Abstract
Off-target toxicity due to the expression of target antigens in normal tissue represents a major obstacle to the use of chimeric antigen receptor (CAR)-engineered T cells for treatment of solid malignancies. To circumvent this issue, we established a clinical platform for engineering T cells with transient CAR expression by using in vitro transcribed mRNA encoding a CAR that includes both the CD3-ζ and 4-1BB co-stimulatory domains. We present two case reports from ongoing trials indicating that adoptive transfer of mRNA CAR T cells that target mesothelin (CARTmeso cells) is feasible and safe without overt evidence of off-tumor on-target toxicity against normal tissues. CARTmeso cells persisted transiently within the peripheral blood after intravenous administration and migrated to primary and metastatic tumor sites. Clinical and laboratory evidence of antitumor activity was demonstrated in both patients and the CARTmeso cells elicited an antitumor immune response revealed by the development of novel anti-self antibodies. These data demonstrate the potential of utilizing mRNA engineered T cells to evaluate, in a controlled manner, potential off-tumor on-target toxicities and show that short-lived CAR T cells can induce epitope-spreading and mediate antitumor activity in patients with advanced cancer. Thus, these findings support the development of mRNA CAR-based strategies for carcinoma and other solid tumors.
Introduction
The adoptive transfer of genetically modified T cells engineered to express a chimeric antigen receptor (CAR) has produced early promising results for the treatment of patients with CD19+ hematological malignancies (1–4). However, the application of CAR T cells to treat solid malignancies has been limited. This is due, at least in part, to the potential of CAR-based therapies to cause on-target off-tumor toxicity through their recognition of healthy cells that express the target antigen (5, 6). Several groups have evaluated safety approaches to circumvent the development of potential adverse outcomes from the adoptive transfer of CAR T cells. Most often these strategies have incorporated a safety, or “suicide”, gene or more recently, an inducible caspase 9 transgene (7). However, the effectiveness of these strategies is potentially limited by their incomplete elimination of the transferred CAR T cells. As a result, there remains a need for an effective strategy to control the lifespan of adoptively transferred CAR T cells that can be evaluated for their safety in early clinical studies (8).
Mesothelin is a tumor-associated antigen that is overexpressed in the majority of malignant pleural mesotheliomas (MPM), pancreatic cancers, ovarian cancers, and some lung cancers (9, 10). Although mesothelin has a relatively limited expression pattern in normal tissues, it is expressed at low levels on normal peritoneal, pleural and pericardial mesothelial surfaces. Mesothelin is a target of an endogenous immune response in MPM, ovarian cancer and pancreatic cancer (11, 12). Clinical trials using antibody-based strategies to target mesothelin-expressing tumors have already demonstrated initial safety and potential activity with serositis identified as a dose-limiting on-target off-tumor toxicity (13, 14). In preclinical studies we observed potent antitumor effects with CAR T cells expressing a scFv-specific for mesothelin (15). Our approach to the clinic was to first evaluate mesothelin as a target using mRNA CAR cells.
We have demonstrated the feasibility of using mRNA electroporation to engineer T cells with transient CAR expression (16–18). This approach produced potent antitumor effects in preclinical xenograft models of human mesothelioma and advanced leukemia, and established a cost-efficient and flexible platform for evaluating the safety and potential efficacy of novel CAR targets. Due to concerns for off-tumor toxicity with mesothelin-redirected T cells, we designed a clinical trial to evaluate the feasibility and safety of targeting mesothelin-positive tumors using T cells engineered to transiently express, by mRNA electroporation, a mesothelin-targeting CAR that incorporates the CD3ζ and 4-1BB signaling domains (CARTmeso cells).
Here, we present two case reports from the first-in-human studies of mesothelin-specific mRNA CAR T cells in patients with mesothelin-expressing solid malignancies. We tested the feasibility of manufacturing mRNA-engineered T cells and the safety of repetitive infusion of CARTmeso cells in patients. Surprisingly, we observed clinical evidence for tumor responses and induction of a broad antitumor immune response consistent with epitope-spreading in these two heavily-pretreated patients with progressive disease. Our data thus support the feasibility of mRNA CAR T cells as a novel strategy for evaluating new therapeutic targets suitable for the treatment of patients with solid malignancies and suggest that mRNA CAR T cells may have therapeutic benefit.
Materials and Methods
Patients
Subject 17510-105 had advanced MPM and was enrolled into a phase I clinical trial (NCT01355965) at the Abramson Cancer Center, University of Pennsylvania (Philadelphia, PA). Inclusion criteria required age >18 years, Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 1, adequate end organ function and histopathologic confirmation of either epithelial or biphasic MPM. Patients must have progressed on first line therapy with a platinum pemetrexed-based doublet or decided to not pursue standard first line chemotherapy. Subject 21211-101 had metastatic pancreatic cancer (PDA) and was included under a compassionate use study under the same approved Investigational New Drug (IND) application for NCT01355965. Exclusion criteria for both studies included active autoimmune disease requiring immunosuppressive therapy, prior gene therapy or therapy with murine monoclonal antibodies, history of allergy to murine proteins, viral infection with HIV, HCV or HBV, pregnancy and any clinically significant pericardial effusion. Written informed consent was required. The study was approved by local institutional review boards. Both patients were elderly and had advanced chemotherapy-refractory cancer with extensive tumor burden at the time of enrollment (Table S1).
Clinical protocols
Phase I clinical trial (NCT01355965) was designed to evaluate the manufacturing feasibility and safety of mRNA-transduced CARTmeso cells in patients with advanced MPM; Subject 17510-105 was given 3 infusions under Schedule 1 and 2 of the protocol (Fig. S1B). PDA Subject 21211-101 was given 8 doses by intravenous infusion and 2 intratumoral injections under Schedule 3 (Fig. S1B). Detailed Study design is described in the Supplemental methods.
Cell Lines
K562 (ATCC CCL-243) human erythroleukemia line; Panc-1 (ATCC CRL-1469) human pancreatic ductal adenocarcinoma; BxPc-3 (CRL-1687) human pancreatic ductal adenocarcinoma; and MiaPaCa (ATCC CRM-CRL-1420D) human pancreatic ductal adenocarcinoma were obtained from American Type Culture Collection (Manassas, VA). All cell lines were banked at early passages and maintained in culture under the six month limit for re-characterization. Vials were thawed and maintained in culture for only several weeks at a time. REN, 208, 213, 302, 307, M30, M60 were all derived from mesothelioma patient samples at the University of Pennsylvania. 21211 is a pancreatic adenocarcinoma line developed from ascites from a patient at the University of Pennsylvania. The K562 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA), where the authenticated K562 cell line is deposited. Isoenzyme analysis was performed and they matched the isoenzymes listed on ATCC website: http://www.atcc.org/Products/All/CCL-243.aspx#A7931A04156C4C7FA40828AEF707302F. The cell lines were free from mycoplasma contamination; except for transgene expression and isoenzyme analysis no additional authentication was performed.
CARTmeso cell manufacturing
The T cells were manufactured as specified by an FDA-approved IND. Patients meeting eligibility criteria underwent a large volume apheresis to isolate T cells for expansion and electroporation with an anti-mesothelin ss1 scFv CAR mRNA construct containing the 4-1BB and TCR-ζ signaling modules, as described previously (15). Clinical grade in vitro transcribed RNA was produced as described (16). For patient 21211 (PDA), autologous T cells were obtained from the patient’s monozygotic twin brother. Briefly, T cells were activated by the addition of bead-immobilized anti-CD3 and anti-CD28 antibodies and cultured for 10±2 days in cell culture medium supplemented with human serum. The expanded T cells were electroporated with mRNA encoding an anti-mesothelin ss1 scFv CAR using a closed system electroporation device (Maxcyte Inc, Rockville, MD). The CARTmeso cells were then cryopreserved in an infusible cryoprotectant supplemented solution. The infused cells were an average of 99.6% and 99.5% CD3+ cells for the MPM and PDA patient, respectively. For the MPM and PDA patient respectively, 98.0% and 99.5% of the infused cells expressed the scFv as assessed by flow cytometry after electroporation. The cell viability was 91.4% (mean) with a range of 87.8%–97.1% for the MPM patient, and 91.2% (mean) with a range of 76.0% to 98.6% for the PDA patient. At the time of cell infusion, frozen CARTmeso cell aliquots were thawed and administered to patients in the Clinical Trials Research Center at the Hospital of the University of Pennsylvania. Figure S1A presents a summary of the manufacturing process for the RNA-modified T cells.
Sample collection and processing
Samples (peripheral blood, bone marrow) were collected in lavender top (K2EDTA) or red top (no additive) Vacutainer® tubes (Becton Dickinson). Research tubes were delivered to the laboratory within 2 hours of blood draw, and samples were generally processed within 30 minutes of receipt according to established laboratory standard operating procedures (SOP). Peripheral blood mononuclear cells were purified, processed, and stored in liquid nitrogen as described previously (19). Serum was isolated from red top tubes by centrifugation, aliquoted in single use 100–200 μL aliquots and stored at −80° C.
Ascites fluid analysis
On days +3 and +15 after beginning CARTmeso therapy, ascites was collected by large volume paracentesis on PDA patient 21211-101 as part of the standard of care. An automated total white blood cell count (cells/μL) of unprocessed ascites was determined and excess fluid was used to isolate cells by Ficoll-Paque processing which were then cryopreserved. For multi-parametric immunophenotyping, cryopreserved cells were thawed and stained at a density of 1×106 cells/100μL PBS for 30 minutes on ice using antibody and reagent concentrations recommended by the manufacturer, washed, resuspended in 0.5% paraformaldehyde and analyzed using a BD Fortessa (BD Immunocytometry systems equipped with Violet (405 nm), Blue (488 nm), Green (532 nm), and Red (633 nm) lasers and appropriate filter sets for detection and separation of the following fluorescently-conjugated antibodies: c-met, mesothelin, EpCAM, and CD45. Tumor cells were identified as mesothelin+c-met+. Tumor cell count within the ascites fluid was determined by multiplying the percentage of mesothelin+c-met+ cells by the total cell count/μL.
Real-time quantitative polymerase chain reaction (qPCR) analysis of RNA transcripts
Total RNA was isolated directly from whole blood, ascites and tumor tissue using Ribopure TM blood kits (Ambion). cDNA synthesis was performed using iScript cDNA synthesis kits (Biorad). cDNA was used in qPCR assays to detect and quantify the abundance of transgene and CD3ε transcripts, using 1 μL cDNA reaction/amplification. The primer/probe set to detect transgene nucleic acid recognizes the 4-1BB-TCRζ junctional fragment in the signaling domain of the CAR molecule and has been described previously. To detect CD3ε transcripts, a Taqman assay (Life Technologies) was utilized. To quantify levels of T cells and transgene in samples, a 9-point standard curve was generated using cDNA synthesized from RNA isolated from each final manufactured product, with a dilution range of 10% to 0.002%. Each data-point (sample, standard curve) was evaluated in triplicate with a positive cycle threshold (Ct) value in 3 of 3 replicates with % coefficient of variance (CV) less than 20% for all quantifiable values. A parallel amplification reaction to control for the quality and quantity of interrogated cDNA was performed using a primer/probe combination specific for the housekeeping gene PP1B, and a ABI Taqman assay (Life Technologies). These amplification reactions generated a normalization factor (NF) to normalize for input cDNA across samples. The frequency of marked sample/CD3+ T cell in each sample was calculated according to the formula: .
Analysis of serum soluble factors
Whole blood was collected in red top (no additive) Vacutainer® tubes (Becton Dickinson), processed to obtain serum using established laboratory SOP, aliquoted for single use and stored at −80°C. Quantification of soluble cytokine factors was performed using Luminex bead array technology and kits purchased from Life technologies. Assays were performed as per the manufacturer protocol with a 9 point standard curve generated using a 3-fold dilution series. The 2 external standard points were evaluated in duplicate and the 5 internal standards in singlet; all samples were evaluated in duplicate at 1:2 dilution; calculated %CV for the duplicate measures were less than 15%. Data were acquired on a FlexMAP-3D (Life Technologies) by percent and analyzed using XPonent 4.0 software (Life Technologies) and 5-parameter logistic regression analysis. Standard curve quantification ranges were determined by the 80–120% (observed/expected value) range. Reported values included those within the standard curve range and those calculated by the logistic regression analysis.
Detailed Study design, CTL assay, Human anti-chimeric antibody (HACA) detection, Protoarray analysis, Immunoblot analysis, and PET data acquisition and analysis are in the Supplemental Methods.
Results
We report results from 2 cases demonstrating the safety, feasibility and antitumor effects of RNA CAR T cell infusions in patients with refractory advanced cancer. Details of the Study design are described in the Supplemental Material. Subject 17510-105 had advanced MPM and was given 3 infusions under Schedule 1 and 2 of the protocol (Fig. S1B). Subject 21211-101 had metastatic pancreatic cancer (PDA) and was given 8 doses by intravenous infusion and 2 intratumoral injections under Schedule 3 (Fig. S1B).
Safety of RNA CARTmeso cell infusions
Treatment-related adverse events are summarized in Table S2. The safety and tolerability of the infusions are described in the Supplemental Materials.
Humoral immune responses directed against CARTmeso cells
IgG human anti-chimeric antibodies (HACA) or human anti-mouse antibody (HAMA) responses were detected in both patients after CARTmeso infusions. Details are in the Supplemental Materials.
Antitumor clinical activity of RNA CARTmeso cells
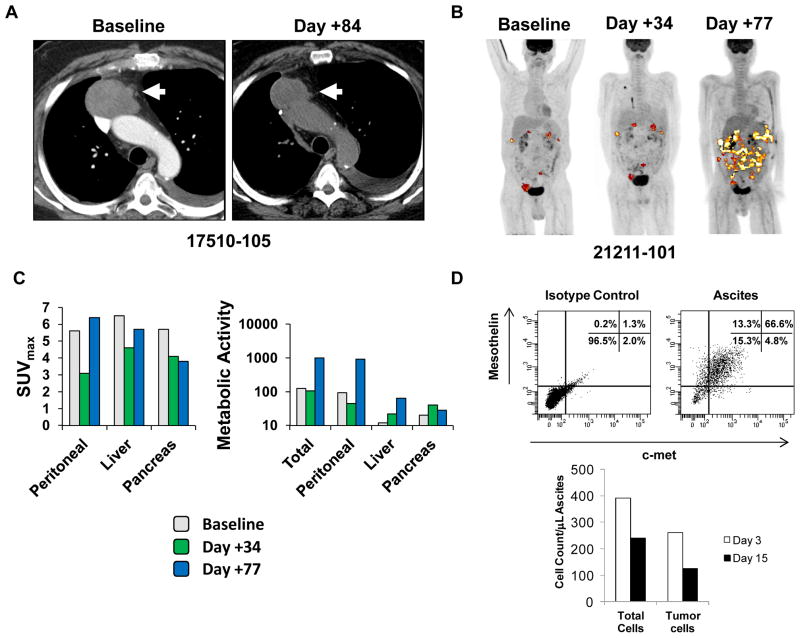
Both cases were evaluated for tumor response by computed tomography (CT) imaging. In addition, patient 21211-101 was evaluated by [18F]2-fluoro-2-deoxy-D-glucose (FDG) avidity on positron emission tomography/computed tomography (PET/CT) imaging before and after infusions (Fig. 1). Patient 17510-105 had stable disease after receiving CARTmeso T cell infusions on Schedule 1. However, this patient developed a confirmed partial response by the modified RECIST criteria after receiving one infusion of CARTmeso cells on Schedule 2 (Fig. 1A). Disease progression was observed 6 months later. PDA patient 21211-101 had stable disease by RECIST 1.1 after completing three weeks of intravenous CARTmeso cell therapy. By FDG PET/CT imaging, a decrease in the maximum standardized uptake value (SUVmax) was seen in all sites of disease. To further quantify this metabolic response, changes in the mean volumetric product (MVPmean) for each disease site were determined (Fig. 1, B and C). A decrease in MVPmean was observed only in the peritoneal lesions. This effect was transient and was not sustained on repeat imaging obtained on day +77 after the completion of IV injections on day +18. To understand the impact of CARTmeso cell therapy on peritoneal tumor burden, ascites fluid was analyzed by flow cytometry at defined time-points after beginning therapy. Analysis of ascitic fluid on days +3 and +15 after beginning therapy revealed a 40% decrease in the concentration of tumor cells that co-expressed mesothelin and c-met (Fig. 1D). Overall, these findings suggest a role for CARTmeso cell infusions in inducing an antitumor effect in these two patients.
Figure 1. Antitumor activity of CARTmeso cells.
(A) CT imaging of MPM patient 17510-105 showing the patient’s dominant mesothelioma mass prior to receiving CARTmeso infusion on Schedule 1 (baseline) and 35 days after receiving the first CARTmeso infusion on Schedule 2 (day +84). (B) Whole body FDG PET/CT imaging of PDA patient 21211-101 obtained at baseline, day +34 after completing intravenous CARTmeso cell infusions and day +77 after completing intra-tumoral CARTmeso cell infusions. (C) Analysis of maximum standardized uptake value (SUVmax) and mean metabolic volumetric product (MVPmean) at baseline, day +34 and day +77 is shown for all lesions (Total) and individual sites of disease (Peritoneal, Liver, and Pancreas) for PDA patient 21211-101. (D) Shown is a representative flow cytometry plot of ascites from PDA patient 21211-101 analyzed with isotype control antibodies versus anti-mesothelin and anti-c-met antibodies to identify mesothelin+ c-met+ tumor cells. Flow cytometry findings were quantified to determine the total number of cells/mL and tumor cells/μL in the ascites which is shown in the bar graph with comparison between day +3 and day +15 after beginning intravenous CARTmeso cell infusions.
We also evaluated the impact of CARTmeso cell therapy using serological tumor markers for both patients. Serum mesothelin-related peptide (SMRP) and CA19-9 levels were measured pre- and post-CARTmeso cell infusion. For patient 17510-105, SMRP levels initially increase during Schedule 1 but then declined from 17 to 12 nM after receiving the 1st infusion of CARTmeso cells on Schedule 2, consistent with a reduction in tumor burden seen by CT imaging. For PDA patient 21211-101, CA19-9 levels increased slowly over the course of treatment from 449 units/mL at baseline to 1429 units/mL after completing intravenous CARTmeso cell therapy but remained stable thereafter for 1 month. However, after completing the intra-tumoral injections of CARTmeso cells, the CA19-9 level began to rise again to 1865 units/mL on day +59 and 2271 units/mL by day +71 consistent with disease progression.
In vivo persistence and trafficking of RNA CARTmeso cells
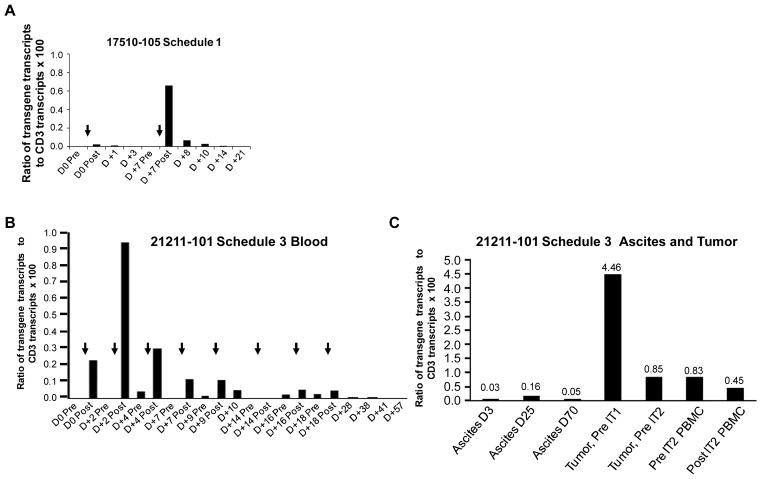
A quantitative polymerase chain reaction (qPCR) assay was developed to detect and quantify the persistence of CARTmeso cells in patients following infusion. Because the CARTmeso transgene is introduced and maintained in T cells as mRNA, reverse transcription was necessary prior to qPCR. Analysis of peripheral blood, ascites and tumor samples from the patients are presented in figure 2. Within the peripheral blood, CARTmeso transgene was detected in both patients immediately following each infusion. Moreover, the levels of detected transgene at each time point during Schedule 1 correlated with the infusion dose. In both cases, maximal levels were detected in the post-infusion time point collected within 2 hours of the infusion. In agreement with the biodegradable nature of the CARTmeso transgene, the levels decreased progressively on successive days. For the PDA patient 21211-101, additional infusions beyond one week produced lower levels of detectable CARTmeso transgene within the peripheral blood compared to the initial infusion (Fig. 2B).
Figure 2. In vivo persistence of CARTmeso cells and trafficking to primary and metastatic tumor sites.
RNA was isolated from whole blood on the indicated day before (Pre) and after (Post) CARTmeso cell infusion (black arrows). The relative amount of CARTmeso and CD3ε transcripts with normalization to the housekeeping gene PP1B was determined. Shown is the ratio of CARTmeso transgene transcripts to CD3ε transcripts × 100 for MPM patient 17510-105 during (A) Schedule 1. The arrows indicate injection of 108 and 109 CARTmeso cells on day 0 and day 7. (B) The ratio of CARTmeso transgene transcripts to CD3ε transcripts × 100 within whole blood for PDA patient 21211-101 during Schedule 3 is shown. The arrows indicate injection of 3×108 CARTmeso cells i.v. (C) The ratio of CARTmeso transgene transcripts to CD3ε transcripts × 100 within ascites on Day +3 and +25 after intravenous CARTmeso cell infusion, within ascites on Day +70 after intratumoral CARTmeso cell injection, within primary pancreatic tumor tissue prior to intratumoral injection with CARTmeso cells on day +35 (Tumor, Pre IT1) and day +57 (Tumor, Pre IT2) and within peripheral blood mononuclear cells before (Pre IT2 PBMC) and after (Post IT2 PBMC) intratumoral injection with CARTmeso cells on day +57 is shown. See Supplemental figure S1 for details on cell injection dose and schedule.
Trafficking of CARTmeso cells to tumor tissues was evaluated in patient 21211-101 by collecting ascites at serial time points and by obtaining a tumor biopsy on day +35 of Schedule 3. In each sample, CARTmeso transgene was detected demonstrating that mRNA CARTmeso cells are capable of trafficking to the extravascular tumor compartments (Fig. 2C). Within the ascites, CARTmeso transgene was detected 3 days after initial infusion and in subsequent samples obtained 6 days after the 8th infusion and 13 days after the 1st intra-tumoral injection of CARTmeso cells. Within the primary pancreatic tumor, high levels of CARTmeso transcripts were detected prior to the 1st intra-tumoral injection, while lower but significant levels were detected 22 days later on subsequent tumor biopsy. These findings demonstrate that CARTmeso cells can infiltrate solid tumors when delivered intravenously.
Bioactivity of RNA CARTmeso cells
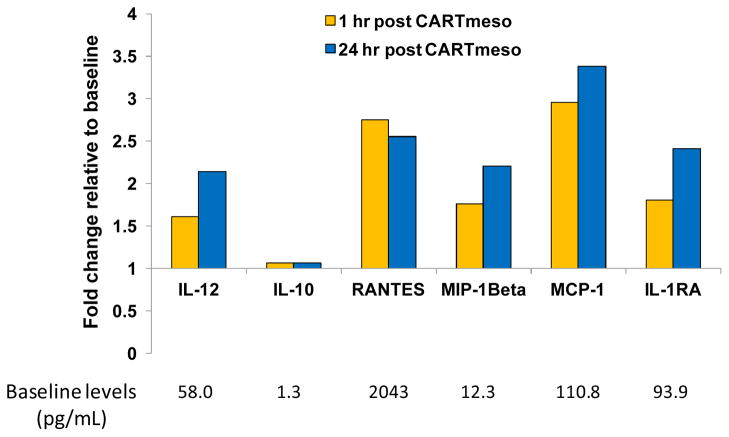
The impact of CARTmeso cell infusion on immune activation in the patients was examined by soluble immune factor profiling of serum samples collected at defined time points. From this analysis of a panel of 30 cytokines, chemokines and other soluble factors, variable responses were seen. No significant changes were seen in patient 17510-105 after the first two doses, however a transient cytokine release syndrome occurred after the third infusion with large elevations in IL-6 and chemokines such as MCP-1 (20). The cytokines remained elevated for 4 days in this patient. In contrast, after the 1st CARTmeso cell infusion in PDA patient 21211-101, modest increases in MIP1β, IL-12, MCP-1 and IL-1Rα were detected within 1 hour (Fig. 3). Changes in the levels of these cytokines persisted throughout the course of treatment with the levels of IL-12 and IL-1Rα returning to near baseline 10 days after the last CARTmeso cell intravenous infusion (Fig. S3). Intra-tumoral injection of CARTmeso cells also produced a modest increase in the levels of IL-12 and IL-1Rα within 3 days of injection. These findings are consistent with the induction of a cellular immune response in vivo following infusion of CARTmeso cells.
Figure 3. Serum cytokines and chemokines after CARTmeso cell infusion.
Fold change in serum cytokines and chemokines in PDA patient 21211-101 on Day 0 at 1 hour and 24 hours later after the first intravenous CARTmeso cell infusion. Baseline levels (pg/mL) of cytokines/chemokines are shown below each corresponding soluble factor.
To confirm the bioactivity of CARTmeso cells, we determined the capacity of CARTmeso cells generated for patient 21211-101 to recognize and lyse human pancreatic tumor cell lines including a cell line derived from the patient’s own ascites fluid (Fig. S4). From this analysis, we determined that CARTmeso cells can recognize and lyse pancreatic tumor cell lines that express mesothelin. Specific and potent lysis was also observed for the patient’s own tumor cells suggesting the possibility that the decrease in tumor cells within the patient’s ascites may have been a result of direct killing by the infused CARTmeso cells.
Induction of humoral epitope-spreading after RNA CARTmeso cell infusion
We hypothesized that CARTmeso cells, if able to recognize and lyse primary tumor cells in vivo, might elicit a systemic antitumor immune response. To examine this hypothesis, we performed high-throughput serological analyses of antibody responses to antigens to detect the development of a polyclonal immune response that may have occurred as a result of tumor destruction and epitope-spreading. These analyses utilized an unbiased interrogation of treatment-induced IgG responses to almost 10,000 independent human proteins. In PDA patient 21211-101, new antibody responses were detected at day +44 to more than 100 proteins. Responses that were elevated at least 10-fold in post-infusion samples compared to baseline were detected for 18 proteins (Table S4). Elevated antibody responses to these proteins had not been observed in a set of 15 additional protoarray samples performed for other studies. Similarly, we observed elevated antibody responses to a subset of proteins for patient 17510-105 (Table S3). For each patient, antibody responses were detected to some proteins at more than one time-point post-CARTmeso cell infusion. Overall, these antibody responses observed in both patients are consistent with CAR T cell-mediated tumor destruction leading to the release of self-proteins that are cross-presented in a classical process of epitope-spreading.
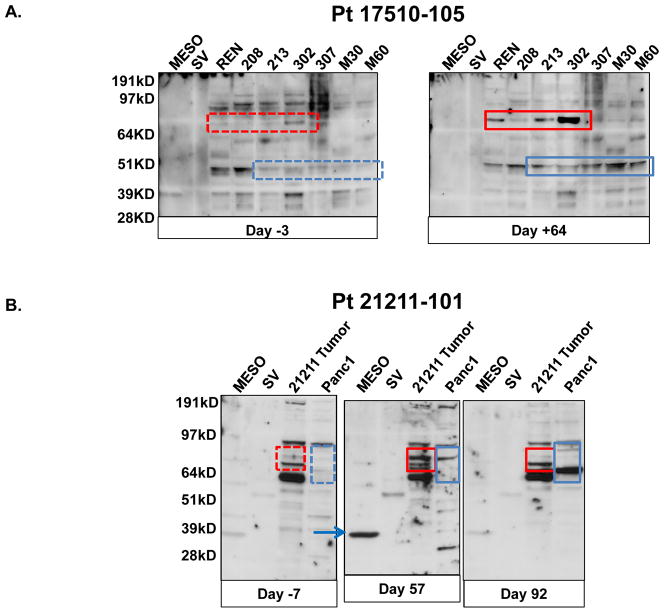
We also examined pre- and post-treatment sera from both patients for the induction of antitumor immune responses by immunoblotting using purified tumor-associated proteins or protein lysates from human MPM or PDA cell lines. Antitumor immune responses were defined by the presence of new bands or increases in the intensity of pre-existing bands on immunoblots (Fig. 4). No detectable SV40 T antigen antibodies were seen at baseline or after CARTmeso infusion in the mesothelioma patient. At baseline, we observed low antibody levels against the 37 kD purified mesothelin protein (Lanes marked Meso in Fig. 4) in patients 17510-105 and 21211-101. After CARTmeso cell administration, no significant change in anti-mesothelin antibodies was observed. However, for patient 21211-101 a marked increase in anti-mesothelin antibodies was detected on day +57 of Schedule 3 (Fig 4B, blue arrow). This antibody response was transient and returned to baseline levels by day +92. Further antibody pattern alterations were observed after CARTmeso cell infusion in patients 17510-105 (Fig. 4A) and 21211-101 (Fig. 4B). For example, in the post-treatment sera of patient 17510-105 there was an increase in antibodies detecting a 48 kD protein and a ~75 kD protein present in multiple allogeneic cell lines (Fig 4A, red boxes). For patient 21211-101, antibodies against multiple proteins present in an allogeneic cell line (Panc-1) and an autologous cell line derived from the patient’s own ascites fluid were detected (Fig 4B, boxes). For instance, several new antibodies were detected in the 64–80 kD regions at day +64 that were less abundant or absent at day +99. In addition, several new antibodies could be detected at day +99 that were not observed at baseline or at day +64. These findings along with the protoarray analysis suggest that an antitumor humoral immune response was induced by CARTmeso cell infusion.
Figure 4. CARTmeso cell induction of antitumor antibodies.
Sera obtained from patients pre-and post-CARTmeso therapy were analyzed for the presence of antibodies reactive with mesothelin and with cell extracts from autologous and allogeneic tumor cell lines. (A) Sera from MPM patient 17510-105 obtained at day −3 and day +64 were analyzed by immunoblotting for reactivity against purified mesothelin protein (MESO), SV40 large T antigen protein (SV), and lysates obtained from seven mesothelioma cell lines (REN, 208, 213, 302, 307, M30 and M60). New bands at ~70 kD (red boxes) and ~45 kD (blue boxes) were seen after CARTmeso cell infusion. (B) Sera from PDA patient 21211-101 obtained at days −7, +57 and +92 were analyzed by immunoblotting for reactivity against mesothelin protein (MESO), SV40 large T antigen protein (SV), lysate from a PDA cell line (Panc1) and lysate from a tumor cell line derived from the patient’s own ascites (21211 Tumor). A strong anti-mesothelin band was noted in the Day +57 sample (blue arrow) and new bands were seen after receiving CARTmeso cell infusion at ~65–80 kD (red boxes) for the autologous 21211 cell line and for the Panc1 cell lines (blue boxes).
Discussion
CAR T cell immunotherapy has shown antitumor potency in patients with hematological malignancies in many trials. However, with the exception of pediatric neuroblastoma (21), CAR T cell approaches have not shown antitumor effects in solid malignancies. This is, in part, due to the development of off-tumor on-target toxicities as has been observed in CAR-based clinical studies that have targeted carbonic anhydrase IX (CAIX), which is overexpressed on renal cell carcinoma but which is also expressed at low levels on normal tissues including the liver (5, 6). For this reason, it is critical to consider potential off-target toxicities and strategies to mitigate these toxicities in the early clinical development of CAR T cell approaches for solid malignancies. Here, we have demonstrated the feasibility of using mRNA CAR T cells to explore the safety and bioactivity of mesothelin-redirected CAR T cells in patients with advanced MPM and PDA. Our findings demonstrate that CARTmeso cells had acceptable safety after delivery by intravenous and intra-tumoral routes of injection and mediated antitumor activity in vivo including the induction of epitope-spreading. We observed evidence of antitumor activity despite the transient nature of CAR T cells and a lack of pre-treatment lymphodepletion. A recent study has demonstrated a promising potential in combining CAR T cell therapy with checkpoint blockade (22).
We are exploring three treatment schedules designed to evaluate the safety of cell doses, repeated infusions, and route of administration. Both patients treated with CARTmeso cells developed anti-murine CARTmeso antibody responses. For the patient with MPM, this antibody response was the cause of an anaphylactic event that occurred upon reinitiating CARTmeso cell infusions after a four week treatment interruption (20). Based on this finding, we have modified our on-going clinical trials evaluating RNA CARTmeso cell therapy in MPM (NCT01355965) and PDA (NCT01897415) to prohibit infusion breaks lasting more than 10 days in order to avoid IgE class switching that can occur during this time interval. Given the reduced levels of CARmeso T cell persistence with repeated infusions, we believe that the use of humanized or fully human scFv will be required, unlike the case with CD19-directed CARs that induce B cell aplasia.
Given the CARTmeso cell bioactivity in vivo, we did not observe overt evidence for off-tumor toxicities (e.g. pleuritis, pericarditis and peritonitis). In previous studies with anti-mesothelin antibody-toxin drug conjugates, pleuritis was a dose-limiting toxicity (23). However, unlike antibody-based therapies targeting mesothelin, which have a biodistribution that is largely dependent on diffusion capacity into tissues, CARTmeso cells must traffic to normal tissues to directly mediate off-tumor toxicities. We hypothesize that in the absence of an inflammatory response to recruit CARTmeso cells into these normal tissues, CARTmeso targeting of mesothelin-expressing normal tissues may be limited. Formal testing of this hypothesis will require further investigation using frequent and repetitive dosing of CARTmeso cells in more patients.
Our finding that the levels of CARTmeso transcripts were detectable only transiently within the peripheral blood after CARTmeso cell infusion was expected based on preclinical studies (16, 17). We found that the levels of CARTmeso transcripts were measurable following each infusion with levels detected correlating with infusion dose. To address the trafficking of CARTmeso cells to tumor tissues, we detected CARTmeso transcripts in ascites fluid three days after the initial CARTmeso cell infusion. Unexpectedly, we also detected CARTmeso transcripts within ascites fluid seven days after the last intravenous infusion of CARTmeso cells and within a tumor biopsy collected seventeen days after the last intravenous infusion of CARTmeso cells. Overall, these findings indicate that CARTmeso cells can traffic to and persist in tumor tissues.
The development of a broad tumor-specific adaptive immune response due to epitope-spreading as a consequence of tumor destruction and inflammation has long been proposed to be an important secondary mechanism underlying the potency of immunotherapy. However, without a clearly identified tumor antigen in a vaccine setting, it is difficult to detect specific antitumor lymphocyte responses (24). For this reason, we used immunoblot and protoarray analyses to evaluate the development of humoral immunity following CARTmeso cell infusion. This approach has been used by other investigators to identify antitumor immunity following various immunotherapies (25–27). In our study, both patients with clinical responses to CARTmeso therapy also developed new antitumor responses detectable on western blot analysis. Antibody responses to a number of self-proteins were also detected by protoarray following CARTmeso cell infusion in both patients. Among the molecules most robustly recognized by antibodies in the patient with PDA were Septin 6 and density-regulated protein. Septin 6 is a GTPase belonging to the septin family of proteins involved in cytokinesis and by virtue of alternative splicing and overexpression has been implicated in neoplasia (28,29). Density-regulated protein modulates the expression of cancer-related transcripts and is overexpressed in epithelial (e.g. ovarian and breast) cancers (30). In patient 17510-105 we detected an antibody response recognizing p21 (CDKN1A)-activated kinase (PAK6). PAKs are key regulators of cell motility/migration, survival, proliferation and gene transcription. PAK hyper-activation has been observed in mesothelioma and other tumor types including breast cancer, colon cancer, and melanoma (31). However, the majority of antibodies detected by the protoarray analysis recognized proteins that are not expressed on the surface of cells, overexpressed by cancer, nor implicated in the malignant process. We believe that this response may reflect the breadth and robustness of the humoral immune response induced by CART immunotherapy. Overall, our findings of humoral epitope-spreading induced by CARTmeso therapy have significant implications for the role of CAR therapy in stimulating broad antitumor adaptive immunity. The clinical activity, trafficking of CART cells to tumor tissues, and evidence of epitope-spreading suggest that mRNA CAR T cell therapy is a promising treatment modality for patients with solid malignancies.
Supplementary Material
Acknowledgments
We thank Andrea Brennan and staff of the Clinical Cell and Vaccine Production Facility for CARTmeso manufacturing and testing; members of the Translational and Correlative Studies Laboratory for technical support: J. Scholler and P. Patel for flow cytometry of ascites fluid; S. McGettigan for CTL assays; A.L Brennan and X. Liu for mRNA production, K. Haines for developing the autologous PDA cell line; E. Veloso, L. Lledo, J. Gilmore, and G. Binder for assistance in clinical research support; S. Katz for performing modified RECIST analysis on patients with MPM; L. Li and others at Maxcyte Inc for assistance with electroporation, and Elizabeth Jaffee for gift of lentiviral vector to express mesothelin.
Funding: This work was supported in part by grants from the National Institutes of Health (G.L.B. – K08 CA138907, M.V.M. - K08 CA166039, S.MA and C.H.J P01CA066726; C.H.J and Y.Z 2R01CA120409), The Prevor Family Fund for Immunotherapy Cancer Research and the Lustgarten Foundation.
Footnotes
Author contributions: The clinical trial was written by A.R.H. The compassionate use study was written by C.H.J. C.H.J. was the regulatory sponsor with assistance from M.V.M and G.P. Clinical treatment was directed by A.R.H for the MPM patients and by G.L.B. for the PDA patient. Laboratory analysis of clinical samples was directed by M.K. M.C.S conducted experimental patient procedures. A.C. supervised mRNA manufacturing. The manuscript was written by G.L.B, A.R.H., and M.K. and edited by C.H.J, S.M.A., D.A.T, M.V.M., and B.L.L. All authors discussed and interpreted results.
Competing interests: C.H.J., B.L.L., Y.Z. and M.K. have financial interests due to intellectual property and patents in the field of cell and gene therapy. Conflicts of interest are managed in accordance with University of Pennsylvania policy and oversight. This arrangement is under compliance with the policies of the University of Pennsylvania. The other authors declare that they have no competing interests.
References
- 1.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. New England Journal of Medicine. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter D, Rheingold S, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. New England Journal of Medicine. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, et al. Treatment of Metastatic Renal Cell Carcinoma With CAIX CAR-engineered T cells: Clinical Evaluation and Management of On-target Toxicity. Mol Ther. 2013;14:904–12. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. JClin Oncol. 2006;24:e20–e2. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 7.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39:49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–8. [PubMed] [Google Scholar]
- 10.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:136–40. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho M, Hassan R, Zhang J, Wang QC, Onda M, Bera T, et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11:3814–20. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 12.Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, Huang LQ, et al. Mesothelin specific CD8+ T cell responses provide evidence of in vivo cross-priming by antigen presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly RJ, Sharon E, Pastan I, Hassan R. Mesothelin-targeted agents in clinical trials and in preclinical development. Molecular cancer therapeutics. 2012;11:517–25. doi: 10.1158/1535-7163.MCT-11-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan R, Miller AC, Sharon E, Thomas A, Reynolds JC, Ling A, et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5:208ra147. doi: 10.1126/scitranslmed.3006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–5. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan A, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9062–72. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett DM, Zhao Y, Liu X, Jiang S, Carpenito C, Kalos M, et al. Treatment of advanced leukemia in mice with mRNA engineered T cells. Hum Gene Ther. 2011;22:1575–86. doi: 10.1089/hum.2011.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett DM, Liu X, Jiang S, June CH, Grupp SA, Zhao Y. Regimen-Specific Effects of RNA-Modified Chimeric Antigen Receptor T Cells in Mice with Advanced Leukemia. Hum Gene Ther. 2013;24:717–27. doi: 10.1089/hum.2013.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells expressing chimeric receptors establish memory and potent antitumor effects in patients with advanced leukemia. Science Translational Medicine. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, et al. T Cells Expressing Chimeric Antigen Receptors Can Cause Anaphylaxis in Humans. Cancer Immunology Research. 2013 doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.John LB, Devaud C, Duong CM, Yong C, Beavis PA, Haynes NM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 23.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–9. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 24.Disis ML. Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunology, Immunotherapy. 2011;60:433–42. doi: 10.1007/s00262-010-0960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fonseca C, Soiffer R, Ho V, Vanneman M, Jinushi M, Ritz J, et al. Protein disulfide isomerases are antibody targets during immune-mediated tumor destruction. Blood. 2009;113:1681–8. doi: 10.1182/blood-2007-09-114157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gnjatic S, Ritter E, Buchler MW, Giese NA, Brors B, Frei C, et al. Seromic profiling of ovarian and pancreatic cancer. Proc Natl Acad Sci U S A. 2010;107:5088–93. doi: 10.1073/pnas.0914213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marina O, Hainz U, Biernacki MA, Zhang W, Cai A, Duke-Cohan JS, et al. Serologic markers of effective tumor immunity against chronic lymphocytic leukemia include nonmutated B-cell antigens. Cancer Res. 2010;70:1344–55. doi: 10.1158/0008-5472.CAN-09-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell SE, Hall PA. Do septins have a role in cancer? British Journal of Cancer. 2005;93:499–503. doi: 10.1038/sj.bjc.6602753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall PA, Jng K, Hillan KJ, Russell SE. Expression profiling the human septin gene family. The Journal of Pathology. 2005;206:269–78. doi: 10.1002/path.1789. [DOI] [PubMed] [Google Scholar]
- 30.Oh JJ, Grosshans DR, Wong SG, Slamon DJ. Identification of differentially expressed genes associated with HER-2/neu overexpression in human breast cancer cells. Nucleic Acids Research. 1999;27:4008–17. doi: 10.1093/nar/27.20.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menges CW, Sementino E, Talarchek J, Xu J, Chernoff J, Peterson JR, et al. Group I p21-activated kinases (PAKs) promote tumor cell proliferation and survival through the AKT1 and Raf-MAPK pathways. Mol Cancer Res. 2012;10:1178–88. doi: 10.1158/1541-7786.MCR-12-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.