Abstract
Inducible nitric oxide synthase (iNOS) is an essential mediator in diabetic vascular lesions and known to be regulated by activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII). The aim of this study was to investigate whether CaMKII affects iNOS-mediated pericyte death in the retina of diabetic mice with early stage disease. Totaland phospho-CaMKII, iNOS, and active caspase-3 protein levels were assessed by Western blotting, and CaMKII activity was measured by kinase assay. iNOS-related pericyte death was assessed by double immunofluorescent staining for iNOS and α-smooth muscle actin, followed by the TUNEL assay. Autocamtide-2-related inhibitory peptide (AIP), a specific inhibitor of CaMKII, was injected into the right vitreous 2 days before sacrifice of mice, to examine the effect of CaMKII inactivation in diabetic retinas. The levels of total- and phospho-CaMKII, iNOS, and active caspase- 3 protein, and CaMKII activity were significantly increased in the diabetic retinas compared with those of control retinas. Furthermore, TUNEL-positive signals colocalized with iNOS-immunoreactive pericytes in the same retinas. However, inactivation of CaMKII by AIP treatment inhibited all these changes, which was accompanied by less pericyte loss. Our results demonstrate that CaMKII contributes to iNOS-related death of pericytes in the diabetic retina and that inactivation of this enzyme may be a potential treatment for retinal vascular lesion.
Keywords: AIP, CaMKII, diabetic retina, iNOS, pericyte death
INTRODUCTION
Vascular damage induced in early stage diabetes results in blood-retinal barrier (BRB) breakdown, and pericyte loss (Cheung et al., 2005; Joussen et al., 2004). These events are an immediate cause of diabetic retinopathy (DR) in patients, which is the major pathology leading to vision loss at advanced stages of the disease. Therefore, results from previous reports indicate that control of these pathological processes from early stage of diabetes is a principal target for preventing diabetesinduced retinal destruction.
Excessive production of nitric oxide (NO) is thought to play an important role in both physiological and pathological conditions (Christopherson and Bredt, 1997; Lipton et al., 1993). In particular, NO generated by inducible NO synthase (iNOS) is involved in chronic diseases, including diabetes (Lee et al., 2010), whereas NO generated by endothelial NOS is involved in physiological functions, such as blood flow, kidney function and smooth muscle action (Carmo et al., 2000; Ellis et al., 2002). Also, iNOS has been known to play a critical role in processes leading to retinal capillary damage in the early stages of DR, including pericyte loss (Leal et al., 2007; Tang et al., 2003; Zheng et al., 2007).
CaMKII is a multifunctional serine-threonine protein kinase and is primarily composed of α- and β-subunits. CaMKII is activated in response to Ca2+ influx or Ca2+/calmodulin binding in an autophosphorylation-dependent manner (Cruzalegui et al., 1992; Soderling, 1996). Activated CaMKII has been shown to be an important mediator in the development of abnormal vascular dysfunction in diabetes (Benter et al., 2005; Kato et al., 2008). Autocamtide-2-related inhibitory peptide (AIP), a potent inhibitor of CaMKII, has been used as a tool for studying the physiological role of CaMKII (Aromolaran and Blatter, 2005; Munevar et al., 2008).
Several reports have suggested that CaMKII activation plays a critical role in iNOS-induced vascular lesions (Jones et al., 2007; Ozveren et al., 2006; Zhang et al., 2003), however there have been no reports on the correlation between CaMKII activation and iNOS induction in diabetes and/or in the retina. In the present study, we investigated whether CaMKII affects iNOS-related pericyte death in diabetic mouse retinas.
MATERIALS AND METHODS
Experimental diabetic model
Male C57BL/6 mice (8 weeks of age) were purchased from KOATEC (Korea). All mice were maintained on a standard rodent diet, given water ad libitum, and handled in strict accordance with the Institutional Animal Care and Use Committee of Gyeongsang National University. To induce diabetes, mice were injected 55 mg/kg of streptozotocin (STZ; Sigma, USA) dissolved in 0.05 M citrate buffer (pH 4.5) into the peritoneal cavity once a day for 5 consecutive days. Control mice received the buffer only. Mice were sacrificed 2 months after the injections. One week after the fifth injection of STZ, mice with blood glucose levels > 13.9 mM were included in the diabetic group. Blood samples were obtained by tail puncture following a 2-h fasting period, and blood glucose was measured using a glucometer (Precision; MEDISENSE Contract Manufacturing; UK).
Antibodies
Rabbit monoclonal and polyclonal antibodies specific for CaMKII and active caspase-3 were purchased from Abcam (UK). Mouse monoclonal antibodies specific for α-smooth muscle actin (ASMA; pericyte marker) and α-tubulin were purchased from Chemicon (USA) and Sigma, respectively. Rabbit polyclonal antibodies specific for phospho-CaMKII (T286) and iNOS were obtained from Cell Signaling (USA). Horseradish peroxidase (HRP)-conjugated anti-mouse and -rabbit IgG secondary antibodies for western blotting, and Alexa Fluor™-350 and -488 goat anti-rabbit and -mouse IgG secondary antibodies for immunofluorescent staining were purchased from Pierce (USA) and Invitrogen (USA), respectively.
Western blot analysis
Two months after STZ and buffer injection, total protein was extracted from the mouse retinas and Western blotting was performed, as previously described (Kim et al., 2005). Thirty micrograms of retinal total protein was resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The membranes were immunoblotted with the appropriate primary and secondary antibodies, and the proteins were visualized using an enhanced chemiluminescent (ECL) kit according to the manufacturer’s instructions (Amersham Biosciences, USA). Each blot was stripped and reprobed with α-tubulin. Protein levels for each antibody were normalised to α-tubulin, and the results expressed as the fold-change compared with each control.
AIP treatment
AIP was completely dissolved in sterile 100 mM phosphatebuffered saline (PBS; pH 7.4) to a final concentration of 500 μM. Two microliters of AIP were injected into the right vitreous 2 days before sacrificing diabetic and non-diabetic mice. The left vitreous was injected with PBS as a control. The efficacy of AIP in inhibiting CaMKII was verified by western blot analysis for total and phospho-CaMKII at 0, 1, 2, and 4 days after the injection. The maximum decrease in the level of phospho-CaMKII protein was observed 2 days after treatment (data not shown); therefore, this time point was used throughout this study.
CaMKII activity assay
CaMKII activity was examined using total protein lysate (100 μg) from each retina using the CaMKII Kinase Assay Kit (Upstate) according to the manufacturer’s directions. CaMKII was stimulated with buffer containing 40 mmol/L HEPES, 1 mM Mgacetate, 100 μM EGTA, 200 μM Ca2+, 50 μM ATP, and 10 μM calmodulin. The reaction was initiated by the addition of calmodulin and terminated by the addition of 1 mM EGTA at the indicated times. Total enzyme activity was measured as pmol of phosphate incorporated into the CaMKII substrate peptide/ min/mg total retinal protein. Results are expressed as the foldchange compared with controls.
Co-labeling with iNOS and ASMA in parallel with the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) assay
To determine the correlation between pericyte death and iNOS expression, double immunofluorescent staining for iNOS and ASMA was performed, followed by the TUNEL assay with TMR red on the same frozen sections, as described previously (Kim et al., 2007). The retinal sections were incubated in Image-iT™ FX signal enhancer (Invitrogen), a mixture of iNOS and ASMA antibodies, and a mixture of Alexa Fluor™-488 and -350 goat anti-rabbit and -mouse IgGs, consecutively, and wet-mounted in ProLong® Gold anti-fade reagent (Invitrogen). All reactions were performed in a moist chamber to avoid evaporative loss throughout the procedure. Pericytes co-stained with TUNEL and iNOS were carefully observed using an IX2-DSU disk scanning biological microscope (Olympus, Germany).
Evans blue leakage assay
Vessel leakage was assessed using the Evans blue leakage assay, following a previously reported protocol (Kim et al., 2007b) without any modifications. The concentration of Evans blue dye in the retinal extracts was calculated using a standard curve for Evans blue in formamide and normalized to the dried retinal weight of each sample. The results are expressed as micrograms of Evans blue per milligram of total protein content.
Image capture and statistical analysis
Retinas were imaged using an IX2-DSU microscope. Images of the retinal sections were captured at a distance of approximately 0.8-1 mm from the optic nerve head. Quantitative analyses were obtained using the Soft Imaging System (System GmbH, Germany) and SigmaGel 1.0 (Jandel Scientific, Germany) software. All diagrams were manufactured by SigmaPlot 4.0 (SPSS Inc., USA) software and Adobe Illustrator CS3 (USA). The significance of inter-group differences was evaluated by the Kruskal Wallis H-test and Mann-Whitney U-test (SPSS). Data are representative of four independent tests and presented as the mean ± SEM. Differences were considered significant at P < 0.05.
RESULTS
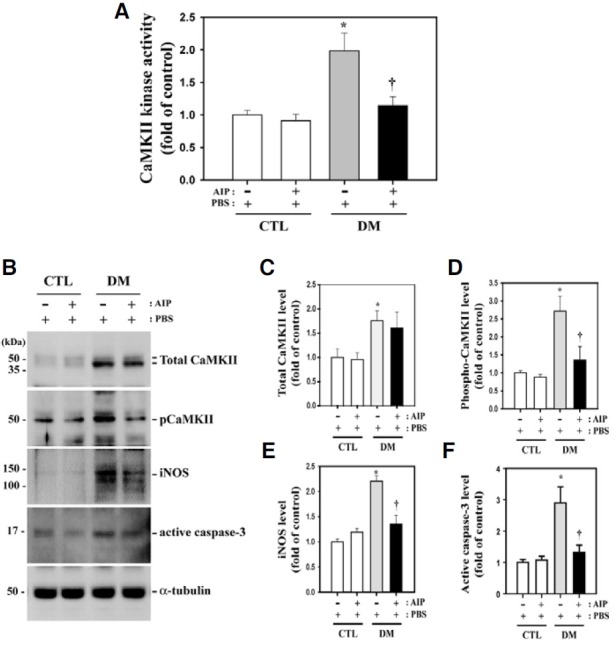
Upregulation of CaMKII, iNOS, and active caspase-3 protein levels and CaMKII kinase activity in diabetic mouse retina and the effect of AIP treatment
CaMKII kinase activity was 1.98-fold higher in retinas of diabetic mice than in the non-diabetic controls (P = 0.013, n = 4) (Fig. 1A). Furthermore, CaMKII activity was reduced by 42% (P = 0.033, n = 4) in diabetic mice after AIP treatment. In agreement with this finding, total and phospho-CaMKII, iNOS, and active caspase-3 protein levels were greatly increased in retinas of diabetic mice compared with non-diabetic control mice (1 ± 0.177 vs. 1.78 ± 0.205, P = 0.0197; 1 ± 0.058 vs. 2.72 ± 0.414, P = 0.0034; 1 ± 0.057 vs. 2.21 ± 0.103, P = 0.00007; 1 ± 0.090 vs. 2.90 ± 0.512, P = 0.0107, respectively; n = 4) (Figs. 1B-1F), while total CaMKII levels remained unchanged after AIP treatment (Fig. 1B). Levels of phospho-CaMKII, iNOS, and active caspase-3 were suppressed by 50%, 39%, and 45%, respectively, in the retinas of AIP-treated mice compared with PBS-treated diabetic mice (P = 0.0405, 0.003, and 0.031, respectively, n = 4). Total CaMKII protein levels did not significantly change (P = 0.813, n = 4).
Fig. 1. CaMKII and caspase-3 activity and total CaMKII and iNOS levels in diabetic mouse retina and the effect of AIP treatment. (A) CaMKII kinase activity and the levels of these proteins increased in the retinas of diabetic mice compared with the controls (*P = 0.013, n = 4). Activity was greatly reduced in the retinas of AIPtreated diabetic mice compared with PBS-treated diabetic mice (†P = 0.033, n = 4). (B) Western blot analysis using the indicated specific antibodies on total cell lysates of retinas from diabetic and control mice. Caspase- 3, iNOS and phospho-CaMKII levels were reduced in AIP-treated diabetic mice, whereas total CaMKII protein levels did not significantly change (P = 0.813, n = 4). (C-F) Quantitative representation of Western blot results for the indicated proteins. All experiments were performed in quadruplicate. Data are presented as the mean ± SEM (n = 4). *P < 0.05, comparing the PBS-treated control and diabetic mice; †P < 0.05, comparing the PBS- and AIP-treated diabetic groups.
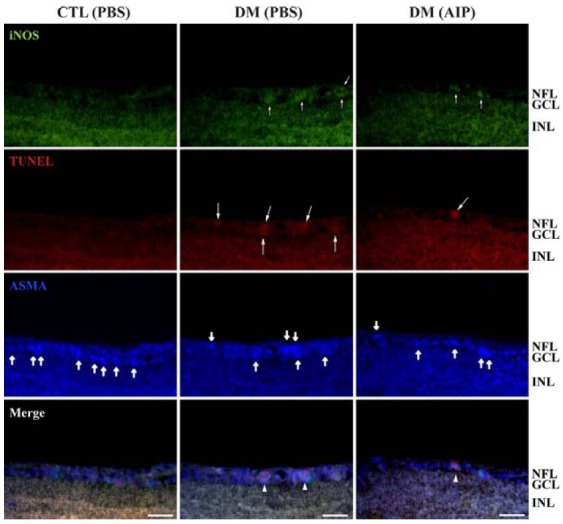
Correlation between iNOS expression and pericyte death in diabetic retinas and the effect of AIP treatment
Increased iNOS expression (small arrows) and TUNEL-positive signals (large arrows) were observed in diabetic mice compared with control mice (Fig. 2). Thick arrows indicate AMPAexpression in pericytes. The diabetes-induced increases in iNOS and TUNEL in retinal pericytes was suppressed by AIP treatment.
Fig. 2. Correlation of CaMKII activity and iNOS expression with pericyte death in diabetic mice retinas. Double immunofluorescent staining for iNOS and ASMA (pericyte marker) was performed, followed by the TUNEL assay with TMR red on the same frozen sections. Increased iNOS and TUNEL-positive cells were observed in diabetic mice retinas (small and large arrows). These signals co-localized with ASMA-positive pericytes (thick arrows). The diabetes-induced increase in iNOS-positive dead pericytes (arrowheads) was suppressed by AIP treatment. GCL, ganglion cell layer; INL, inner nuclear layer; NFL, nerve fiber layer. Scale bar, 12.5 μm.
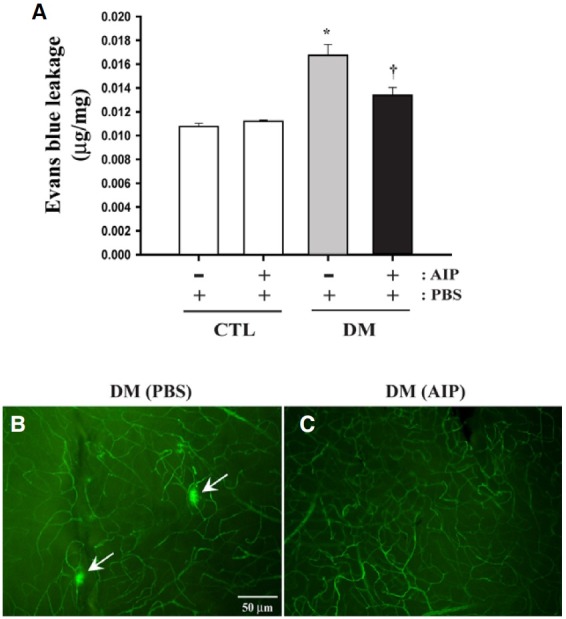
Diabetes-induced retinal vascular leakage and the effect of AIP treatment
Results from the Evans blue leakage assay revealed that vascular leakage increased over 1.55-fold in diabetic mouse retinas compared with the non-diabetic controls (P = 0.0011, n = 4). However, AIP treatment effectively blocked vascular leakage without affecting normal BRB in the control mice (Fig. 3A) (P = 0.0083, n = 4). In agreement with this result, fluorescence angiography shows vascular leakage in the retinal flat mounts of diabetic mice (arrows, Fig. 3B), which was prevented with AIP treatment (Fig. 3C). No leakage was observed in the retinas of PBS- or AIP-treated non-diabetic control mice (data not shown).
Fig. 3. BRB breakdown in diabetic mouse retinas and the effect of AIP treatment. BRB breakdown was assessed using the Evans blue leakage assay (A) and angiography using TMR-D infusion (B, C). (A) Vascular leakage increased in diabetic mouse retinas compared with non-diabetic controls (*P = 0.0011). Diabetes-induced vascular leakage was significantly inhibited in diabetic mice compared with control mice by AIP treatment (†P = 0.0083). Experiments were performed in quadruplicate and the results expressed as micrograms of Evans blue per milligram of total protein content. Data are presented as the mean ± SEM (n = 4). *P < 0.05, comparing the PBS-treated control groups and others; †P < 0.05, comparing the PBS- and AIPtreated diabetic groups. (B) TMR-D angiography confirmed vascular leakage in the retinal flat mounts of PBS-treated diabetic mice (arrows). (C) AIP treatment prevents vascular leakage in diabetic mice. CTL, nondiabetic control group; DM, diabetic group. Scale bar, 50 μm.
DISCUSSION
Diabetes induces vascular lesions and pericyte loss in the retina and iNOS has been shown to mediate these events (Leal et al., 2007; Tang et al., 2003; Zheng et al., 2007). In the present study, we investigated whether CaMKII activity contributes to iNOS-mediated diabetes-induced pericyte death in the retina.
Total and active CaMKII, iNOS, and active caspase-3 levels were greatly increased in diabetic mice compared to non-diabetic control mice, and these increases were prevented by AIP treatment expect for total CaMKII. These results show that CaMKII activity is required for upregulation of iNOS and caspase- 3 in the diabetic retinas. CaMKII is known to be a potent upstream regulator of iNOS (Jones et al., 2007; Ozveren et al., 2006). In addition, overexpression of CaMKII or iNOS can trigger caspase-3-dependent cell death in damaged retinas (Cooper et al., 2008; Goebel, 2009; She et al., 2007) and both contribute to diabetic pathology (Ko et al., 2008; Li and Mahato, 2008). On the basis of these facts, we hypothesised that CaMKII blockade may inhibit iNOS-mediated cell death in diabetic mice.
We confirm that diabetes leads to the death of retinal pericytes and iNOS-immunopositive signals induced by diabetes co-localised with the dead pericytes. Furthermore, diabetesinduced iNOS expression and pericyte death were suppressed by inhibition of CaMKII. In multiple diabetic animal models, induction and activation of CaMKII have been reported to aggravate hyperglycemia-induced abnormal vascular pathology (Aromolaran and Blatter, 2005; Munevar et al., 2008; Yousif et al., 2008). The inflammatory enzyme iNOS also plays an important role in the pathogenesis of vascular lesions characteristic of the early stages of DR, including cell loss in retinal pericytes (Leal et al., 2007; Zheng et al., 2007). Therefore, our results suggest that increased iNOS expression induces pericyte death in diabetic retinas and that this process requires CaMKII activity.
Vascular leakage through BRB breakdown is an early characteristic of diabetes-induced vascular destruction in retina and closely connected to damage of pericyte (Cheung et al., 2005; Joussen et al., 2004). Vessel leakage was highly increased in diabetic mouse retinas. Results show that CaMKII inactivation reduces diabetes-induced retinal vascular leakage. Although, a correlation between CaMKII activity and vessel leakage has not been previously reported, CaMKII induction of iNOS has been shown to lead vascular leakage (Kaur et al., 2009; Leal et al., 2007). Therefore, the present study suggests that CaMKII mediates increases in iNOS levels in the retinas of diabetes, inducing vessel leakage. Taken together, our study demonstrates that CaMKII activation due to diabetes may contribute to iNOSmediated pericyte death in mouse retinas.
Acknowledgments
This work was supported by the National Research Foundation (NRF) of Korea grant funded by the Korea government (MEST) (R13-2005-012-01001-0), a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A084575).
References
- 1.Aromolaran A.A., Blatter L.A. Modulation of intracellular Ca2+ release and capacitative Ca2+ entry by CaMKII inhibitors in bovine vascular endothelial cells. Am. J. Physiol. Cell Physiol. (2005);289:C1426–1436. doi: 10.1152/ajpcell.00262.2005. [DOI] [PubMed] [Google Scholar]
- 2.Benter I.F., Yousif M.H., Canatan H., Akhtar S. Inhibition of Ca2+/calmodulin-dependent protein kinase II, RASGTPase and 20-hydroxyeicosatetraenoic acid attenuates the development of diabetes-induced vascular dysfunction in the rat carotid artery. Pharmacol. Res. (2005);52:252–257. doi: 10.1016/j.phrs.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Carmo A., Cunha-Vaz J.G., Carvalho A.P., Lopes M.C. Nitric oxide synthase activity in retinas from non-insulindependent diabetic Goto-Kakizaki rats: correlation with bloodretinal barrier permeability. Nitric Oxide. (2000);4:590–596. doi: 10.1006/niox.2000.0312. [DOI] [PubMed] [Google Scholar]
- 4.Cheung A.K., Fung M.K., Lo A.C., Lam T.T., So K.F., Chung S.S., Chung S.K. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes. (2005);54:3119–3125. doi: 10.2337/diabetes.54.11.3119. [DOI] [PubMed] [Google Scholar]
- 5.Christopherson K.S., Bredt D.S. Nitric oxide in excitable tissues: physiological roles and disease. J. Clin. Invest. (1997);100:2424–2429. doi: 10.1172/JCI119783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper N.G., Laabich A., Fan W., Wang X. The relationship between neurotrophic factors and CaMKII in the death and survival of retinal ganglion cells. Prog. Brain Res. (2008);173:521–540. doi: 10.1016/S0079-6123(08)01136-9. [DOI] [PubMed] [Google Scholar]
- 7.Cruzalegui F.H., Kapiloff M.S., Morfin J.P., Kemp B.E., Rosenfeld M.G., Means A.R. Regulation of intrasteric inhibition of the multifunctional calcium/calmodulin-dependent protein kinase. Proc. Natl. Acad. Sci. USA. (1992);89:12127–12131. doi: 10.1073/pnas.89.24.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis E.A., Guberski D.L., Hutson B., Grant M.B. Time course of NADH oxidase, inducible nitric oxide synthase and peroxynitrite in diabetic retinopathy in the BBZ/WOR rat. Nitric Oxide. (2002);6:295–304. doi: 10.1006/niox.2001.0419. [DOI] [PubMed] [Google Scholar]
- 9.Goebel D.J. Selective blockade of CaMKII-alpha inhibits NMDA-induced caspase-3-dependent cell death but does not arrest PARP-1 activation or loss of plasma membrane selectivity in rat retinal neurons. Brain Res. (2009);1256:190–204. doi: 10.1016/j.brainres.2008.12.051. [DOI] [PubMed] [Google Scholar]
- 10.Jones R.J., Jourd'heuil D., Salerno J.C., Smith S.M., Singer H.A. iNOS regulation by calcium/calmodulin-dependent protein kinase II in vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. (2007);292:H2634–2642. doi: 10.1152/ajpheart.01247.2006. [DOI] [PubMed] [Google Scholar]
- 11.Joussen A.M., Poulaki V., Le M.L., Koizumi K., Esser C., Janicki H., Schraermeyer U., Kociok N., Fauser S., Kirchhof B., et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. (2004);18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 12.Kato I., Oya T., Suzuki H., Takasawa K., Ichsan A.M., Nakada S., Ishii Y., Shimada Y., Sasahara M., Tobe K., et al. A novel model of insulin-dependent diabetes with renal and retinal lesions by transgenic expression of CaMKIIalpha (Thr286Asp) in pancreatic beta-cells. Diabetes Metab. Res. Rev. (2008);24:486–497. doi: 10.1002/dmrr.864. [DOI] [PubMed] [Google Scholar]
- 13.Kaur C., Sivakumar V., Foulds W.S., Luu C.D., Ling E.A. Cellular and vascular changes in the retina of neonatal rats after an acute exposure to hypoxia. Invest. Ophthalmol. Vis. Sci. (2009);50:5364–5374. doi: 10.1167/iovs.09-3552. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y.H., Kim Y.S., Kang S.S., Noh H.S., Kim H.J., Cho G.J., Choi W.S. Expression of 14-3-3 zeta and interaction with protein kinase C in the rat retina in early diabetes. Diabetologia. (2005);48:1411–1415. doi: 10.1007/s00125-005-1774-7. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y.H., Choi M.Y., Kim Y.S., Han J.M., Lee J.H., Park C.H., Kang S.S., Choi W.S., Cho G.J. Protein kinase C delta regulates anti-apoptotic alphaB-crystallin in the retina of type 2 diabetes. Neurobiol. Dis. (2007);28:293–303. doi: 10.1016/j.nbd.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Ko S.H., Ryu G.R., Kim S., Ahn Y.B., Yoon K.H., Kaneto H., Ha H., Kim Y.S., Song K. H. Inducible nitric oxide synthase- nitric oxide plays an important role in acute and severe hypoxic injury to pancreatic beta cells. Transplantation. (2008);85:323–330. doi: 10.1097/TP.0b013e31816168f9. [DOI] [PubMed] [Google Scholar]
- 17.Leal E.C., Manivannan A., Hosoya K., Terasaki T., Cunha-Vaz J., Ambrosio A.F., Forrester J.V. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and bloodretinal barrier breakdown in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. (2007);48:5257–5265. doi: 10.1167/iovs.07-0112. [DOI] [PubMed] [Google Scholar]
- 18.Lee B.R., Lee Y.P., Kim D.W., Song H.Y., Yoo K.Y., Won M.H., Kang T.C., Lee K.J., Kim K.H., Joo J.H., et al. Amelioration of streptozotocin-induced diabetes by Agrocybe chaxingu polysaccharide. Mol. Cells. (2010);29:349–354. doi: 10.1007/s10059-010-0044-9. [DOI] [PubMed] [Google Scholar]
- 19.Li F., Mahato R.I. iNOS gene silencing prevents inflammatory cytokine-induced beta-cell apoptosis. Mol. Pharm. (2008);5:407–417. doi: 10.1021/mp700145f. [DOI] [PubMed] [Google Scholar]
- 20.Lipton S.A., Choi Y.B., Pan Z.H., Lei S.Z., Chen H.S., Sucher N.J., Loscalzo J., Singel D.J., Stamler J.S. A redoxbased mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. (1993);364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 21.Munevar S., Gangopadhyay S.S., Gallant C., Colombo B., Sellke F.W., Morgan K.G. CaMKIIT287 and T305 regulate history-dependent increases in alpha agonist-induced vascular tone. J. Cell Mol. Med. (2008);12:219–226. doi: 10.1111/j.1582-4934.2007.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozveren E., Korkmaz B., Buharalioglu C.K., Tunctan B. Involvement of calcium/calmodulin-dependent protein kinase II to endotoxin-induced vascular hyporeactivity in rat superior mesenteric artery. Pharmacol. Res. (2006);54:208–218. doi: 10.1016/j.phrs.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 23.She H., Nakazawa T., Matsubara A., Hisatomi T., Young T.A., Michaud N., Connolly E., Hafezi-Moghadam A., Gragoudas E.S., Miller J.W. Reduced photoreceptor damage after photodynamic therapy through blockade of nitric oxide synthase in a model of choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. (2007);48:2268–2277. doi: 10.1167/iovs.06-0979. [DOI] [PubMed] [Google Scholar]
- 24.Soderling T.R. Structure and regulation of calcium/calmodulin- dependent protein kinases II and IV. Biochim. Biophys. Acta. (1996);1297:131–138. doi: 10.1016/s0167-4838(96)00105-7. [DOI] [PubMed] [Google Scholar]
- 25.Tang J., Mohr S., Du Y.D., Kern T.S. Non-uniform distribution of lesions and biochemical abnormalities within the retina of diabetic humans. Curr. Eye Res. (2003);27:7–13. doi: 10.1076/ceyr.27.2.7.15455. [DOI] [PubMed] [Google Scholar]
- 26.Yousif M.H., Akhtar S., Walther T., Benter I.F. Role of Ca2+/calmodulin-dependent protein kinase II in development of vascular dysfunction in diabetic rats with hypertension. Cell Biochem. Funct. (2008);26:256–263. doi: 10.1002/cbf.1446. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S., Yang Y., Kone B.C., Allen J.C., Kahn A.M. Insulin-stimulated cyclic guanosine monophosphate inhibits vascular smooth muscle cell migration by inhibiting Ca/calmo-dulindependent protein kinase II. Circulation. (2003);107:1539–1544. doi: 10.1161/01.cir.0000056766.45109.c1. [DOI] [PubMed] [Google Scholar]
- 28.Zheng L., Du Y., Miller C., Gubitosi-Klug R.A., Ball S., Berkowitz B.A., Kern T.S. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin- induced diabetes. Diabetologia. (2007);50:1987–1996. doi: 10.1007/s00125-007-0734-9. [DOI] [PubMed] [Google Scholar]


