scholar]
- 19.Gassmann M., Fandrey J., Bichet S., Wartenberg M., Marti H.H., Bauer C., Wenger R.H., Acker H. Oxygen supply and oxygen-dependent gene expression in differentiating embryonic stem cells. Proc. Natl. Acad. Sci. USA. (1996);93:2867–2872. doi: 10.1073/pnas.93.7.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu C.J., Iyer S., Sataur A., Covello K.L., Chodosh L.A., Simon M.C. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol. Cell. Biol. (2006);26:3514–3526. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaffe R., Jauniaux E., Hustin J. Maternal circulation in the first-trimester human placenta-myth or reality? Am. J. Obstet. Gynecol. (1997);176:695–705. doi: 10.1016/s0002-9378(97)70572-6. [DOI] [PubMed] [Google Scholar]
- 22.Johkura K., Cui L., Asanuma K., Okouchi Y., Ogiwara N., Sasaki K. Cytochemical and ultrastructural characterization of growing colonies of human embryonic stem cells. J. Anat. (2004);205:247–255. doi: 10.1111/j.0021-8782.2004.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J.H., Panchision D., Kittappa R., McKay R. Generating CNS neurons from embryonic, fetal, and adult stem cells. Meth. Enzymol. (2003);365:303–327. doi: 10.1016/s0076-6879(03)65022-6. [DOI] [PubMed] [Google Scholar]
- 24.Kim S.E., Kim B.K., Gil J.E., Kim S.K., Kim J.H. Comparative analysis of the developmental competence of three human embryonic stem cell lines in vitro. Mol. Cells. (2007);23:49–56. [PubMed] [Google Scholar]
- 25.Lee E.Y., Xia Y., Kim W.S., Kim M.H., Kim T.H., Kim K.J., Park B.S., Sung J.H. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repr. Regen. (2009);17:540–547. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 26.Lennon D.P., Edmison J.M., Caplan A.I. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J. Cell. Physiol. (2001);187:345–355. doi: 10.1002/jcp.1081. [DOI] [PubMed] [Google Scholar]
- 27.Li J., Foote R.H. Culture of rabbit zygotes into blastocysts in protein-free medium with one to twenty per cent oxygen. J. Reprod. Fertil. (1993);98:163–167. doi: 10.1530/jrf.0.0980163. [DOI] [PubMed] [Google Scholar]
- 28.Loboda A., Jozkowicz A., Dulak J. HIF-1 and HIF-2 transcription factors-similar but not identical. Mol. Cells. (2010);29:436–442. doi: 10.1007/s10059-010-0067-2. [DOI] [PubMed] [Google Scholar]
- 29.Oh S.K., Kim H.S., Ahn H.J., Seol H.W., Kim Y.Y., Park Y.B., Yoon C.J., Kim D.W., Kim S.H., Moon S.Y. Derivation and characterization of new human embryonic stem cell lines: SNUhES1, SNUhES2, and SNUhES3. Stem Cells. (2005);23:211–219. doi: 10.1634/stemcells.2004-0122. [DOI] [PubMed] [Google Scholar]
- 30.Patel J., Landers K., Mortimer R.H., Richard K. Regulation of hypoxia inducible factors (HIF) in hypoxia and normoxia during placental development. Placenta. (2010);31:951–957. doi: 10.1016/j.placenta.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Pereda T.J., Motta P.M. New advances in human embryology: morphofunctional relationship between the embryo and the yolk sac. Med Electron Microsc. (1999);32:67–78. doi: 10.1007/s007950050011. [DOI] [PubMed] [Google Scholar]
- 32.Peura T., Bosman A., Chami O., Jansen R.P., Texlova K., Stojanov T. Karyotypically normal and abnormal human embryonic stem cell lines derived from PGD-analyzed embryos. Cloning Stem Cells. (2008);10:203–216. doi: 10.1089/clo.2007.0062. [DOI] [PubMed] [Google Scholar]
- 33.Pick M., Azzola L., Mossman A., Stanley E.G., Elefanty A.G. Differentiation of human embryonic stem cells in serum-free medium reveals distinct roles for bone morphogenetic protein 4, vascular endothelial growth factor, stem cell factor, and fibroblast growth factor 2 in hematopoiesis. Stem Cells. (2007);25:2206–2214. doi: 10.1634/stemcells.2006-0713. [DOI] [PubMed] [Google Scholar]
- 34.Pringle K.G., Kind K.L., Sferruzzi-Perri A.N., Thompson J.G., Roberts C.T. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Hum. Reprod. Update. (2010);16:415–431. doi: 10.1093/humupd/dmp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramirez-Bergeron D.L., Simon M.C. Hypoxia-inducible factor and the development of stem cells of the cardiovascular system. Stem Cells. (2001);19:279–286. doi: 10.1634/stemcells.19-4-279. [DOI] [PubMed] [Google Scholar]
- 36.Reubinoff B.E., Pera M.F., Fong C.Y., Trounson A., Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. (2000);18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 37.Richards S., Leavesley D., Topping G., Upton Z. Development of defined media for the serum-free expansion of primary keratinocytes and human embryonic stem cells. Tissue Eng. Part C Methods. (2008);14:221–232. doi: 10.1089/ten.tec.2007.0428. [DOI] [PubMed] [Google Scholar]
- 38.Robins J.C., Akeno N., Mukherjee A., Dalal R.R., Aronow B.J., Koopman P., Clemens T.L. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. (2005);37:313–322. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 39.Semenza G.L., Agani F., Feldser D., Iyer N., Kotch L., Laughner E., Yu A. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv. Exp. Med. Biol. (2000);475:123–130. doi: 10.1007/0-306-46825-5_12. [DOI] [PubMed] [Google Scholar]
- 40.Son Y.S., Park J.H., Kang Y.K., Park J.S., Choi H.S., Lim J.Y., Lee J.E., Lee J.B., Ko M.S., Kim Y.S., et al. Heat shock 70-kDa protein 8 isoform 1 is expressed on the surface of human embryonic stem cells and downregulated upon differentiation. Stem Cells. (2005);23:1502–1513. doi: 10.1634/stemcells.2004-0307. [DOI] [PubMed] [Google Scholar]
- 41.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. (1998);282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 42.Umaoka Y., Noda Y., Narimoto K., Mori T. Effects of oxygen toxicity on early development of mouse embryos. Mol. Reprod. Dev. (1992);31:28–33. doi: 10.1002/mrd.1080310106. [DOI] [PubMed] [Google Scholar]
- 43.Ungrin M.D., Joshi C., Nica A., Bauwens C., Zandstra P.W. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS ONE. (2008);3:e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D.W., Fermor B., Gimble J.M., Awad H.A., Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J. Cell. Physiol. (2005);204:184–191. doi: 10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
- 45.Westfall S.D., Sachdev S., Das P., Hearne L.B., Hannink M., Roberts R.M., Ezashi T. Identification of oxygensensitive transcriptional programs in human embryonic stem cells. Stem Cells Dev. (2008);17:869–881. doi: 10.1089/scd.2007.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida Y., Takahashi K., Okita K., Ichisaka T., Yamanaka S. Hypoxia enhances the generation of induced pluripo-tent stem cells. Cell Stem Cell. (2009);5:237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]


