Abstract
Glutathione peroxidases (Gpxs) are the key anti-oxidant enzymes found in Saccharomyces cerevisiae. Among the three Gpx isoforms, glutathione peroxidase 3 (Gpx3) is ubiquitously expressed and modulates the activities of redox-sensitive thiol proteins involved in various biological reactions. By using a proteomic approach, glyceralde-hyde-3-phosphate dehydrogenase 2 (GAPDH2; EC 1.2.1.12) was found as a candidate protein for interaction with Gpx3. GAPDH, a key enzyme in glycolysis, is a multi-functional protein with multiple intracellular localizations and diverse activities. To validate the interaction between Gpx3 and GAPDH2, immunoprecipitation and a pull-down assay were carried out. The results clearly showed that GAPDH2 interacts with Gpx3 through its carboxyl-terminal domain both in vitro and in vivo. Additionally, Gpx3 helps to reduce the S-nitrosylation of GAPDH upon nitric oxide (NO) stress; this subsequently increases cellular viability. On the basis of our findings, we suggest that Gpx3 protects GAPDH from NO stress and thereby contributes to the maintenance of homeostasis during exposure to NO stress.
Keywords: Apoptosis, GAPDH, glutathione peroxidase 3, nitosylation, NO stress
INTRODUCTION
Living organisms suffer from various sources of stresses, including environmental factors. A series of stresses induce oxidative stress in the host organism, leading to damage to all cellular constituents (Sies et al., 1985; Soriano et al., 2009; Yoon et al., 2002). The molecular basis of the responses to various stresses has been studied extensively in Saccharomyces cerevisiae. S. cerevisiae contains three glutathione peroxidase (Gpx) proteins: Gpx1 (YKL026C), Gpx2 (YBR244W), and Gpx3 (YIR037W). The basal expression level of the gpx3 gene is constitutively higher than those of the other two gpx genes. Additionally, the Δgpx3 mutant is hypersensitive to peroxides. However, disruption of the gpx1 or gpx2 does not result in any obvious phenotypes with respect to the tolerance to oxidative stress (Inoue et al., 1999). Therefore, the gpx3 gene product is thought to have a major function in scavenging peroxides in S. cerevisiae. Delaunay et al. identified novel roles of Gpx3 in yeast as both sensor and transducer of the stress response to hydrogen peroxide (Delaunay et al., 2002). Previously, we performed a proteomic analysis to screen for the Gpx3 interactome (Lee et al., 2008). Among the identified candidate proteins, we focused on GAPDH2, because of its involvement in stress sensing and apoptosis.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; EC 1.2.1.12) is a key enzyme in glycolysis. GAPDH is a multifunctional protein with multiple intracellular localizations and performs diverse activities independent of its traditional role in glycolysis (Hara et al., 2005; Michael, 1999). These new activities include regulation of the cytoskeleton, membrane fusion and transport, and glutamate accumulation into presynaptic vesicles (Ikemoto et al., 2003; Tisdale, 2001). A role of GAPDH in the nucleus is also suggested on the basis of its ability to activate transcription in neurons, export nuclear RNA, and affect DNA repair (Meyer-Sieglar et al., 1991; Singh and Green, 1993). Hara et al. (2005) reported that GAPDH plays an important role in the cell death cascade induced by nitric oxide (NO) stress. NO is a highly diffusible free radical with di-chotomous regulatory roles in numerous physiological and pathological events (Delledonne, 2005; Hess et al., 2005; Ignarro et al., 1987; Nathan, 1992). Diverse cellular functions can be directly or indirectly affected by NO through post-translational modification of proteins. The most widespread and functionally relevant modification is S-nitrosylation, which is defined as the covalent attachment of NO to the thiol side chain of a cysteine residue. S-nitrosylation has been shown to regulate the functions of an increasing number of intracellular proteins (Abat et al., 2008; Stamler et al., 2001). GAPDH, one of such proteins, is translocated into the nucleus after S-nitrosylation of active cysteine. It then triggers apoptosis in mammalian cells (Hara et al., 2005). However, there are no existing reports regarding the S-nitrosylation of GAPDH and its functional roles in S. cerevisiae.
In this study, we demonstrated that GAPDH directly interacts with Gpx3 through a region containing a highly conserved catalytic domain regardless of oxidative stress. Moreover, the interaction between Gpx3 and GAPDH reduces the S-nitrosylation of GAPDH and consequently contributes to cell survival against NO stress.
MATERIALS AND METHODS
Strains
S. cerevisiae strain YPH499 (MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1) and its isogenic derivatives were used in all experiments. The gene disruption of YIR037w (Δgpx3) was carried out using the PCR-mediated gene deletion method as previously described (Kho et al., 2006).
DNA construction
DNA fragments from the S. cerevisiae cDNA, which encoded Gpx3 and GAPDH2 ORF, were amplified. The pASK-IBA13- plus-strep-Gpx3 construct was generated by inserting gpx3 between the BamHI and SalI sites of pASK-IBA13-plus. The GAPDH-His construct (pET21a-GAPDH2) was generated by inserting the gapdh2 gene between the BamHI and NotI sites of pET-21a(+). For binding domain mapping, we constructed HIStagged N-terminal NAD-binding domain and C-terminal catalytic domain of GAPDH. The N terminal NAD-binding domain of GAPDH (1-148 amino acids) was obtained using the primers, 5′-CGGAATTCATGGTTAGAGTTGCTATTAACGGT-3′ and 5′- ATTTGCGGCCGCAGCGTTGGAAACAATCTTCAAGTC-3′, and inserted into pET-21a(+). The C-terminal catalytic domain of GAPDH (149-332 amino acids) was obtained by PCR using the primers, 5′-CGGAATTCATGTCTTGTACCACCAACTGTT TG-3′ and 5′-ATTTGCGGCCGCAGCCTTGGCAACGTGTTC AAC-3′, and inserted into the pET-21a(+). pESC-LEU-Myc- Gpx3, and pESC-URA-FLAG-GAPDH2 were constructed by subcloning gpx3 into the BamHI and SalI sites of pESC-LEU and gapdh2 into the EcoRI and NotI sites of pESC-LEU.
Growth condition and protein extraction
Yeast cells were grown at 30℃ in YPD (1% yeast extract, 2% Bactopeptone, and 2% glucose), SD media (0.17% yeast nitrogen base without amino acids, 5% ammonium sulfate, 2% glucose, 0.03% adenine hemisulfate, and appropriate amino acids and bases), or galactose induction media (YPD or SD media containing 2% galactose, 1% raffinose, and 0.03% hemisulfate). Cells were cultured until the mid/late exponential phases in YPD medium for the preparation of yeast cell extracts. The cells were then suspended in a lysis buffer (50 mM Tris, pH 8.0, 1 mM PMSF, 100 mM NaCl, 1 mM EDTA, and protease inhibitors) and were disrupted using glass beads (0.4-0.6 mm diameter, Sigma).
S-nitrosylation assay
Cells were harvested by centrifugation and immediately stored in the dark at -80℃ to minimize the loss of endogenous Snitrosylated proteins. HEN buffer [0.5 ml; 25 mM HEPES (pH 7.7), 0.1 mM EDTA, 10 mM neocuproine, and 1% SDS] per 50 to 100 ml of cell solution was added, and the sample was homogenized using glass beads. After homogenization, the samples were centrifuged for 10 min at 2,000 × g at 4℃. The protein concentration of the sample was determined using the Bradford assay kit from Bio-RAD. The extracts were diluted to 0.8 mg/ml with HEN buffer plus 0.4% CHAPS, 1% SDS and 20 mM MMTS and incubated at 50℃ for 20 min with frequent agitation in order to block free thiols. After the removal of excess MMTS by acetone precipitation, the samples were resuspended in HEN buffer containing 1% SDS at a protein concentration of 1 to 2 mg/ml. The samples were subsequently incubated with 5 mM ascorbate and 0.4 mM biotin-HPDP for 1 h at room temperature. Another acetone precipitation was performed to remove excess biotin-HPDP. The samples were resuspended in 300 μl of HEN buffer containing 1% SDS. Additional 600 μl of neutralization buffer [20 mM HEPES (pH 7.7), 0.1 M NaCl, 1 mM EDTA and 0.5% Triton X-100] was added to the solution. Then, 50 μl of streptavidin-agarose beads were added to the samples and incubated for 1 h at room temperature with agitation. The sample was then washed five times in a wash buffer (neutralization buffer plus 600 mM NaCl) followed by single wash with phosphate-buffered saline (PBS). For the experiments in which total endogenous S-nitrosylated proteins were measured, 50 μl of PBS and 50 μl of 2% SDS sample buffer without 2-mercaptoethanol was added to the beads. After this mixture was boiled, SDS-PAGE was performed and biotinylated proteins were detected by immunoblot analysis using the anti-Biotin-M antibody (Sigma) and ECL reagent (Pierce). An increase in the biotin labeling of proteins in the presence of ascorbic acid is indicative of protein S-nitrosylation (Derakhshan et al., 2007).
Pull-down assay using tagged proteins
pET-21a derivatives encoding for His-GAPDH2 and His-N or His-C terminal domains of GAPDH2 and pGEX-6p-1-Gpx3 encoding GST-Gpx3 were transformed into E. coli cells. The expression of GST-Gpx3 or His-GAPDH2 derivatives was induced in 2× YT media. The cell extracts were centrifuged at 13,000 rpm for 30 min. The soluble fractions were incubated with 100 μl of Ni-NTA agarose and glutathione sepharose beads for 4 h at 4℃ with rotation. After incubation, the beads were collected by centrifugation at 3,000 rpm for 1 min and washed three times in the lysis buffer. Next, cell lysates with overexpression of the other tagged proteins were added and then incubated for 2 h at 4℃ with rotation, and washed three times. The bound proteins were eluted by the SDS-PAGE sample buffer and separated by SDS-PAGE, followed by immunoblotting with anti-His or anti-Strep antibodies. The proteins bands were visualized by the ECL detection system (Pierce).
GAPDH activity assay
Triplicate samples of different amounts of exponentially growing cells were incubated with and without 4.0 mM glyceraldehydes- 3-phosphate (G-3-P) in the presence of NAD (100 μl of a 10 mM solution; Boehringer Mannheim), 10 mM EDTA and 0.1 mM dithiothreitol in an assay buffer to a final volume of 1 ml. After incubation of the reaction mixtures at 28℃, the cells were removed by centrifugation, and the supernatants were analyzed for the presence of NADH formation was monitored spectrophotometrically at 340 nm. Background absorbance measured in negative controls (without substrate) was subtracted from positive absorbance values.
RESULTS
Analysis of interactome for mining the proteins that interact with Gpx 3
Although Gpx3 has been accepted as a major antioxidant enzyme in the detoxification of ROS, it remains unclear whether the regulation and defense mechanisms of Gpx3 in cellular homeostasis are adapted from oxidative stress (Inoue et al., 1999). Therefore, to further the study of other functions of Gpx3, the novel interacting proteins of Gpx3 were analyzed using proteomics. By using this approach, several candidate proteins were identified and validated (Kho et al., 2006; Lee et al., 2009). In the present study, we focused on GAPDH2.
Interaction between Gpx3 and GAPDH2 both in vivo and in vitro
To confirm whether GAPDH2 protein actually interacts with Gpx3, an in vitro pull-down assay with His-tagged GAPDH2 and GST-tagged Gpx3 was performed. After pull-down using Ni-NTA agarose beads with protein extracts from E. coli overexpressing His-GAPDH2, a crude protein lysate from E. coli overexpressing GST-Gpx3 was applied under the normal condition. After incubation, immunoblot analyses with anti-GST or anti-His antibodies were performed. As shown in Fig. 1A, Gpx3 interacts with GAPDH2 in vitro. Next, to test whether the interaction between Gpx3 and GAPDH2 also occurs in vivo and whether this interaction is dependent on redox conditions, S. cerevisiae Δgpx3 strain overexpressing both FLAG-GAPDH2 and Myc-Gpx3 was treated with DTT (1 mM) and H2O2 (4 mM), respectively. The lysates were then used for a pull-down assay with FLAG-agarose beads. As shown in Fig. 1B, Gpx3 also interacts with GAPDH2 in vivo, and its interaction does not depend on the redox status.
Fig. 1. Gpx3 interacts with GAPDH2 both in vivo and in vitro. (A) Gpx3 associates directly with GAPDH2 in vitro. Crude protein lysates from E. coli overexpressing His-tagged GAPDH2 and Strep-tagged Gpx3 were used for pull-down assays with Ni-NTA agarose beads. The interaction was detected by immunoblotting using anti-Gpx3 antibody. Data show a representative of three independent experiments. (B) Gpx3 associates directly with GAPDH2 in vivo. The Δgpx3 strain overexpressing both GAPDH and Gpx3 was treated with DTT (final 4 mM) or H2O2 (final 1 mM). The lysates were used for pull-down assays with FLAG-agarose beads. The results show that Gpx3 also interacts with GAPDH2 in vivo. (C) The carboxyl catalytic domain of GAPDH2 and Gpx3 were expressed in E. coli. Pull-down assays with Ni-NTA agarose beads were performed. The interaction was detected by immunoblotting using anti-Gpx3 antibody. The carboxyl catalytic domain (also termed the mixed alpha/antiparallel beta-fold domain) of GAPDH2 interacts with Gpx3.
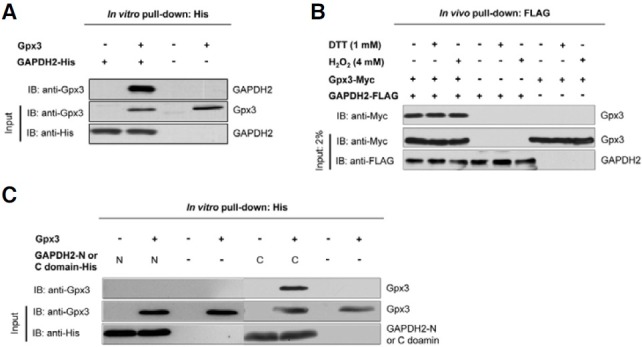
Gpx3 interacts with the C-terminal catalytic domain of GAPDH2
GAPDH exists as a tetramer of identical subunits, each containing 2 conserved functional domains: an NAD-binding domain (1-148 amino acids) and a highly conserved catalytic domain (or mixed alpha/antiparallel beta-fold domain; 149-332 amino acids) (Meyer-Sieglar et al., 1991). To test the region involved in interaction with Gpx3, two functional domains of GAPDH2 were expressed. The interaction between Gpx3 and these GAPDH2 fragments was then examined. The results clearly showed that the catalytic domain of GAPDH2 interacts with Gpx3 (Fig. 1C).
Gpx3 protects GAPDH2 from S-nitrosylation under NO stress
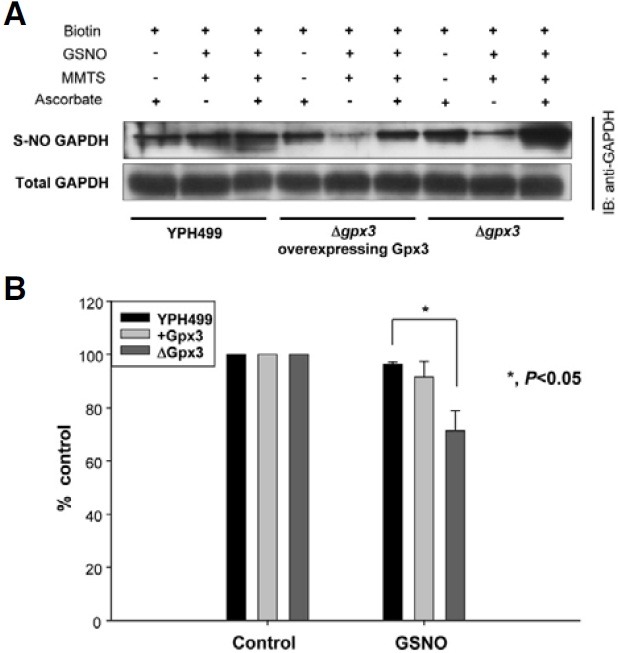
S-nitrosylation, the modification of a cysteine thiol by NO, has emerged as an important post-translational modification of signaling proteins. Previous studies have shown that many proteins are inactivated by S-nitrosylation of active cysteine residues. It has also been demonstrated that mammalian GAPDH is S-nitrosylated upon exposure to NO stress (Meyer-Sieglar et al., 1991; Nathan, 1992; Nilkantha et al., 2008). Therefore, we speculated that GAPDH in S. cerevisiae would also be S-nitrosylated under NO stress. Interestingly, Δgpx3 strain showed elevated S-nitrosylation level of GAPDH, while the S-nitrosylation level of GAPDH recovered by re-introduction of Gpx3 (Fig. 2A). Next, we investigated whether Gpx3 contributes to the protection of GAPDH activity during NO stress (Fig. 2B). In the Δgpx3 strain, GAPDH activity decreased by more than 30%, compared with that of the control strain (YPH499). However, GAPDH activity decreased only by approximately 8% in the Δgpx3 strain overexpressing Gpx3. Therefore, this finding indicates that GAPDH activity may be protected by Gpx3 through the lowering of the S-nitrosylation level of active cysteine residue.
Fig. 2. Effect of Gpx3 on S-nitrosylation and activity of GAPDH. (A) S-nitrosylation of GAPDH was affected by Gpx3. After treatment of wild-type YPH499, Δgpx3 strain, and Δgpx3 strain overexpressing Gpx3 with GSNO, the lysates were subjected to a biotin switch assay. (B) The activity of endogenous GAPDH was influenced by Gpx3. The crude protein extracts prepared from various yeast strains (wild-type, Δgpx3, and Δgpx3 overexpressing Gpx3) were treated with or without GSNO (2 mM). Endogenous GAPDH activity was then measured. Data show a representative of three independent experiments.
Cell growth is affected by Gpx3 after exposure to NO stress
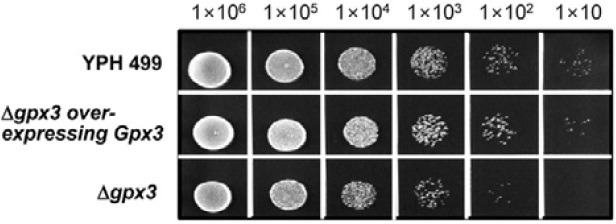
We next investigated the effect of Gpx3 on cell growth after exposure to NO stress (2 mM and 4 mM of GSNO) (Fig. 3). As shown in Fig. 3B, the growth retardation was observed in the Δgpx3 strain between 12 and 16 h of culture time after treatment with 2 mM GSNO. The spot assay also showed that cell viability was slightly reduced in the Δgpx3 strain (Fig. 4). These results strongly suggest a relationship between Gpx3 and cell viability via S-nitrosylation of GAPDH.
Fig. 3. The effect of Gpx3 on cell viability under NO stress. The growth curves of various yeast strains (wild-type (A), Δgpx3 strain (B), and Δgpx3 overexpressing Gpx3 (C)) were measured. The Δgpx3 strain showed greater sensitivity to GSNO during the early exponential phase. This sensitivity disappeared by reintroduction of Gpx3.
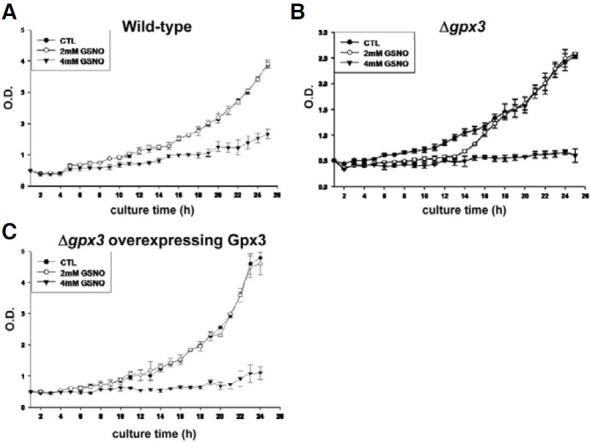
Fig. 4. Spot assays for evaluating the effect of Gpx3 on cell viability. The YPH499, Δgpx3, and Δgpx3 overexpressing Gpx3 strains were examined for their viability against GSNO stress by spotting cells (106, 105, 104, 103, 102, and 10 cells) on YPD media in the presence of GSNO (2 mM).
DISCUSSION
It has been well documented that GAPDH is a multi-functional protein with multiple intracellular localizations and diverse activities, independent of its traditional role in glycolysis. These other activities include regulation of the cytoskeleton, membrane fusion and transport (Hara et al., 2005), glutamate accumulation into presynaptic vesicles (Tisdale, 2001), and roles of GAPDH in nuclear fraction. Recently, the role of GAPDH as a stress sensor and transducer in apoptosis was reported in other studies (Chuang et al., 2005; Hara et al., 2006). Apoptotic stimuli induce the generation of NO either by induction of iNOS or by activation of nNOS. NO S-nitrosylates GAPDH at the catalytic cysteine residue, which is critical for the catalytic function of the enzyme. This abolishes catalytic activity but confers GAPDH the ability to bind to Siah-1, an E3 ubiquitin ligase. Siah-1 possesses a nuclear localization signal and escorts GAPDH to the nucleus where GAPDH increases the stability of Siah-1 (Meyer- Sieglar et al., 1991; Nilkantha et al., 2008). This enables Siah-1 to degrade selected protein targets eliciting cell death. It is, however, unclear whether GAPDH performs similar roles in S. cerevisiae.
In this study, we suggest that Gpx3, one of the major antioxidant enzymes in S. cerevisiae, is linked to GAPDH2 under NO stress. Through immunoprecipitation and pull-down assays, the interaction between Gpx3 and GAPDH2 was confirmed both in vivo and in vitro (Figs. 1A and 1B). GAPDH2 interacts with Gpx3 through its C-terminal catalytic domain (Fig. 1C). S. cerevisiae has three isoforms of GAPDH. In addition, the homology among the isoforms is above 90% at the nucleotide level. Therefore, Gpx3 may also interact with other GAPDH proteins. Gpx3 prevents GAPDH from undergoing S-nitrosylation and thereby contributes to the maintenance of GAPDH activity (Fig. 2). Thus, in S. cerevisiae, Gpx3 increased cell viability during NO stress (Figs. 3 and 4). From these data, we suggest that Gpx3 is possibly involved in a defense mechanism against NO stress, through lowering the S-nitrosylation level of GAPDH. Further studies are necessary to determine the details of the mechanisms by which Gpx3 lowers S-nitrosylation level of GAPDH.
Acknowledgments
We thank Dr. Eui-Jeon Woo for helpful advices and Dr. Sunghyun Kang for critical reading of the manuscript. This research was supported by grants from Korea Research Institute of Bioscience and Biotechnology Open Innovation Program (to K.-H. Bae), National Research Foundation of Korea (to S.G. Park, Grant No. 2010-0008754), Mid-career Research Program (to K.-H. Bae, Grant No. 2010-0022319) and Happy Technology Program through the National Research Foundation of Korea (to B.C. Park, Grant No. 2010-0020765).
References
- 1.Abat J.K., Saigal P., Deswal R. S-nitrosylation - another biological switch like phosphorylation. Physiol. Mol. Biol. Plants. (2008);14:119–130. doi: 10.1007/s12298-008-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chuang D.M., Hough C., Senatorov V.V. Glyceraldehyde-3-phosphate dehydrogenase, apoptosis, and neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. (2005);45:269–290. doi: 10.1146/annurev.pharmtox.45.120403.095902. [DOI] [PubMed] [Google Scholar]
- 3.Delaunay A., Pflieger D., Barrault M.B., Vihn J., Toledano M.B. A thiol peroxidase is an H2O2 receptor and redoxtransducer in gene activation. Cell. (2002);111:471–481. doi: 10.1016/s0092-8674(02)01048-6. [DOI] [PubMed] [Google Scholar]
- 4.Delledonne M. NO news is good news for plants. Curr. Opin. Plant Biol. (2005);8:390–396. doi: 10.1016/j.pbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Derakhshan B., Wille P.C., Gross S.S. Unbiased identification of cysteine S-nitrosylation sites on proteins. Nat. Prot. (2007);2:1685–1691. doi: 10.1038/nprot.2007.210. [DOI] [PubMed] [Google Scholar]
- 6.Hara M.R., Agrawal N., Kim S.F., Cascio M.B., Fujimuro M., Ozeki Y., Takahashi M., Cheah J.H., Tankou S.K., Hester L.D., et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. (2005);7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 7.Hara MR., Cascio M.R., Sawa A. GAPDH as a sensor of NO stress. Biochim. Biophys. Acta. (2006);1762:502–509. doi: 10.1016/j.bbadis.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Hess D.T., Matsumoto A., Kim S.O., Marshall H.E., Stamler J.S. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. (2005);6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 9.Ignarro L.J., Buga G.M., Wood K.S., Byrns R.E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA. (1987);84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikemoto A., Bole D.G., Ueda T. Glycolysis and glutamate accumulation into synaptic vesicles. J. Biol. Chem. (2003);278:5929–5940. doi: 10.1074/jbc.M211617200. [DOI] [PubMed] [Google Scholar]
- 11.Inoue Y., Matsuda T., Sugiyama K., Izawa I., Kimura A. Genetic analysis of glutathione peroxidase in oxidative stress response of Saccharomyces cerevisiae. J. Biol. Chem. (1999);274:27002–27009. doi: 10.1074/jbc.274.38.27002. [DOI] [PubMed] [Google Scholar]
- 12.Kho C.W., Lee P.Y., Bae K.-H., Cho S., Lee Z.W., Park B.C., Kang S., Lee D.H., Park S.G. Glutathione peroxidase 3 of Saccharomyces cerevisiae regulates the activity of methionine sulfoxide reductase in a redox state-dependent way. Biochem. Biophys. Res. Commun. (2006);348:25–35. doi: 10.1016/j.bbrc.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 13.Lee P.Y., Bae K.-H., Kho C.W., Kang S., Lee D.H., Cho S., Kang S., Lee S.C., Park B.C., Park S.G. Interactome analysis of yeast glutathione peroxidase 3. J. Microbiol. Biotechnol. (2008);18:1364–1367. [PubMed] [Google Scholar]
- 14.Lee H., Chi S.-W., Lee P.Y., Kang S., Cho S., Lee C.-K., Bae K.-H., Park B.C., Park S.G. Reduced formation of advanced glycation endproducts via interactions between glutathione peroxidase 3 and dihydroxyacetone kinase 1. Biochem. Biophys. Res. Commun. (2009);389:178–180. doi: 10.1016/j.bbrc.2009.08.116. [DOI] [PubMed] [Google Scholar]
- 15.Meyer-Sieglar K., Mauro D.J., Seal G., Wurzer J., Deriel J.K., Sirover M.A. A human nuclear uracil DNA glycosylase is the 37-kDa subunit of glyceraldehyde-3-phosphate dehydrogenase. Proc. Natl. Acad. Sci. USA. (1991);88:8460–8464. doi: 10.1073/pnas.88.19.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michael A.S. New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim. Biophys. Acta. (1999);1432:159–184. doi: 10.1016/s0167-4838(99)00119-3. [DOI] [PubMed] [Google Scholar]
- 17.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. (1992);6:3051–3064. [PubMed] [Google Scholar]
- 18.Nilkantha S., Hara M.R., Kornberg M.D., Cascio M.B., Bae B.I., Shahani N., Thomas B., Dawson T.D., Dawson V.L., Snyder S.H., et al. Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nat. Cell Biol. (2008);10:866–873. doi: 10.1038/ncb1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sies H., Cadenas E. Oxidative stress: damage to intact cells and organs. Philos. Trans. R. Soc. Lond. B. Biol. Sci. (1985);311:617–631. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- 20.Singh R., Green M.R. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science. (1993);259:365–368. doi: 10.1126/science.8420004. [DOI] [PubMed] [Google Scholar]
- 21.Soriano F.X., Baxter P., Murray L.M., Sporn M.B., Gillingwater T.H., Hardingham G.E. Transcriptional regulation of the AP-1 and Nrf2 target gene sulfiredoxin. Mol. Cells. (2009);27:279–282. doi: 10.1007/s10059-009-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamler J.S., Lamas S., Fang F.C. Nitrosylation: the prototypic redox-based signaling mechanism. Cell. (2001);106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 23.Tisdale E.J. Glyceraldehyde-3-phosphate dehydrogenase is required for vesicular transport in the early secretory pathway. J. Biol. Chem. (2001);276:2480–2486. doi: 10.1074/jbc.M007567200. [DOI] [PubMed] [Google Scholar]
- 24.Yoon S.O., Yun C.H., Chung A.S. Dose effect of oxidative stress on signal transduction in aging. Mech. Aging Dev. (2002);123:1597–1604. doi: 10.1016/s0047-6374(02)00095-7. [DOI] [PubMed] [Google Scholar]


