Abstract
Background
Cardiovascular disease is a major cause of morbidity and mortality for women and men with diabetes. Previous cross-sectional studies of prevalent diabetes have found that women are less likely to meet ADA and AHA guidelines for control of cardiovascular risk factors (hemoglobin A1c, LDL cholesterol, and blood pressure), but have not studied the critical period immediately after diagnosis.
Methods
To assess gender differences in cardiovascular risk factors at the time of diabetes diagnosis (baseline) and one year later (follow-up), we conducted a retrospective cohort study of 6,547 individuals with incident diabetes in an integrated care delivery system. We assessed mean cardiovascular risk factor values by gender and adjusted odds ratios of attaining ADA goals.
Findings
Compared with men, at baseline women had lower hemoglobin A1c (7.9% vs. 8.2%, P<0.001), higher LDL cholesterol (118.9 vs. 111.5 mg/dL, P < 0.001), higher systolic blood pressure (131.9 vs. 130.5 mmHg, P<0.001), and lower diastolic blood pressure (79.1 vs. 79.7 mmHg, P=0.006). At follow-up, the hemoglobin A1c gender gap had closed (6.9% vs. 6.9%, P=0.39), and the gender gaps had decreased for blood pressure (129.8/77.0 vs. 128.9/77.6, P=0.009) and LDL cholesterol (104.0 vs 98.2 mg/dL, P<0.001). These associations varied by age. Adjusted odds ratios showed similar relationships.
Conclusions
In this cohort of individuals with incident diabetes, men and women had important differences in risk factor control at the time of diabetes diagnosis. These differences varied by age, and decreased over time.
Introduction and Background
Cardiovascular disease is a major cause of morbidity and mortality for both men and women with diabetes (Gregg, Gu, Cheng, Narayan, & Cowie, 2007). In the U.S., mortality rates have declined among men with diabetes, but not among women (Gregg et al., 2007). A potential reason for differences in outcomes between men and women with diabetes is differential cardiovascular risk factor control and management. Practice guidelines from the American Diabetes Association (ADA) and the American Heart Association (AHA) emphasize similar treatment targets for men and women except during pregnancy (American Diabetes Association, 2013; Mosca et al., 2011). Despite these recommendations, previous studies have indicated that women with diabetes are less likely than men with diabetes to achieve some cardiovascular risk factor targets (Bertoni et al., 2008; Bird et al., 2007; Casagrande, Fradkin, Saydah, Rust, & Cowie, 2013; Chou et al., 2007; Ferrara et al., 2008; Larkin et al., 2010; Tseng et al., 2006; Wexler, Grant, Meigs, Nathan, & Cagliero, 2005; Winston, Barr, Carrasquillo, Bertoni, & Shea, 2009). These studies have primarily been cross-sectional and studied individuals with prevalent diabetes, and so were unable to examine changes in risk factor control and management over time and were not able to study the crucial period immediately after diabetes diagnosis. This period of time is of special interest due to the legacy effect, where tight glucose control early in the course of the diabetes can have long lasting effects on complications (Holman, Paul, Bethel, Matthews, & Neil, 2008). In addition, while other areas of cardiovascular care have shown age dependent gender differences (Daugherty et al., 2011; Vaccarino, Parsons, Every, Barron, & Krumholz, 1999; Vaccarino et al., 2009), few studies in diabetes have examined potential effect modification by age.
We therefore compared cardiovascular risk factor control and management between men and women during the first year after diabetes diagnosis in a cohort of individuals with newly diagnosed (incident) diabetes. The objectives of this study were to: 1) compare hemoglobin A1c (HbA1c), LDL cholesterol, and blood pressure levels between men and women at the time of diabetes diagnosis (baseline) and one year after diagnosis (follow-up), 2) explore the effect of age on the relationship between gender and cardiovascular risk factor control, and 3) examine disease management (medication use and treatment intensification) as a function of gender during this same time period.
Methods
Study Population
The cohort was drawn from the adult diabetes registry of an integrated, group model, not-for-profit HMO. Electronic data on blood pressure, medication dispensing, laboratory test results, diagnoses, and health care utilization were available from electronic health records and administrative databases from January 2000. In addition to membership in a validated diabetes registry, we required a minimum of two years of continuous enrollment and at least two diabetes diagnoses (ICD-9 codes of 250 with a fifth digit of 0 or 2) at any point between January 2000 and September 2008, and limited analyses to individuals aged 21 or greater (Bayliss, Blatchford, Newcomer, Steiner, & Fairclough, 2011; Zgibor et al., 2007). Follow-up data were available through December 2009.
The cohort consisted of all individuals with incident diabetes. We considered individuals to have incident diabetes if all of following criteria were met: 1) a first diabetes diagnosis occurred after at least one year of continuous health plan enrollment, 2) a second diabetes diagnosis occurred within 2 years of the first diagnosis, 3) there was at least 1 primary care visit before the first diagnosis, 4) no diabetes medications were prescribed more than 90 days prior to the first diagnosis, and 5) there was a full year of enrollment after the first diagnosis (n=9,272). We excluded women who were pregnant during that year (n=33), and individuals without baseline and follow-up information on HbA1c, LDL cholesterol, and blood pressure (n=2,692), for a final sample size of 6,547.
Study Measures
We used the date of the first diabetes diagnosis as the baseline date. The baseline HbA1c and LDL cholesterol were defined as the measurement closest to baseline that was less than one year prior to baseline or six months after baseline (an 18 month window; Figure 1). The follow-up HbA1c and LDL cholesterol were defined as the closest measurement to one year after baseline that was between 6 and 24 months after baseline (an 18 month window). The baseline and follow-up blood pressures were defined similarly, except that the mean of the three closest measurements within the 18 month time windows was used.
Figure 1. Timeline of assessment of baseline and follow-up risk factors.
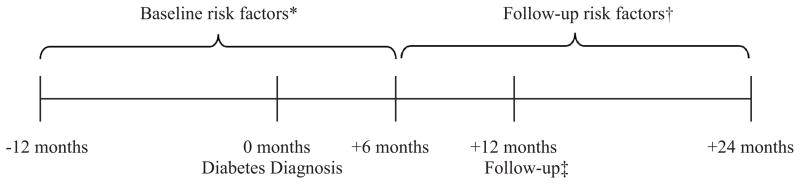
* The baseline risk factor value was the value within this window that was closest to the diabetes diagnosis date. For blood pressure, the three closest values were averaged.
† The follow-up risk factor value was the value within this window that was closest to the 12 month follow-up date. For blood pressure, the three closest values were averaged.
‡ All individuals had continuous enrollment from one year prior to the diabetes diagnosis date to 12 months after the diabetes diagnosis date.
We defined risk factor control using the 2012 ADA guidelines and the 2011 AHA guidelines: HbA1c less than 7.0%, systolic blood pressure less than 130 mmHg and diastolic blood pressure less than 80 mmHg, and fasting LDL cholesterol less than 100 mg/dL (American Diabetes Association, 2012; Mosca et al., 2011). Because the ADA has recently proposed new blood pressure targets, we conducted a secondary analysis using a blood pressure target of less than 140/80 mmHg (American Diabetes Association, 2013). Due to findings from several large clinical trials, over the past several years the optimal HbA1c target has been a matter of debate (Montori & Fernandez-Balsells, 2009; Skyler et al., 2009). Therefore, we conducted an additional sensitivity analysis using a HbA1c target of less than 8%. We assessed whether individuals had met the target for each risk factor at baseline and follow-up. We used the same target values for baseline and follow-up, although prior to diabetes diagnosis patients may have had higher targets for LDL cholesterol and blood pressure. This allowed us to assess how far individuals were from the diabetes specific goals at the time of diagnosis.
Potential socio-demographic and clinical predictors were derived from registration files, visit claims, laboratory databases, and pharmacy records. There was a substantial amount of missing race and ethnicity data due to lack of systematic collection during the earlier portions of the study period. In previous analyses, imputation of missing race information using a RAND Bayesian algorithm did not substantially change the overall racial distribution (Elliott, Fremont, Morrison, Pantoja, & Lurie, 2008; Schroeder et al., 2012). We did not impute missing race information for this paper, but instead included “unknown” race as a race category. We counted the overall number of comorbid diagnoses using the Quan version of the Elixhauser index based on ICD-9 codes in the year prior to baseline (Quan et al., 2005). We defined coronary artery disease (CAD) as the presence of an appropriate ICD-9 code in the year prior to baseline (410, 411, 412, 413, 414, V36.0, or V36.3). Body mass index (BMI) was calculated using height and weight closest to baseline. Smoking was defined using self-reported smoking status closest to baseline. Low socioeconomic status (SES) was defined as living in a census block group with ≥ 20% of individuals under the poverty line and/or ≥ 25% of individuals without a high school education (Krieger, 1992).
Medication information was obtained from pharmacy dispensing data. Baseline medications were dispensed in the 100 days prior to baseline, and follow-up medications were dispensed in the 100 days prior to the follow-up date. To assess medication use, we counted the number of different antihypertensive and lipid-lowering medications dispensed at an internal pharmacy at baseline, and the number of different oral antihyperglycemic, antihypertensive, and lipid-lowering medications at follow-up. We also recorded whether insulin was dispensed at follow-up. For blood pressure and cholesterol, treatment intensification was determined using a standard-based method score, as described by Rose et al (Rose, Berlowitz, Manze, Orner, & Kressin, 2009). The treatment intensification score assesses the number of times that treatment intensification appropriately occurs. The treatment intensification score is the number of observed treatment intensifications minus the number of expected treatment intensifications divided by the number of expected treatment intensifications. Observed treatment intensification occurred when a new class of medication was prescribed, a current dose increased, or medication was changed within class with an increase in the bioequivalent dose. Expected treatment intensification occurred when the measured risk factor was elevated. Accordingly, the treatment intensification score could range from −1 to 1, with −1 indicating no treatment intensification, 0 indicating treatment intensification at equal to expected treatment intensification, and 1 indicating treatment intensification every time the risk factor was measured regardless of its value. To account for potential clinical ambiguity in intensifying therapy when risk factors were close to goal, we required a systolic blood pressure > 150 mmHg or LDL cholesterol > 110 mg/dL to count as elevated. Because no cohort members were on glucose lowering agents at baseline, and because of the difficulty of assessing changes in insulin doses from pharmacy dispensing information, we did not assess treatment intensification for glucose lowering agents.
Statistical Analysis
We conducted bivariate analyses to identify associations between gender and risk factor levels (HbA1c, LDL cholesterol, and blood pressure), other covariates, medication use, and treatment intensification using t-tests for continuous variables, and chi-square tests for dichotomous or categorical variables. We conducted similar analyses after stratifying by age categories (young: 21–49, middle-aged: 50–64, elderly: ≥ 65). These age categories were chosen by balancing a number of factors: the sample size in each group, identifying a post-menopausal group, and preserving the age 65 cut-off because of its association with Medicare coverage. ANOVA was used to assess the statistical significance of the age-gender interaction on risk factor levels. We used logistic regression to estimate the odds of achieving ADA goals for each risk factor for women compared to men, adjusting for baseline covariates: age (three categories), race/ethnicity, smoking, BMI, CAD, comorbidities, SES, and baseline use of antihypertensive and lipid-lowering medications. We then added age by gender interaction terms to the models.
This study was approved by the local institutional review board. Access to the data was granted via a limited Health Insurance Portability and Accountability Act waiver that allowed access to the electronic patient medical records. Analyses were conducted with SAS Version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Of 9,272 individuals who met the incident diabetes criteria, we excluded 33 women who were pregnant and 2,692 for missing information on baseline or follow-up HbA1c, LDL cholesterol, or blood pressure, for a final sample size of 6,547. Compared to individuals with complete risk factor information, those excluded for incomplete baseline or follow-up risk factor information were equally likely to be male (53.6% vs 53.6%). However, excluded individuals were slightly younger (60.0 vs 60.8 years, P = 0.006), less likely to have CAD (10.8% vs 15.8%, P < 0.001) more likely to have missing race information (32.8% vs 22.0%, P < 0.001), had slightly lower BMI (32.6 vs 32.9 kg/m2, P = 0.008) and more likely to live in a low SES neighborhood (20.9% vs 17.6%, P < 0.001) (Table 1). Individuals with unknown race were more likely to be male, younger, and have fewer comorbidities (Supplemental Table 1), and were included in the subsequent analyses.
Table 1. Comparison of individuals with incident diabetes with complete (n=6,547) and incomplete (n=2,692) baseline and follow-up information on HbA1c, LDL cholesterol, and blood pressure.
N(%) or mean (SD) / median.
| Complete baseline and follow-up risk factor information (N=6,547) | Incomplete baseline or follow-up risk factor information (N=2,692) | P Value | |
|---|---|---|---|
|
| |||
| Male | 3,509 (53.6%) | 1,444 (53.6%) | 0.97 |
|
| |||
| Age (years) | 60.8 (12.1) / 61.0 | 60.0 (14.0) / 59.2 | 0.006 |
|
| |||
| Age | <0.001 | ||
| 21–49 | 1,302 (19.9%) | 706 (26.2%) | |
| 50–64 | 2,614 (39.9%) | 949 (35.3%) | |
| ≥ 65 | 2,631 (40.2%) | 1,037 (38.5%) | |
|
| |||
| Race/ethnicity | <0.001 | ||
| Black | 315 (4.8%) | 130 (4.8%) | |
| Non-Black Hispanic | 767 (11.7%) | 353 (13.1%) | |
| Non-Hispanic White | 3,707 (56.6%) | 1,216 (45.2%) | |
| Other | 320 (4.9%) | 109 (4.1%) | |
| Unknown | 1,438 (22.0%) | 884 (32.8%) | |
|
| |||
| Current smoking | 840 (12.8%) | 354 (13.2%) | 0.68 |
|
| |||
| Body mass index (kg/m2) (N=9,012) | 32.9 (6.9) / 31.8 | 32.6 (7.4) / 31.2 | 0.008 |
|
| |||
| Coronary artery disease | 1,033 (15.8%) | 290 (10.8%) | <0.001 |
|
| |||
| Comorbidities* | 0.01 | ||
| 1–2 | 3,357 (51.3%) | 1,438 (53.4%) | |
| 3–4 | 2,298 (35.1%) | 860 (32.0%) | |
| 5 or more | 892 (13.6%) | 394 (14.6%) | |
|
| |||
| Low socioeconomic status† (N=8,923) | 1,114 (17.6%) | 538 (20.9%) | <0.001 |
Comorbidities were defined using the Quan version of the Elixhauser index based on the ICD-9 codes in the year prior to the baseline date (Quan et al., 2005).
Low socioeconomic status was defined as living in a census block group with ≥ 20% of individuals under the poverty line and/or ≥ 25% of individuals without a high school education.
Of the 6,547 individuals with complete data on baseline and follow-up risk factors, women were more likely to be a racial or ethnic minority, had higher BMI, more comorbidities, were more likely to live in a low SES neighborhood, and were less likely to have coronary artery disease (all P < 0.001) (Table 2).
Table 2. Cohort characteristics at the time of diabetes diagnosis by gender.
N(%) or mean (SD) / median.
| Female (N=3,038) | Male (N=3,509) | Total (N=6,547) | P value | |
|---|---|---|---|---|
|
| ||||
| Age (years) | 61.1 (12.2) / 61.1 | 60.6 (12.0) / 60.9 | 60.8 (12.1) / 61.0 | 0.13 |
|
| ||||
| Age | 0.64 | |||
| 21–49 | 595 (19.6%) | 707 (20.1%) | 1,302 (19.9%) | |
| 50–64 | 1,204 (39.6%) | 1,410 (40.2%) | 2,614 (39.9%) | |
| ≥ 65 | 1,239 (40.8%) | 1,392 (39.7%) | 2,631 (40.2%) | |
|
| ||||
| Race/ethnicity | ||||
| Black | 170 (5.6%) | 145 (4.1%) | 315 (4.8%) | <0.001 |
| Non-Black Hispanic | 398 (13.1%) | 369 (10.5%) | 767 (11.7%) | |
| Non-Hispanic White | 1,694 (55.8%) | 2,013 (57.4%) | 3,707 (56.6%) | |
| Other | 152 (5.0%) | 168 (4.8%) | 320 (4.9%) | |
| Unknown | 624 (20.5%) | 814 (23.2%) | 1,438 (22.0%) | |
|
| ||||
| Current smoking | 379 (12.5%) | 461 (13.1%) | 840 (12.8%) | 0.42 |
|
| ||||
| Body mass index (kg/m2) (N=6,458) | 34.0 (7.5) / 32.9 | 31.9 (6.1) / 31.0 | 32.9 (6.9) / 31.8 | <0.001 |
|
| ||||
| Coronary artery disease | 300 (9.9%) | 733 (20.9%) | 1,033 (15.8%) | <0.001 |
|
| ||||
| Comorbidities* | ||||
| 1–2 | 1,462 (48.1%) | 1,895 (54.0%) | 3,357 (51.3%) | <0.001 |
| 3–4 | 1,136 (37.4%) | 1,162 (33.1%) | 2,298 (35.1%) | |
| 5 or more | 440 (14.5%) | 452 (12.9%) | 892 (13.6%) | |
|
| ||||
| Low socioeconomic status† (N=6,344) | 576 (19.6%) | 538 (15.8%) | 1,114 (17.6%) | <0.001 |
Comorbidities were defined using the Quan version of the Elixhauser index based on the ICD-9 codes in the year prior to the baseline date (Quan et al., 2005).
Low socioeconomic status was defined as living in a census block group with > 20% of individuals under the poverty line and/or > 25% of individuals without a high school education.
At the time of diabetes diagnosis, compared with men, women had lower HbA1c (7.9% vs. 8.2%), higher LDL cholesterol (118.9 vs. 111.5 mg/dL), higher systolic blood pressure (131.9 vs. 130.5 mmHg), and slightly lower diastolic blood pressure (79.1 vs. 79.7 mmHg) (Table 3, all P < 0.01). By one year after diagnosis, the HbA1c gap had closed (6.9% vs. 6.9%) and there was a less than 1mmHg difference in systolic and diastolic blood pressure (129.8/77.0 vs. 128.9/77.6 mmHg, P = 0.009). LDL cholesterol values had decreased in both women and men (104.0 vs 98.2 mg/dL, P < 0.001), with the gap closing 20% from 7.4 mg/dL at baseline to 5.8 mg/dL at follow-up (Table 3). After multivariate adjustment, women were more likely to be at goal for blood pressure and HbA1c, and less likely to be at goal for LDL cholesterol at baseline (Table 4). By follow-up, women remained more likely to be at goal for blood pressure and likely less likely to be at goal for cholesterol (Table 4).
Table 3. Mean (SD) cardiovascular risk factor values at baseline and follow-up by gender and age.
| Baseline | Follow-up | |||||||
|---|---|---|---|---|---|---|---|---|
| HbA1c % |
LDL-c mg/dL |
SBP mmHg |
DBP mmHg |
HbA1c % |
LDL-c mg/dL |
SBP mmHg |
DBP mmHg |
|
| Total | ||||||||
| Women | 7.9 (2.2) | 118.9 (37.7) | 131.9 (14.3) | 79.1 (8.7) | 6.9 (1.2) | 104.0 (34.1) | 129.8 (14.1) | 77.0 (8.3) |
| Men | 8.2 (2.5) | 111.5 (36.8) | 130.5 (13.7) | 79.7 (9.1) | 6.9 (1.4) | 98.2 (31.2) | 128.9 (13.5) | 77.6 (9.1) |
| P value | <0.001 | <0.001 | <0.001 | 0.006 | 0.39 | <0.001 | 0.009 | 0.009 |
| Young (21–49) | ||||||||
| Women | 8.4 (2.3) | 119.3 (37.1) | 126.2 (12.9) | 81.1 (8.5) | 7.2 (1.5) | 105.2 (31.7) | 124.8 (12.7) | 79.2 (8.1) |
| Men | 9.3 (2.7) | 117.7 (40.0) | 128.0 (13.0) | 83.2 (8.9) | 7.4 (1.8) | 105.1 (33.1) | 127.4 (13.5) | 81.7 (8.9) |
| P value | <0.001 | 0.45 | 0.01 | <0.001 | 0.04 | 0.98 | <0.001 | <0.001 |
| Middle-aged (50–64) | ||||||||
| Women | 8.1 (2.3) | 122.2 (38.9) | 131.6 (13.7) | 80.5 (8.1) | 7.0 (1.2) | 106.5 (36.0) | 129.0 (13.8) | 78.2 (8.0) |
| Men | 8.5 (2.5) | 115.0 (37.4) | 130.3 (13.6) | 81.2 (8.7) | 6.9 (1.3) | 99.7 (31.7) | 128.7 (13.4) | 79.2 (8.4) |
| P value | <0.001 | <0.001 | 0.01 | 0.02 | 0.71 | <0.001 | 0.61 | 0.002 |
| Elderly (≥ 65) | ||||||||
| Women | 7.4 (1.9) | 115.5 (36.5) | 135.0 (14.6) | 76.7 (8.8) | 6.7 (0.9) | 101.2 (33.1) | 133.0 (14.2) | 74.9 (8.3) |
| Men | 7.4 (1.9) | 104.8 (33.4) | 131.9 (13.9) | 76.3 (8.6) | 6.7 (1.0) | 93.1 (28.7) | 129.9 (13.5) | 74.0 (8.6) |
| P value | 0.39 | <0.001 | <0.001 | 0.20 | 0.66 | <0.001 | <0.001 | 0.005 |
| Interaction P value* | <0.001 | 0.002 | <0.001 | <0.001 | 0.038 | 0.001 | <0.001 | <0.001 |
HbA1c = hemoglobin A1c; LDL-c = LDL cholesterol; SBP = systolic blood pressure; DBP = diastolic blood pressure.
P value for interaction between age and gender, from ANOVA.
Table 4. Adjusted* odds ratios (95% CI) for women vs. men of achieving ADA guidelines for hemoglobin A1c (HbA1c), LDL cholesterol (LDL-c), and blood pressure (BP), overall and by age group, N=6,265.
| Age Group † | |||||
|---|---|---|---|---|---|
| Total | Young | Middle-aged | Elderly | Interaction P value | |
| Baseline | |||||
| HbA1c ≤ 7% | 1.23 (1.10–1.37) | 1.80 (1.41–2.30) | 1.21 (1.03–1.43) | 1.05 (0.89–1.24) | 0.002 |
| HbA1c ≤ 8% | 1.37 (1.22–1.53) | 1.72 (1.36–2.17) | 1.42 (1.20–1.69) | 1.07 (0.87–1.31) | 0.008 |
| LDL-c ≤ 2.59 mmol/L | 0.81 (0.73–0.91) | 0.90 (0.71–1.15) | 0.81 (0.68–0.97) | 0.78 (0.65–0.92) | 0.62 |
| BP ≤ 130/80 mmHg | 1.13 (1.01–1.26) | 1.75 (1.38–2.21) | 1.18 (0.99–1.39) | 0.87 (0.74–1.03) | <0.001 |
| BP ≤ 140/80 mmHg | 1.19 (1.07–1.32) | 1.75 (1.38–2.21) | 1.28 (1.09–1.50) | 0.91 (0.78–1.08) | <0.001 |
| Follow-up | |||||
| HbA1c ≤ 7% | 0.97 (0.87–1.09) | 1.01 (0.81–1.27) | 0.99 (0.83–1.17) | 0.93 (0.77–1.12) | 0.81 |
| HbA1c ≤ 8% | 1.04 (0.88–1.22) | 1.28 (0.98–1.68) | 1.01 (0.80–1.28) | 0.79 (0.57–1.09) | 0.07 |
| LDL-c ≤ 2.59 mmol/L | 0.86 (0.77–0.95) | 1.10 (0.87–1.38) | 0.80 (0.68–0.94) | 0.81 (0.69–0.96) | 0.06 |
| BP ≤ 130/80 mmHg | 1.15 (1.03–1.27) | 1.79 (1.42–2.26) | 1.20 (1.02–1.41) | 0.88 (0.75–1.03) | <0.0001 |
| BP ≤ 140/80 mmHg | 1.22 (1.09–1.35) | 1.86 (1.48–2.35) | 1.29 (1.10–1.52) | 0.90 (0.76–1.07) | <0.0001 |
Adjusted for age, race/ethnicity, smoking, body mass index, coronary artery disease, comorbidities, socioeconomic status, and baseline use of antihypertensive and lipid-lowering medications.
Model also contains age*gender interaction terms.
Effect Modification by Age
Stratification by age showed effect modification. For HbA1c, baseline gender differences were limited to the young and middle-aged groups, while gender differences at follow-up were found only among younger individuals. In contrast, for LDL cholesterol at baseline and follow-up, gender differences were limited to the middle-aged and elderly groups. For blood pressure at baseline and follow-up, young men and elderly women had higher blood pressure levels. Similar effect modification was found for the odds ratios for achieving treatment targets after adjustment for age, race, comorbidities, smoking, BMI, SES, and use of baseline use of antihypertensive and lipid-lowering medications (Table 4).
Medication Use
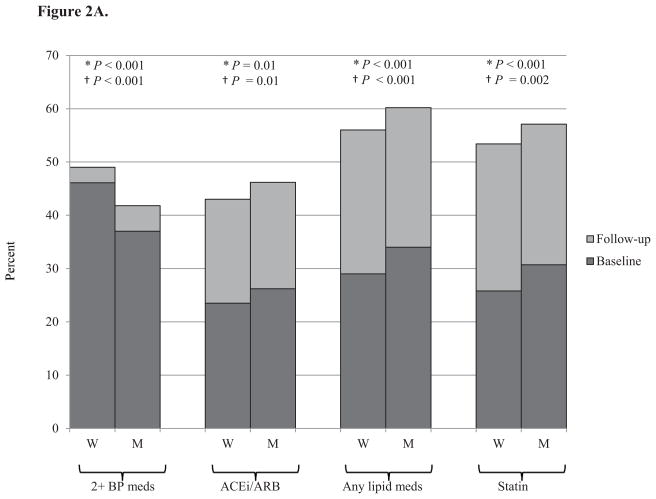
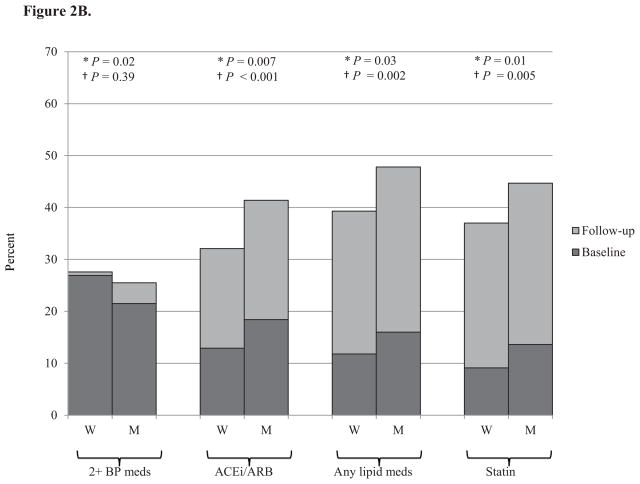
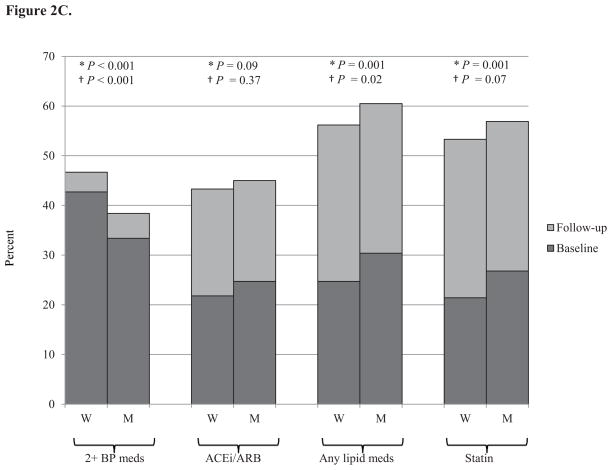
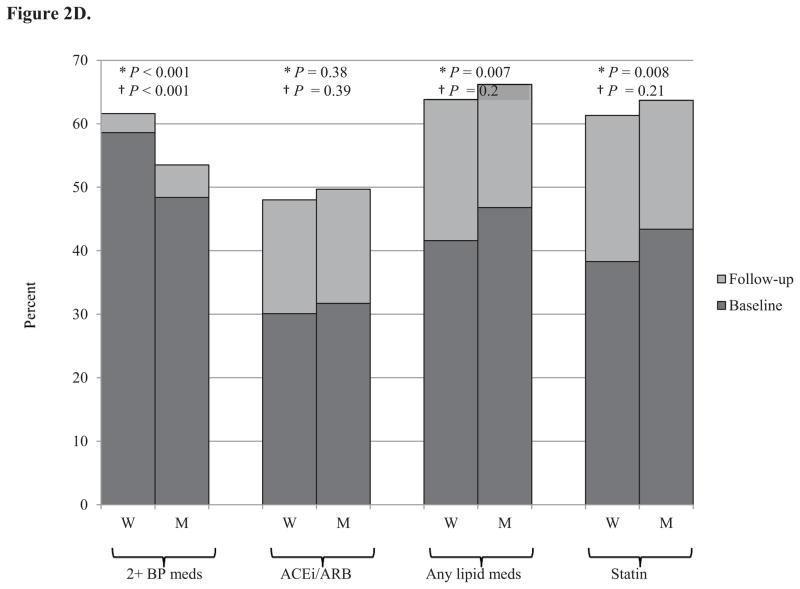
Medication use for blood pressure and lipids increased in both men and women from baseline to follow-up (Figure 2). At both baseline and follow-up, women were on more antihypertensive medications, but were less likely to be on an angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), while men were on more cholesterol lowering medications and were more likely to be on a statin.
Figure 2. Percent of individuals on blood pressure and cholesterol medications at baseline and follow-up by gender and age.
Panel A. Total cohort, N=6,547
Panel B. Young (21–49 years), N=1,302
Panel C. Middle-aged (50–64 years), N=2,614
Panel D. Elderly (≥ 65 years), N=2,631
M = men; W = women; BP = blood pressure; ACEi/ARB = angiotensin converting enzyme inhibitor/angiotensin receptor blocker. * = P value at baseline. † = P value at follow-up.
Medication use increased with increasing age. The only age-gender interaction effects with for ACE inhibitors and ARBs. While young women were less likely than young men to be on an ACE inhibitor or ARB, their use was similar in the middle-aged and elderly for men and women (Figure 2). There were no gender differences in diabetes medication use (data not shown).
Baseline medication use of antihypertensive and lipid-lowering medications was predictive of achieving treatment targets (Table 5). Baseline use of antihypertensive medications was associated with greater likelihood of meeting HbA1c and LDL targets and lower likelihood of meeting blood pressure targets. Baseline use of lipid-lowering medications was associated with greater likelihood of meeting almost all targets. However, it was not a confounder of the age and gender relationship with achieving treatment targets. Eliminating the medication variables from the multivariate model did not result in any substantial changes to the odds ratios shown in Table 4 (data not shown).
Table 5. Adjusted* odds ratios (95% CI) for any vs. no use of baseline antihypertensive or lipid-lowering medications of achieving ADA guidelines for hemoglobin A1c (HbA1c), LDL cholesterol (LDL-c), and blood pressure (BP) N=6,265.
| Any vs. no use of baseline antihypertensive medications | Any vs. no use of baseline lipid-lowering medications | |
|---|---|---|
| Baseline | ||
| HbA1c ≤ 7% | 1.09 (0.98–1.23) | 1.41 (1.25–1.60) |
| HbA1c ≤ 8% | 1.19 (1.05–1.35) | 1.70 (1.48–1.96) |
| LDL-c ≤ 2.59 mmol/L | 1.29 (1.15–1.46) | 1.56 (1.38–1.77) |
| BP ≤ 130/80 mmHg | 0.70 (0.62–0.79) | 1.13 (0.99–1.27) |
| BP ≤ 140/80 mmHg | 0.75 (0.67–0.85) | 1.22 (1.08–1.38) |
| Follow-up | ||
| HbA1c ≤ 7% | 1.26 (1.11–1.43) | 1.08 (0.95–1.24) |
| HbA1c ≤ 8% | 1.37 (1.14–1.64) | 1.24 (1.01–1.51) |
| LDL-c ≤ 2.59 mmol/L | 1.16 (1.03–1.30) | 1.27 (1.12–1.44) |
| BP ≤ 130/80 mmHg | 0.78 (0.70–0.88) | 1.20 (1.06–1.35) |
| BP ≤ 140/80 mmHg | 0.78 (0.69–0.87) | 1.29 (1.14–1.46) |
Adjusted for age, gender, age*gender, race/ethnicity, smoking, body mass index, coronary artery disease, comorbidities, socioeconomic status, and baseline use of antihypertensive and lipid-lowering medications.
Overall, women had less treatment intensification than men for blood pressure (Rose treatment intensification score (SD) of 0.01 (0.27) for women vs. 0.07 (0.29) for men, P < 0.001) and for cholesterol (−0.20 (0.45) for women and −0.16 (0.43) for men, P < 0.001). Overall, most visits with an elevated blood pressure or LDL cholesterol were not associated with subsequent treatment intensification. Elderly individuals had higher levels of treatment intensification for cholesterol and lower levels of treatment intensification for blood pressure (data not shown). There was no evidence of an age-gender interaction for treatment intensification (data not shown). Results using a systolic blood pressure cut off of 140 were in a similar direction.
Discussion
In this cohort of individuals with incident diabetes, initial risk factor differences between women and men were mitigated over the first year after diabetes diagnosis. We showed that the specific patterns varied by risk factor and age. Risk factor differences may be due to biological factors, behavioral differences, or clinician and health care system factors leading to differential diagnosis patterns, baseline treatments, treatment initiation, or treatment intensification (Ferrara et al., 2008; Wexler et al., 2005), and the relative contributions of these factors may differ between HbA1c, LDL cholesterol, and blood pressure.
Men were diagnosed with diabetes at a higher HbA1c than women. This was especially true for young and middle-aged men. This may reflect lower routine health care utilization and therefore delayed diabetes diagnosis by young and middle-aged men compared to young and middle-aged women, and may be due to behavioral differences or health care system factors. Alternatively, it could reflect more rapidly worsening disease in young and middle-aged men. The lower HbA1c in older individuals compared to younger individuals has been reported by others (Casagrande et al., 2013). By one year after diagnosis, the HbA1c levels were similar in men and women.
Conversely, men had lower LDL cholesterol values than women at baseline. While both genders had a marked decrease in LDL cholesterol over the year following diagnosis, LDL cholesterol remained lower in men. Middle-aged and elderly men were more likely to be at goal for LDL cholesterol than middle-aged and elderly women, while young men and women were equally likely to be at goal. These differences may represent a combination of biological factors, behavioral differences, and clinician and health care system factors. LDL cholesterol tends to be lower in pre-menopausal women and increases at menopause due to decreasing estrogen levels (Pilote et al., 2007). While we controlled for baseline CAD status, clinicians or patients may perceive greater CAD risk for men. In support of this, men were on more cholesterol lowering medications at baseline and follow-up and had higher levels of treatment intensification than women.
Women had slightly higher systolic blood pressure and lower diastolic blood pressure at baseline and follow-up. This corresponded to women being more likely to be at goal blood pressure (<140/80 or <130/80) then men. Older individuals had lower diastolic blood pressure, reflecting the widening pulse pressure with age (Pimenta & Oparil, 2012). Similar to previous work by Daugherty et al (Daugherty et al., 2011), we found that there was a gender-age interaction, with young men and elderly women having higher blood pressure. In the prior study, older women were less likely to have their elevated blood pressure recognized and less likely to have antihypertensive medication initiated than older men (Daugherty et al., 2011). Similarly, we found that although women were on more antihypertensive medications, men had higher levels of treatment intensification than women overall and in each age group. Taken together, these studies suggest that clinician differences in management may underlie some of these age-based gender differences in blood pressure outcomes. Biological differences in vascular stiffness and sex hormones may also contribute (Daugherty et al., 2011; Pilote et al., 2007). Of note, young women were on fewer ACE inhibitors or ARBs than young men, which may reflect concerns about use of these medications during pregnancy.
Baseline medication use of antihypertensive and lipid-lowering medications was predictive of achieving treatment targets, but was not a confounder of the age-gender relationship with achieving treatment targets. Interestingly, use of antihypertensive medications at baseline was associated with a lower likelihood of achieving blood pressure targets. This likely reflects confounding by indication (Cox et al., 2009), which is when those who are prescribed a given medication differ clinically or prognostically from those who are not prescribed the medication. In this circumstance, we hypothesize that individuals with elevated blood pressure are more likely to be prescribed antihypertensive medications, and that the medications are not always sufficient to control their blood pressure. In contrast, use of lipid-lowering medications at baseline was associated with a higher likelihood of achieving LDL cholesterol targets, perhaps due to the high effectiveness of these medications. Of note, there are other statistical methods for accounting for antihypertensive medications in order to estimate the effect of determinants on underlying blood pressures (i.e., the blood pressure that an individual would have had without treatment) (Tobin, Sheehan, Scurrah, & Burton, 2005). However, we were primarily interested in the association between gender and the observed blood pressure.
Thus we found that risk factor relationships between men and women vary longitudinally. As previous studies examined cross-sectional prevalent diabetes populations, it is difficult to compare our results with previous research. Most previous studies have reported that women with diabetes are less likely to be at goal for LDL or total cholesterol than men (Bertoni et al., 2008; Bird et al., 2007; Casagrande et al., 2013; Ferrara et al., 2008; Tseng et al., 2006; Wexler et al., 2005; Winston et al., 2009), although this has not been entirely consistent (Ferrara et al., 2008; Larkin et al., 2010). Some of these studies also found that women were less likely to be treated with cholesterol lowering agents (Ferrara et al., 2008; Larkin et al., 2010; Wexler et al., 2005). In contrast, the majority of studies have not found a gender difference in glycemic control (Bertoni et al., 2008; Bird et al., 2007; Chou et al., 2007; Ferrara et al., 2008; Tseng et al., 2006; Winston et al., 2009), with a few exceptions (Casagrande et al., 2013; Larkin et al., 2010; Wexler et al., 2005). Blood pressure differences have been the least consistent, with some studies finding greater control among women (Wexler et al., 2005; Winston et al., 2009), some no difference (Bertoni et al., 2008), some less control (Larkin et al., 2010), and some with mixed results (Bird et al., 2007; Casagrande et al., 2013; Chou et al., 2007). Three of these studies reported exploring potential age-gender interactions (Bertoni et al., 2008; Larkin et al., 2010; Tseng et al., 2006). While age-gender interactions have been found for blood pressure in other settings (Daugherty et al., 2011), none of these studies of prevalent diabetes found an interaction (Bertoni et al., 2008; Larkin et al., 2010; Tseng et al., 2006). One study only looked at two age groups (Larkin et al., 2010), and another was limited to Medicare-enrolled veterans (Tseng et al., 2006).
This study has several limitations. First, since data were obtained in routine clinical practice, the timing of measurements was variable, and missing data were common. Second, we do not have information on some important individual level covariates, such as education and income. Third, we were unable to measure lifestyle interventions and did not assess medication adherence. Fourth, the study was conducted within a single healthcare system, and therefore these findings may not be generalizable to other healthcare settings.
Implications for Practice and/or Policy
Due to the longitudinal nature of this study, we were able to examine a cohort of individuals with incident diabetes, and follow them for a year. This allowed us to assess how individuals’ control changed over a crucial period in their diabetes care. We also found significant interactions between age and gender, emphasizing that gender differences can change as a population ages.
The HbA1c findings indicate that men, especially young men, may be diagnosed later in their diabetes course than women, and therefore greater attention to diabetes screening may be needed in this population. On the other hand, the LDL cholesterol findings suggest that clinicians or patients may perceive greater CAD risk for men. Therefore greater efforts may be necessary to ensure that women with diabetes are meeting their LDL cholesterol targets, and that their high cardiovascular risk is being appropriately recognized and treated. Finally, the blood pressure findings suggest that greater attention to blood pressure and blood pressure treatment may be needed in both young men and older women.
Overall, our findings were encouraging, as initial risk factor differences decreased over the first year after diagnosis, and were typically small. However, as this study took place within an integrated health care system with strong population management activities, in many ways this study represents a practical “best case scenario.” Previous studies have shown that features of integrated delivery systems can lessen disparities (Adams et al., 2013).
In summary, in this cohort of individuals with incident diabetes, men and women had important differences in risk factor control at the time of diabetes diagnosis. These differences varied by age, and decreased over time.
Supplementary Material
N(%) or mean (SD) / median.
Acknowledgments
This study was supported by the Agency for Healthcare Research and Quality grants 1 R21 HS017627-01 and 1 R01 HS019859-01. S.L.D. is supported by K08 HL103776-02 from the National Heart, Lung and Blood Institute. Emily B. Schroeder had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A portion of this data was presented at the AHA Epidemiology and Prevention/Physical Activity, Nutrition and Metabolism 2012 Scientific Sessions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams AS, Uratsu C, Dyer W, Magid D, O’Connor P, Beck A, et al. Health system factors and antihypertensive adherence in a racially and ethnically diverse cohort of new users. JAMA Intern Med. 2013;173:54–61. doi: 10.1001/2013.jamainternmed.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss EA, Blatchford PJ, Newcomer SR, Steiner JF, Fairclough DL. The effect of incident cancer, depression and pulmonary disease exacerbations on type 2 diabetes control. J Gen Intern Med. 2011;26:575–581. doi: 10.1007/s11606-010-1600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni AG, Clark JM, Feeney P, Yanovski SZ, Bantle J, Montgomery B, et al. Suboptimal control of glycemia, blood pressure, and LDL cholesterol in overweight adults with diabetes: the Look AHEAD Study. J Diabetes Complications. 2008;22:1–9. doi: 10.1016/j.jdiacomp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Bird CE, Fremont AM, Bierman AS, Wickstrom S, Shah M, Rector T, et al. Does quality of care for cardiovascular disease and diabetes differ by gender for enrollees in managed care plans? Womens Health Issues. 2007;17:131–138. doi: 10.1016/j.whi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Casagrande SS, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The Prevalence of Meeting A1C, Blood Pressure, and LDL Goals Among People With Diabetes, 1988–2010. Diabetes Care. 2013 doi: 10.2337/dc12-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou AF, Brown AF, Jensen RE, Shih S, Pawlson G, Scholle SH. Gender and racial disparities in the management of diabetes mellitus among Medicare patients. Womens Health Issues. 2007;17:150–161. doi: 10.1016/j.whi.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Cox E, Martin BC, Van ST, Garbe E, Siebert U, Johnson ML. Good research practices for comparative effectiveness research: approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effects using secondary data sources: the International Society for Pharmacoeconomics and Outcomes Research Good Research Practices for Retrospective Database Analysis Task Force Report--Part II. Value Health. 2009;12:1053–1061. doi: 10.1111/j.1524-4733.2009.00601.x. [DOI] [PubMed] [Google Scholar]
- Daugherty SL, Masoudi FA, Ellis JL, Ho PM, Schmittdiel JA, Tavel HM, et al. Age-dependent gender differences in hypertension management. J Hypertens. 2011;29:1005–1011. doi: 10.1097/HJH.0b013e3283449512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MN, Fremont A, Morrison PA, Pantoja P, Lurie N. A new method for estimating race/ethnicity and associated disparities where administrative records lack self-reported race/ethnicity. Health Serv Res. 2008;43:1722–1736. doi: 10.1111/j.1475-6773.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara A, Mangione CM, Kim C, Marrero DG, Curb D, Stevens M, et al. Sex disparities in control and treatment of modifiable cardiovascular disease risk factors among patients with diabetes: Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care. 2008;31:69–74. doi: 10.2337/dc07-1244. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med. 2007;147:149–155. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin ME, Backlund JY, Cleary P, Bayless M, Schaefer B, Canady J, et al. Disparity in management of diabetes and coronary heart disease risk factors by sex in DCCT/EDIC. Diabet Med. 2010;27:451–458. doi: 10.1111/j.1464-5491.2010.02972.x. [DOI] [PubMed] [Google Scholar]
- Montori VM, Fernandez-Balsells M. Glycemic control in type 2 diabetes: time for an evidence-based about-face? Ann Intern Med. 2009;150:803–808. doi: 10.7326/0003-4819-150-11-200906020-00008. [DOI] [PubMed] [Google Scholar]
- Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the american heart association. Circulation. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilote L, Dasgupta K, Guru V, Humphries KH, McGrath J, Norris C, et al. A comprehensive view of sex-specific issues related to cardiovascular disease. CMAJ. 2007;176:S1–44. doi: 10.1503/cmaj.051455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta E, Oparil S. Management of hypertension in the elderly. Nat Rev Cardiol. 2012;9:286–296. doi: 10.1038/nrcardio.2012.27. [DOI] [PubMed] [Google Scholar]
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Berlowitz DR, Manze M, Orner MB, Kressin NR. Comparing methods of measuring treatment intensification in hypertension care. Circ Cardiovasc Qual Outcomes. 2009;2:385–391. doi: 10.1161/CIRCOUTCOMES.108.838649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder EB, Hanratty R, Beaty BL, Bayliss EA, Havranek EP, Steiner JF. Simultaneous control of diabetes, hypertension, and hyperlipidemia in two health systems. Circ Cardiovasc Qual Outcomes. 2012;5:645–653. doi: 10.1161/CIRCOUTCOMES.111.963553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol. 2009;53:298–304. doi: 10.1016/j.jacc.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- Tseng CL, Sambamoorthi U, Rajan M, Tiwari A, Frayne S, Findley P, et al. Are there gender differences in diabetes care among elderly Medicare enrolled veterans? J Gen Intern Med. 2006;21(Suppl 3):S47–S53. doi: 10.1111/j.1525-1497.2006.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Parsons L, Peterson ED, Rogers WJ, Kiefe CI, Canto J. Sex differences in mortality after acute myocardial infarction: changes from 1994 to 2006. Arch Intern Med. 2009;169:1767–1774. doi: 10.1001/archinternmed.2009.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler DJ, Grant RW, Meigs JB, Nathan DM, Cagliero E. Sex disparities in treatment of cardiac risk factors in patients with type 2 diabetes. Diabetes Care. 2005;28:514–520. doi: 10.2337/diacare.28.3.514. [DOI] [PubMed] [Google Scholar]
- Winston GJ, Barr RG, Carrasquillo O, Bertoni AG, Shea S. Sex and racial/ethnic differences in cardiovascular disease risk factor treatment and control among individuals with diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2009;32:1467–1469. doi: 10.2337/dc09-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgibor JC, Orchard TJ, Saul M, Piatt G, Ruppert K, Stewart A, et al. Developing and validating a diabetes database in a large health system. Diabetes Res Clin Pract. 2007;75:313–319. doi: 10.1016/j.diabres.2006.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
N(%) or mean (SD) / median.