Abstract
Objective
To examine the effectiveness of hospital-based comprehensive care programs in improving the quality of care for children with special health care needs.
Data Sources
A systematic review was conducted using Ovid MEDLINE, CINAHL, EMBASE, PsycINFO, Sociological Abstracts SocioFile, and Web of Science.
Study Selection
Evaluations of comprehensive care programs for categorical (those with single disease) and noncategorical groups of children with special health care needs were included. Selected articles were reviewed independently by 2 raters.
Data Extraction
Models of care focused on comprehensive care based at least partially in a hospital setting. The main outcome measures were the proportions of studies demonstrating improvement in the Institute of Medicine’s quality-of-care domains (effectiveness of care, efficiency of care, patient or family centeredness, patient safety, timeliness of care, and equity of care).
Data Synthesis
Thirty-three unique programs were included, 13 (39%) of which were randomized controlled trials. Improved outcomes most commonly reported were efficiency of care (64% [49 of 76 outcomes]), effectiveness of care (60% [57 of 95 outcomes]), and patient or family centeredness (53% [10 of 19 outcomes). Outcomes less commonly evaluated were patient safety (9% [3 of 33 programs]), timeliness of care (6% [2 of 33 programs]), and equity of care (0%). Randomized controlled trials occurred more frequently in studies evaluating categorical vs noncategorical disease populations (11 of 17 [65%] vs 2 of 16 [17%], P = .008).
Conclusions
Although positive, the evidence supporting comprehensive hospital-based programs for children with special health care needs is restricted primarily to nonexperimental studies of children with categorical diseases and is limited by inadequate outcome measures. Additional high-quality evidence with appropriate comparative groups and broad outcomes is necessary to justify continued development and growth of programs for broad groups of children with special health care needs.
Children with special health care needs (CSHCN) are those “who have or are at increased risk of a chronic physical, developmental, behavioral, or emotional condition and who also require health care and related services of a type or amount beyond that required by children generally.”1(p2749) This is the most commonly used definition of childhood chronic disease in the literature. Children with special health care needs represent a small group (approximately 13%–19% of all children, excluding the “at risk” group)2 who are at increased risk of hospitalization and intensive care admission,3 school absence,4 frequent medical errors,5 poor care coordination,6 and overwhelming challenges for their families. Such adverse outcomes are probably even more likely among more complex subpopulations of CSHCN who, despite being small in number, are increasingly using acute care resources7–9 and are particularly dependent on care coordination to achieve optimal health outcomes.10–12 Not surprisingly, populations of CSHCN have been targeted for various interventions aimed at improving their care. The Institute of Medicine (IOM)13 has identified the comparative effectiveness of programmatic models in childhood chronic disease as one of its top priority areas of research.
Conceptually, programmatic models for CSHCN can be roughly divided into those for which the primary focus of care coordination is in the community and those for which the primary focus of care coordination is in a specialized institution, usually a hospital. Various community-based models have been described in the literature, including the medical home,4,14,15 hospital to medical home transitions,16 and home care.17–19 However, given the frequent interface of CSHCN with hospitals,20 several hospital- based comprehensive care programs have been created with the potential benefits of provider expertise, one-stop shopping, and organizational efficiencies. These models have been growing in popularity, particularly for the increasing medically complex subpopulations of CSHCN who frequently use hospital ambulatory and inpatient services for much of their health care delivery.7,8
Comprehensive hospital-based care programs for CSHCN aim to streamline care, improve health outcomes, and support families and primary care providers.21 However, little is known to date about the effect of hospital-based programs focused on comprehensive care for CSHCN. The objective of this research article was to determine the effectiveness of such programs for CSHCN. Specifically, our research questions were 2-fold: (1) Does a hospital-based comprehensive service delivery model improve the quality of care for CSHCN? (2) Is there a difference in the body of evidence for these care delivery models between categorical (those with single disease) vs noncategorical subpopulations of CSHCN?
METHODS
A systematic review of the published literature was facilitated by an experienced librarian. All searches were updated to August 25 and 26, 2010. The following databases were searched using the OvidSP platform MEDLINE (no beginning month listed in MEDLINE 1950),EBMReviews–Cochrane Central Register of Controlled Trials <2nd Quarter 2010>, EMBASE (no month listed 1980 to 2010 Week 33), PsycINFO (no month listed 1967 to August Week 3, 2010), EBSCOHost CINAHL (no month listed in CINAHL 1982 to August 25, 2010), CSA platform Sociofile/Sociological Abstracts SocioFile (1952 to August 26, 2010) and ISI Thomson Web of Science Science Citation Index Expanded (SCI-EXPANDED)—1899 to present from inception (eTable 1 [available at: http://www.archpediatrics.com] gives the Ovid MEDLINE search strategy). References listed in articles were also reviewed, and experts were consulted for additional studies. Included were published studies of comprehensive care programs forCSHCN18 years or younger based (at least partially) clinically or administratively in a hospital setting. They included evaluative study designs, such as randomized controlled trials (RCTs); controlled observational studies; pre-post studies; or descriptive cross-sectional studies. Comprehensive care has been defined by the American Academy of Family Physicians as “concurrent prevention and management of multiple physical and emotional health problems of a patient over a period of time in relationship to family, life events and environment.”22 For this study, we used an operational definition that included models of service delivery focusing on care coordination for a broad set of health needs or programs in which care was delivered by a single clinician or team who actively led multiple components of care longitudinally across time and settings (eg, hospital and home).
Articles not written in English and those evaluating pediatric to adult care transition programs were excluded. Publications were selected in a 2-step process independently by 2 of us (E.C. and V.J.). In the first phase, the raters reviewed the titles and, if available, the abstracts derived from the search. In the second phase, any potentially relevant articles were examined in full for the inclusion criteria. The raters met regularly to discuss the classification and coding of data. Disagreements between the raters were resolved through discussion and adjudication by a third reviewer (S.M.). Publications were classified by study design, setting (completely hospital based vs hospital and community based), and disease populations (noncategorical [diverse conditions and age groups] vs categorical [eg, single disease or age group, such as cystic fibrosis, diabetes mellitus, or very low-birth-weight infants]).
Evaluative outcome data from relevant articles were categorized according to the IOM’s quality-of-care aims, which define high quality of care based on the following variables: effectiveness of care (improved health or functioning of patients, including reduced contact with the health care system), efficiency of care (in resource use), patient (focus on the patient’s experience of illness and health care and on the systems that work or fail to work to meet individual patients’ needs) or family (consideration of the needs of the whole family) centeredness, patient safety (reduction in errors and deaths), timeliness of care (minimization of wait times and other delays), and equity of care (consistent distribution of the health care system).23 Outcomes reported that were likely to affect more than 1 quality-of-care domain were categorized accordingly. For instance, a report of a decrease in emergency department visits leading to a consequent reduction in health care resource use was classified as outcomes of both effectiveness of care and efficiency of care. A positive outcome was defined as a significant change (P<.05) reported in an IOM quality-of-care domain.
The unit of analysis for descriptive and statistical purposes was the program evaluated; therefore, multiple publications evaluating a single program were analyzed as a single study. Given the anticipated heterogeneity of study design, quality was assessed by comparing the study design (eg, RCT vs non-RCT) and the comprehensiveness of outcome measures as defined by the number of IOM quality-of-care domains assessed. In addition, quality scores were calculated using a scoring system derived for disparate study designs.24 This system scores studies on a 36-point scale based on 9 domains (abstract and title, introduction and aims, methods and data, sampling, data analysis, ethics and bias, results, transferability or generalizability, and implications and usefulness). Two authors (E.C. and V.J.) scored the studies, and disputes were resolved by consensus. Populations studied were compared based on study design, quality domains, and quality scores using Fisher exact test or independent t test where appropriate. Statistical significance was set at the conventional P = .05 level.
RESULTS
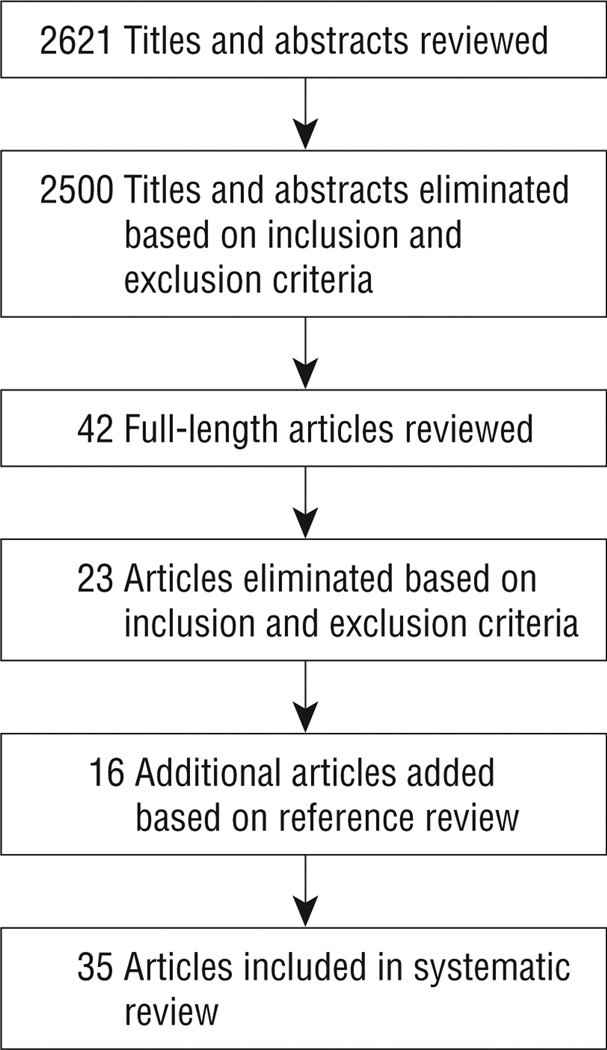
The literature review yielded 2621 potential titles and abstracts, of which 35 articles reporting on 33 unique programs were included. The Figure shows the article selection process. Seventeen programs targeted categorical (single disease) populations (Table 1), while 16 programs targeted noncategorical disease populations (Table 2) (eTable 2 gives more detailed descriptions).
Figure.
Article selection process.
Table 1.
Characteristics and Evaluation of Programs Meeting Inclusion Criteria for Categorical Patient Populations
| Program | Patient Population |
Intervention | Study Design |
Outcomes (Quality-of-Care Domains and Result) |
|---|---|---|---|---|
| Early hospital discharge and home follow-up, Philadelphia, Pennsylvania25 | Low-birth-weight infants | Home follow-up RN care, promoting parent-infant interaction and education | RCT | LOS (e1 and e2↓), physical development (e1∅), rehospitalization (e1 and e2 ∅), acute care visits (e1 and e2 ∅), program cost (e2 ↓), physician’s charges (e2 ↓) |
| Comprehensive follow-up care, Dallas, Texas26 | Low-birth-weight infants | Comprehensive care (well-child and chronic illness care, RN or MD available 24 h for acute problems; home visits provided) | RCT | Treatment compliance (e1 ↑), ED visits (e1 and e2↓), life-threatening illness (e1 and s ↓), ICU services (e1 ↓), ICU admissions and days (e1 and e2 ↓), cost (e2 ↓), death (e1 and s ∅), hospital admissions (e1 and e2 ∅), LOS (e1 and e2 ∅) |
| Pediatric asthma intervention, Chicago, Illinois27 | Asthma | Reinforced asthma education and case management (monthly contact by team and encouragement to call and ask questions, action plan provided) | RCT | Clinic visits (e1 and e2 ↓), hospital admissions (e1 and e2 ∅), LOS (e1 and e2 ∅), ED visits (e1 and e2 ∅), health care reimbursement (e2 ∅), program cost savings (e2 ∅) |
| Education and phone case management for children with type 1 diabetes mellitus, Philadelphia, Pennsylvania28 | Type 1 diabetes mellitus | Education and phone case management intervention (review guidelines, health, and safety; problem solve; meal planning; behavior and parenting) | RCT | Adherence to treatment (e1 ↑), parent-child teamwork for disease management (p ↑), parents’ knowledge of child’s condition (p ∅), glycemic control (e2 ∅) |
| Care ambassador program, Boston, Massachusetts29 | Type 1 diabetes mellitus | Care ambassador (care coordination [appointment scheduling, addressing questions, direct families to resources], clinic attendance monitoring and outreach for missed appointments, psychoeducation) | RCT | Severe hypoglycemia (e1 and s ↓), hospital admissions (e1 and e2 ↓), ED visits (e1 and e2 ↓), glycemic control (e2 ↑) |
| Pediatric asthma center comprehensive inner-city asthma program, Bronx, New York30 | Asthma | Multidisciplinary hospital-based specialty clinic (intense medical and environmental control, education and monitoring, 24-h access, 24-h availability) | RCT | ED visits (e1 and e2 ↓), hospital admissions (e1 and e2 ↓) |
| Earlier discharge with community-based intervention, Winnipeg, Manitoba, Canada31 | Low-birth-weight infants | Early discharge with follow-up in the community (public health nurse and homemaker services for 8 wk after discharge; assessment, education, support, and referral or liaison to other services; home visit or phone contact; nurse always available) | RCT | LOS (e1 and e2 ↓), rehospitalization rate (e1 and e2 ∅), illness rate (e2 ∅), health care team home visits and phone contacts (e1, e2, and p ↑), physical development (e1 ∅), quality of home environment (e1 and p↑), program cost (e2 ↓) |
| Home and ambulatory program for children with asthma, Halifax, Nova Scotia, Canada32 | Asthma | Comprehensive home and ambulatory program (education and home visits by specially trained nurse), control group continued to received standard care | RCT | Illness severity (e1 ↓), illness symptoms (e1 ∅), medication requirements (e1 ∅), primary care physician visits (e1 and e2 ↓), hospital admissions (e1 and e2 ∅), multiple hospital admissions (e1 and e2 ↓), LOS (e1 and e2 ∅), pulmonary function (e1 ↑), school absenteeism (e1 ↓), metered aerosol technique (e1 ↑), reduction of smokers living at home (e1 ∅), reduction in No. of pets (e1 ∅), asthma education questionnaire (e1 ↑), family satisfaction (p ∅), family wanting more information (p ↓) |
| Home-based management, Montreal, Quebec, Canada33 | Type 1 diabetes mellitus | Home-based intervention (diabetes treatment nurse accompanied family home, offered flexible education sessions, implemented insulin treatment plan with diabetologist) | RCT | Metabolic control (e1 ↑), illness-related adverse events (e1 and s ∅) parents’ knowledge of child’s condition (p ∅), parent and child adherence to treatment (p ∅), effect of child’s illness on family (p ∅), parental perceived stress (p ∅), family satisfaction with care (p ∅), child stress scale (e1 ↑), parental out-of-pocket expenses (e2 ↓), parental time spent with hospitalized child (p ↓), parental hours missed from work (p ∅) |
| Aftercare services, Los Angeles, California34 | Low-birth-weight infants | Home health intervention (home care in first 1–4 wk after discharge, physician available for consult 24 h/d), home visit intervention (provided prevention and intervention services, development and health monitoring of infant, parental support, and social service referrals) | RCT | ED visits (e1 and e2 ∅), rehospitalization (e1 and e2∅), immunization status (e1 ↑) |
| Follow-up care for infants with chronic lung disease, Winston-Salem, North Carolina35 | Chronic lung disease | Community-based follow-up (nurse specialist monitored infants’ and parents’ health and resources use, made referrals) | RCT | Physical and mental development (e1 ∅), rehospitalization (e1 and e2 ∅), respiratory illness (e1 ∅) |
| Military community asthma program, Honolulu, Hawaii36 | Asthma | Run by team coordinator, parent educator, and pulmonologist; outpatient management plan, education | Pre-post | Hospital admissions (e1 and e2 ↓) |
| Community link team, London, England37 | Visual impairment and ophthalmic disorders | Hospital-based community link team members accompanied families during assessments, reinforced and clarified clinical information, and advised families about visual stimulation programs; education and social services information | Pre-post | Family centeredness of care (p ∅), family satisfaction with care (p ↑) |
| Comprehensive clinical care program, Cotonou, Republic of Benin38 | Sickle cell anemia | Intensive parental education and information sessions; education was repeated with encouragement for vaccination, attending appointments, improving nutrition, malaria prophylaxis | Pre-post | Disease-related acute events (e1 ↓), general status and physical growth (e1 ↑), hospitalization frequency (e1 and e2 ↓) |
| Multidisciplinary clinic for children with epilepsy, Little Rock, Arkansas39 | Epilepsy | Medical management, treatment plan involving optimal service control and multifaceted education, direct intervention for psychosocial difficulties | Descriptive | Family satisfaction with care (p ↑) |
| Ocular genetics program, Toronto, Ontario, Canada40 | Ocular genetics diseases | Comprehensive and multidisciplinary hospital-based care; centralized medical services and molecular diagnosis; optimized use of alternative caregivers and diverse resources, aim to minimize visits | Descriptive | Family satisfaction with care (p ↑) |
| Cystic fibrosis outreach services, Brisbane, Australia41 | Cystic fibrosis | Outreach in 7 remote sites, multidisciplinary team (respiratory physician, physiotherapist, dietitian and nurse, local pediatricians, eneral practitioners, or health workers) | Retrospective cohort | Pulmonary function (e1 ∅), sputum bacteriology (e1 ∅), physical development (e1 ∅), hospital admissions (e1 and e2 ↓) |
Abbreviations: ED, emergency department; e1, effectiveness of care; e2, efficiency of care; LOS, hospital length of stay; p, patient or family centeredness; RCT, randomized controlled trial; s, patient safety;↓, decrease in outcome measure; ↑ increase in outcome measure;∅, no change in outcome measure.
Table 2.
Characteristics and Evaluation of Programs Meeting Inclusion Criteria for Noncategorical Patient Populations
| Program | Intervention | Study Design |
Outcomes (Quality-of-Care Domains and Result) |
|---|---|---|---|
| Pediatric home care, Bronx, New York17,19,42 | Community- and hospital-based intervention (multidisciplinary team, comprehensive services, case management, coordination of services, monitoring, direct care, education, advocacy) | RCT | Family satisfaction with care (p ↑), child’s psychological adjustment (e1 and e2 ↑), parents’ well-being (p ↑), child’s function status (e1 ∅), effect of child’s illness on family (p ∅) |
| Project CATCH, Columbus, Ohio43 | Hospital run community based (multidisciplinary transition team) | RCT | Services accessed by families (p ↑), parental social support (p ↑), physical and mental development (e1 ↑) |
| Integrated health care program for children with special needs, Michigan44 | Hospital-only integrated clinic (collaborative interdisciplinary model of care, visits in 1 place and time, nonmedical interventions, yearly comprehensive evaluation) | Prospective cohort | Child behavior (e1 ↑), parental coping and well-being (p ∅), child coping and well-being (e1 ∅) |
| Special primary care clinic, Denver, Colorado45 | Hospital-based multidisciplinary team, comprehensive primary care clinic, care coordination, case management | Pre-post | LOS (e1 and e2 ↓), use of needed services (e1 ↑) |
| Pediatric alliance for coordinated care, Boston, Massachusetts46 | Joint hospital and community intervention, pediatric primary care providers and specialists providing integrated care, managed by pediatric nurse practitioner, individualized health plan developed and shared with stakeholders | Pre-post | Ease of family care delivery (e1 ↑), access to medical team and resources (e1 ↑), parents’ knowledge of child’s condition (p ↑), family satisfaction with care (p ↑), relationship with medical team (p ↑), parental days missed from work (p ↓), hospital admissions (e1 and e2 ↓) |
| Accelerated care through emergency program, Melbourne, Australia47 | Hospital-based ED program, 24-h care with nurses in conjunction with subspecialists, clinical pathway with individual care plans developed | Pre-post | Family satisfaction with care (p ↑), avoided ED visits (e1 and e2 ↑), program cost savings (e2 ↑), ED wait times (t ∅) |
| Access to better care program, Columbus, Ohio48 | Staffed by community- and hospital-based physicians and case managers (social workers and clinical nurse specialists), 24-h phone line | Pre-post | Parents’ knowledge of child’s condition (p ↑), family satisfaction with care (p ↑), hospital admissions (e1 and e2 ↓), program cost savings (e2 ∅) |
| Children with special needs disease management program, Baltimore, Maryland49 | Staffed by advanced practice nurse case managers; assessments completed to develop care plan to meet short- and long-term goals; coordination, facilitation of communication and collaboration, advocating for patients and families | Pre-post | Family satisfaction with care (p ↑), hospital admissions (e1 and e2 ↓), length of hospital stay (e1 and e2 ↓), program cost (e2 ↓), program cost savings (e2 ↑), ED visits (e1 and e2 ↑) |
| Tertiary care–primary care partnership model, Milwaukee, Wisconsin50 | Care coordination provided by nurse case manager (children with more frequent and longer hospitalizations were also treated by MD); single point of contact at hospital between patients and families, primary care providers, and community resources; care plans developed and psychosocial support provided | Pre-post | Hospital admissions (e1 and e2 ↓), No. of hospital days (e1 and e2 ↓), hospital charges (e2 ↓), use of outpatient services (e1 and e2 ↑) |
| Chronic complex center, Tampa, Florida51 | Hospital-based medical home | Pre-post | ED visits (e1 and e2 ↓), hospital admissions (e1 and e2 ↓), hospital days (e1 and e2 ↓), costs (e2 ↓) |
| Complex care clinic, Toronto, Ontario, Canada52 | Staffed by pediatrician and nurse practitioner focusing on management and coordination, comprehensive ambulatory follow-up in coordination with the child’s primary care physician, written care plans, communication by e-mail and phone promoted when possible | Pre-post | Hospitalized days (e1 and e2 ↓), hospitalizations (e1 and e2 ∅), ED visits (e1 and e2 ∅), hospital outpatient visits (e1 and e2 ↑), community outpatient visits (e1 and e2 ∅), parental quality of life (p ↑), family centeredness of care (p ↑), parental satisfaction (p ↑) |
| U Special Kids program, Minneapolis, Minnesota53 | Coordinates communication between family, tertiary care services, social services, primary care provider, specialists, schools, insurers; documentation in electronic health record; issues addressed by phone when possible | Pre-post | Unplanned admissions/d (e1 and e2 ↓), planned admissions/d (e1 and e2 ∅) |
| Pediatric medical home program at UCLA, University of California, Los Angeles54 | 60-min Initial visit for comprehensive evaluation, follow-up appointments twice the length of standard visits, “family liaison” served as primary contact for families, “All About Me” binder containing comprehensive information | Pre-post | ED visits (e1 and e2 ↓), outpatient visits (e1 and e2 ∅), urgent care visits (e1 and e2 ∅), hospital admissions (e1 and e2 ∅), hospital days (e1 and e2 ∅), LOS (e1 and e2 ∅) |
| Project Continuity, Omaha, Nebraska55 | Hospital-based comprehensive care coordination intervention, individual and team care management, assessment of family’s needs and priorities, intervention plan developed, agency referrals and follow-up care for continuity and community transition | Descriptive | Timely access to appropriate services (t ↑), parents’ knowledge of child’s condition (p ↑), parents’ participation in child’s care (p ↑) |
| SABH project, Stockholm, Sweden56 | Hospital-managed advanced inpatient medical care at home, 24-h support from pediatricians and specialized medical staff | Descriptive | Hospital admissions (e1 and e2 ↓) |
| Comprehensive ambulatory services, Rochester, New York57 | Multidisciplinary team to expand ambulatory care coordination and provide “wraparound” services | Descriptive | LOS (e1 and e2 ↓), hospital admissions (e1 and e2 ↓), hospital charges (e2 ↓) |
Abbreviations: CATCH, Collaborative Approach to the Transition from Hospital to Community and Home; ED, emergency department; e1, effectiveness of care; e2, efficiency of care; LOS, hospital length of stay; p, patient or family centeredness; RCT, randomized controlled trial; SABH, Sjukhusansluten Avancerad Barnsjukvård I Hemmet; t, timeliness of care; ↓, decrease in outcome measure; ↑ increase in outcome measure; ∅, no change in outcome measure.
STUDY DESIGN
Thirty-three unique programs were evaluated using several study designs. Thirteen evaluations (39%) were RCTs, 13 evaluations (39%) were pre-post study designs, 5 evaluations (15%) were descriptive studies, and 2 evaluations (1 prospective and 1 retrospective) (6%) were cohort study designs with a control group. The RCT designs occurred more frequently in studies evaluating categorical vs noncategorical disease populations (11 of 17 [65%] vs 2 of 16 [17%], P = .008). Of 2 RCTs among noncategorical disease populations, both focused on a joint hospital-based and community-based program. Publications describing noncategorical disease populations most frequently used the pre-post study design (10 of 16 [63%]). Quality scores were similar between the categorical vs noncategorical disease populations (mean [SD], 27.6 [4.3] vs 26.5 [5.1]; P = .51).
INTERVENTION PROGRAM COMPONENTS
Of 33 unique programs, 17 (52%) were entirely hospital based, while the remaining 16 (48%) contained varying degrees of community-based parts. The program components were wide ranging. Seventeen interventions (52%) involved multidisciplinary teams. Fifteen programs (45%) contained education components, including disease-specific awareness, health maintenance information, wellbeing guidelines, and parenting skills training. Fifteen programs (45%) also contained a specific care or treatment plan component. Fourteen programs (42%) included health monitoring and case management or coordination. Thirteen programs (39%) included care management or care coordination. Ten programs (30%) emphasized family-centered needs, promoting parent-child interactions and offering parental support. Eleven programs (33%) also offered health care provider (ie, physician or nurse) availability for phone, e-mail, or in-person consultations. Nine programs (27%) provided families with referrals to appropriate services. Eight programs (24%) included a home visit component.
REPORTED OUTCOMES
Four programs (12%) were evaluated using at least 4 of 6 IOM quality-of-care aims23; no programs were evaluated using all 6 aims. Effectiveness of care was the outcome most frequently studied (30 programs [91%]), followed by efficiency of care (24 programs [73%]), patient or family centeredness (16 programs [48%]), patient safety (3 programs [9%]), and timeliness of care (2 programs [6%]). Positive evidence for comprehensive hospital-based interventions was reported by 32 programs (97%). One study35 found no difference between the comprehensive hospital-based intervention being studied and its control group.
Effectiveness of Care
Thirty programs (91%) evaluated the effectiveness of care provided to patients, and 95 outcomes were assessed. Improved effectiveness was reported for 57 outcomes (60%). Some outcomes measured were disease specific. For instance, a program aimed at children with sickle cell anemia38 found that participants in an intervention group experienced less painful crises and frequency of transfusions compared with a control group, and another intervention aimed at children with type 1 diabetes mellitus found no difference in glycated hemoglobin levels compared with those in control subjects.28 Adherence to treatment plans (eg, improved care techniques and enhanced ability by the child to manage care) was reported as an effectiveness outcome in 3 RCTs focused on categorical disease populations28,32,33 but in no programs evaluated among noncategorical populations. Examples of other effectiveness outcomes reported included mental and physical health status17,19,38,42 and accessibility of the medical team and resources.46 Fiftynine of 95 (62%) reports on the effectiveness of care focused on health care use outcomes that were also coded as efficiency of care (eg, hospitalization rates). A focus on health care use in outcomes of the effectiveness of care was more common among categorical vs noncategorical populations (28 of 58 [50%] vs 30 of 37 [81%], P = .001).
Efficiency of Care
Twenty-four programs (73%) evaluated the efficiency of care provided to patients, and 76 outcomes were assessed. Of these, 49 (64%) reported positive outcomes, generally relative to hospital-based health care use. Examples of these included shorter hospital stays, cost savings for the institutions providing the program, and fewer hospitalizations (including emergency department and intensive care unit).
Patient or Family Centeredness
Sixteen programs (48%) evaluated aspects of patient or family centeredness on 19 outcomes, with 10 (53%) of those reporting positive findings. Examples of specific outcomes reported were diverse, including parental access to information,32 ability to care for their children,55 out-of-pocket expenses,33 general quality of home environment,31 competency to provide age-appropriate supervision for their children’s care management, or simply overall satisfaction with care.* There were no reports of child or youth satisfaction with care.
Patient Safety
Only 3 programs evaluated outcomes regarding the safety of patients. All were RCTs focused on categorical disease populations. One study26 demonstrated a decrease in life-threatening illnesses but no difference in death rates associated with comprehensive follow-up of very low-birth-weight infants. Two RCTs on diabetes evaluated patient safety; one found a decrease in rates of severe hypoglycemia,29 while the other found no difference in illness-related adverse events associated with home-based management.33
Timeliness of Care
Two programs (6%) evaluated timeliness outcomes. Both focused on noncategorical populations with a descriptive55 or pre-post47 design. One study55 reported a decrease in timely access to appropriate services, while the other study47 found no difference in emergency department wait times associated with an intervention.
Equity of Care
The final IOM quality-of-care domain assessed was equity of care. There were no studies examining such outcomes.
COMMENT
The evidence for comprehensive hospital-based programs for improving the quality of care for CSHCN is generally positive but is limited primarily to studies of children with categorical diseases and by inadequate outcome measures in major domains of health care quality. Most evaluative studies used weak study designs. Although RCTs are considered the gold standard in comparative research, most studies reviewed were noncontrolled, particularly those among children with noncategorical conditions. Despite decades of thought supporting the creation of chronic care models for noncategorical disease populations in pediatrics,58,59 most high-quality (eg, RCT) evidence has continued to focus on single disease groups. Little evidence supports the existence of hospital-based comprehensive care programs for CSHCN, particularly for populations of children outside of single disease services, such as sickle cell anemia or diabetes. Therefore, this review article supports the recent prioritization by the IOM of research focused on evaluation of care coordination programs for chronic disease populations.
The findings herein need to be contextualized in reference to other frameworks for models of care for CSHCN. One conceptualization that has been recently reviewed is that of the community-based medical home model. The medical home is a community-based model of care that is “accessible, family centered, continuous, comprehensive, coordinated, compassionate and culturally effective.”60Homer et al61 reviewed 30 studies of interventions that incorporate part or all of the medical home model. Similar to our findings, despite the largely positive findings reported, the authors found generally weak study designs, inconsistent outcome measures, and a lack of comparison groups in most studies. Although the medical home model is meant to be a primary care community-based model, there was some overlap in their article and ours owing to liberal inclusion criteria in both reviews; 6 studies26,39,42,49,50,57 among the 30 were included in our review. Some studies in the review by Homer et al61 were hospital based and included only parts of the medical home model, while some studies herein included a substantial community-based component. Unfortunately, some of the overlap can be traced to the multiplicity of the term medical home, which has been operationally defined variously in different studies.62 Medical home has been used to describe a model of a community-based primary care practice as well as a conceptual model of an ideal system of care focused around a team of providers in the hospital or community. The definitional vagaries have led to lack of clarity as to whether one model is superior to another for specific populations of CSHCN.
This review has several important limitations. Chiefly, the studies were heterogeneous, with varied definitions, designs, interventions, and outcome measures, limiting comparisons that could be made between them (particularly the pooling of data for meta-analysis). Given the many studies with nonrandom assignment or no comparison group, the risk is high for unaccounted confounders and bias. Furthermore, although rigorous attempts were made to search for all relevant articles, limitations in the search strategy to English-language studies and a finite number of search terms meant that some informative studies may have been missed.
In recent years, important developments in clinical care for CSHCN include the emergence of hospital-based programs for complex subpopulations of CSHCN,21 controversy as to whether community-based physicians or hospital-based generalists or subspecialists are better at leading the care of a wide variety of chronic conditions of childhood,63 and challenges about how to best promote comanagement and collaborative care involving bidirectional coordination and remuneration for care coordination activities.21 The implications of our findings are that, despite a large body of literature, we know little about the optimal model of care for CSHCN. This will be particularly relevant as more comprehensive systems of care for CSHCN are created through new pediatric accountable or integrated care organizations. It is possible that hospital-based programs, such as those reviewed herein, will have a growing role in health system efforts to improve quality and to reduce costs using global payments and shared savings. Although there are ethical, logistical, and financial challenges to conducting randomized trials in this area, pragmatic RCTs comparing well-delineated alternate models of care with consistent outcome measures are essential to make evidence-informed policy decisions for optimal models of care for CSHCN.
In conclusion, most studies of comprehensive hospital-based programs report positive results, but the quality of the evidence is modest overall. The evidence supporting the development of programs for CSHCN is restricted primarily to studies of children with categorical diseases and is limited by inadequate outcome measures. Additional high-quality evidence with appropriate comparative groups and broad holistic outcomes is necessary to determine the effect of hospital-based comprehensive care programs.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by the Pediatric Consultants Partnership at The Hospital for Sick Children. Drs Cohen and Mahant have received operational support from the Norman Saunders Complex Care Initiative and the SickKids Foundation. Dr Kuo receives support from award KL2RR029883 from the National Center for Research Resources.
Role of the Sponsors: The funding bodies had no role in the design or conduct of the study; collection, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Cohen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Cohen, Jovcevska, and Mahant. Analysis and interpretation of data: Cohen, Jovcevska, Kuo, and Mahant. Drafting of the manuscript: Cohen. Critical revision of the manuscript for important intellectual content: Cohen, Jovcevska, Kuo, and Mahant. Statistical analysis: Cohen, Kuo, and Mahant. Obtained funding: Cohen and Mahant. Administrative, technical and material support: Cohen, Jovcevska, and Mahant.
Financial Disclosure: None reported.
Previous Presentations: Portions of this study were presented at the 2009 Pediatric Academic Societies Annual Meeting; May 5, 2009; Baltimore, Maryland.
Additional Contributions: Elizabeth Uleryk, MLS, assisted with the database searches, and Jennifer MacInnis, MSc, assisted with the data analysis.
Online-Only Material: The eTables are available at http://www.archpediatrics.com.
REFERENCES
- 1.van der Lee JH, Mokkink LB, Grootenhuis MA, Heymans HS, Offringa M. Definitions and measurement of chronic health conditions in childhood: a systematic review. JAMA. 2007;297(24):2741–2751. doi: 10.1001/jama.297.24.2741. [DOI] [PubMed] [Google Scholar]
- 2.Bethell CD, Read D, Blumberg SJ, Newacheck PW. What is the prevalence of children with special health care needs? Matern Child Health J. 2008;12(1):1–14. doi: 10.1007/s10995-007-0220-5. [DOI] [PubMed] [Google Scholar]
- 3.Dosa NP, Boeing NM, Kanter RK. Excess risk of severe acute illness in children with chronic health conditions. Pediatrics. 2001;107(3):499–504. doi: 10.1542/peds.107.3.499. [DOI] [PubMed] [Google Scholar]
- 4.Cooley WC, McAllister JW. Building medical homes. Pediatrics. 2004;113(suppl)(5):1499–1506. [PubMed] [Google Scholar]
- 5.Sacchetti A, Sacchetti C, Carraccio C, Gerardi M. The potential for errors in children with special health care needs. Acad Emerg Med. 2000;7(11):1330–1333. doi: 10.1111/j.1553-2712.2000.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 6.Matlow AG, Wright JG, Zimmerman B, Thomson K, Valente M. How can the principles of complexity science be applied to improve the coordination of care for complex pediatric patients? Qual Saf Health Care. 2006;15(2):85–88. doi: 10.1136/qshc.2005.014605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon TD, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647–655. doi: 10.1542/peds.2009-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns KH, Casey PH, Lyle RE, Bird TM, Fussell JJ, Robbins JM. Increasing prevalence of medically complex children in US hospitals. Pediatrics. 2010;126(4):638–646. doi: 10.1542/peds.2009-1658. [DOI] [PubMed] [Google Scholar]
- 9.Cohen E, Kuo DZ, Agrawal R, et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538. doi: 10.1542/peds.2010-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava R, Stone BL, Patel R, et al. Delays in discharge in a tertiary care pediatric hospital. J Hosp Med. 2009;4(8):481–485. doi: 10.1002/jhm.490. [DOI] [PubMed] [Google Scholar]
- 11.Cohen E, Friedman J, Nicholas DB, Adams S, Rosenbaum P. A home for medically complex children: the role of hospital programs. J Healthc Qual. 2008;30(3):7–15. doi: 10.1111/j.1945-1474.2008.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava R, Stone BL, Murphy NA. Hospitalist care of the medically complex child. Pediatr Clin North Am. 2005;52(4):1165–1187. doi: 10.1016/j.pcl.2005.03.007. x. [DOI] [PubMed] [Google Scholar]
- 13.Initial National Priorities for Comparative Effectiveness Research. Washington, DC: Institute of Medicine; 2009. Institute of Medicine. [Google Scholar]
- 14.Cooley WC. American Academy of Pediatrics Committee on Children With Disabilities. Providing a primary care medical home for children and youth with cerebral palsy. Pediatrics. 2004;114(4):1106–1113. doi: 10.1542/peds.2004-1409. [DOI] [PubMed] [Google Scholar]
- 15.Cooley WC. Redefining primary pediatric care for children with special health care needs: the primary care medical home. Curr Opin Pediatr. 2004;16(6):689–692. doi: 10.1097/01.mop.0000146440.79293.5b. [DOI] [PubMed] [Google Scholar]
- 16.Kelly A, Golnik A, Cady R. A medical home center: specializing in the care of children with special health care needs of high intensity. Matern Child Health J. 2008;12(5):633–640. doi: 10.1007/s10995-007-0271-7. [DOI] [PubMed] [Google Scholar]
- 17.Stein RE, Jessop DJ. Does pediatric home care make a difference for children with chronic illness? Pediatrics. 1984;73(6):845–853. [PubMed] [Google Scholar]
- 18.Jessop DJ, Stein RE. Who benefits from a pediatric home care program? Pediatrics. 1991;88(3):497–505. [PubMed] [Google Scholar]
- 19.Jessop DJ, Stein RE. Providing comprehensive health care to children with chronic illness. Pediatrics. 1994;93(4):602–607. [PubMed] [Google Scholar]
- 20.Newacheck PW, Halfon N. Prevalence and impact of disabling chronic conditions in childhood. Am J Public Health. 1998;88(4):610–617. doi: 10.2105/ajph.88.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke RT, Alverson B. Impact of children with medically complex conditions. Pediatrics. 2010;126(4):789–790. doi: 10.1542/peds.2010-1885. [DOI] [PubMed] [Google Scholar]
- 22.American Academy of Family Physicians. [Accessed October 6, 2010]; Comprehensive care, definition of. http://www.aafp.org/online/en/home/policy/policies/c/comprehensivecare2.html. [Google Scholar]
- 23.Envisioning the National Health Care Quality Report. Washington, DC: National Academy Press; 2001. Institute of Medicine. [Google Scholar]
- 24.Hawker S, Payne S, Kerr C, Hardey M, Powell J. Appraising the evidence: reviewing disparate data systematically. Qual Health Res. 2002;12(9):1284–1299. doi: 10.1177/1049732302238251. [DOI] [PubMed] [Google Scholar]
- 25.Brooten D, Kumar S, Brown LP, et al. A randomized clinical trial of early hospital discharge and home follow-up of very-low-birth-weight infants. N Engl J Med. 1986;315(15):934–939. doi: 10.1056/NEJM198610093151505. [DOI] [PubMed] [Google Scholar]
- 26.Broyles RS, Tyson JE, Heyne ET, et al. Comprehensive follow-up care and life-threatening illnesses among high-risk infants: a randomized controlled trial. JAMA. 2000;284(16):2070–2076. doi: 10.1001/jama.284.16.2070. [DOI] [PubMed] [Google Scholar]
- 27.Karnick P, Margellos-Anast H, Seals G, Whitman S, Aljadeff G, Johnson D. The pediatric asthma intervention: a comprehensive cost-effective approach to asthma management in a disadvantaged inner-city community. J Asthma. 2007;44(1):39–44. doi: 10.1080/02770900601125391. [DOI] [PubMed] [Google Scholar]
- 28.Howe CJ, Jawad AF, Tuttle AK, et al. Education and telephone case management for children with type 1 diabetes: a randomized controlled trial. J Pediatr Nurs. 2005;20(2):83–95. doi: 10.1016/j.pedn.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Svoren BM, Butler D, Levine BS, Anderson BJ, Laffel LMB. Reducing acute adverse outcomes in youths with type 1 diabetes: a randomized, controlled trial. Pediatrics. 2003;112(4):914–922. doi: 10.1542/peds.112.4.914. [DOI] [PubMed] [Google Scholar]
- 30.Harish Z, Bregante AC, Morgan C, et al. A comprehensive inner-city asthma program reduces hospital and emergency room utilization. Ann Allergy Asthma Immunol. 2001;86(2):185–189. doi: 10.1016/S1081-1206(10)62689-0. [DOI] [PubMed] [Google Scholar]
- 31.Casiro OG, McKenzie ME, McFadyen L, et al. Earlier discharge with community-based intervention for low birth weight infants: a randomized trial. Pediatrics. 1993;92(1):128–134. [PubMed] [Google Scholar]
- 32.Hughes DM, McLeod M, Garner B, Goldbloom RB. Controlled trial of a home and ambulatory program for asthmatic children. Pediatrics. 1991;87(1):54–61. [PubMed] [Google Scholar]
- 33.Dougherty G, Schiffrin A, White D, Soderstrom L, Sufrategui M. Home-based management can achieve intensification cost-effectively in type I diabetes. Pediatrics. 1999;103(1):122–128. doi: 10.1542/peds.103.1.122. [DOI] [PubMed] [Google Scholar]
- 34.Finello KM, Litton KM, deLemos R, Chan LS. Very low birth weight infants and their families during the first year of life: comparisons of medical outcomes based on after care services. J Perinatol. 1998;18(5):365–371. [PubMed] [Google Scholar]
- 35.O’Shea TM, Nageswaran S, Hiatt DC, et al. Follow-up care for infants with chronic lung disease: a randomized comparison of community- and center-based models. [Accessed March 11, 2011];Pediatrics. 2007 119(4):e947–e957. doi: 10.1542/peds.2006-1717. http://pediatrics.aappublications.org/cgi/content/full/119/4/e947. [DOI] [PubMed] [Google Scholar]
- 36.Chan DS, Callahan CW, Moreno C. Multidisciplinary education and management program for children with asthma. Am J Health Syst Pharm. 2001;58(15):1413–1417. doi: 10.1093/ajhp/58.15.1413. [DOI] [PubMed] [Google Scholar]
- 37.Rahi JS, Manaras I, Tuomainen H, Hundt GL. Meeting the needs of parents around the time of diagnosis of disability among their children: evaluation of a novel program for information, support, and liaison by key workers. [Accessed March 21, 2011];Pediatrics. 2004 114(4):e477–e482. doi: 10.1542/peds.2004-0240. http://pediatrics.aappublications.org/cgi/reprint/114/4/e477. [DOI] [PubMed] [Google Scholar]
- 38.Rahimy MC, Gangbo A, Ahouignan G, et al. Effect of a comprehensive clinical care program on disease course in severely ill children with sickle cell anemia in a sub-Saharan African setting. Blood. 2003;102(3):834–838. doi: 10.1182/blood-2002-05-1453. [DOI] [PubMed] [Google Scholar]
- 39.Williams J, Sharp GB, Griebel ML, et al. Outcome findings from a multidisciplinary clinic for children with epilepsy. Child Health Care. 1995;24(4):235–244. doi: 10.1207/s15326888chc2404_3. [DOI] [PubMed] [Google Scholar]
- 40.Morad Y, Sutherland J, DaSilva L, et al. Ocular Genetics Program: multidisciplinary care of patients with ocular genetic eye disease. Can J Ophthalmol. 2007;42(5):734–738. doi: 10.3129/i07-144. [DOI] [PubMed] [Google Scholar]
- 41.Thomas CL, O’Rourke PK, Wainwright CE. Clinical outcomes of Queensland children with cystic fibrosis: a comparison between tertiary centre and outreach services. Med J Aust. 2008;188(3):135–139. doi: 10.5694/j.1326-5377.2008.tb01554.x. [DOI] [PubMed] [Google Scholar]
- 42.Stein RE, Jessop DJ. Long-term mental health effects of a pediatric home care program. Pediatrics. 1991;88(3):490–496. [PubMed] [Google Scholar]
- 43.Gillette Y, Hansen NB, Robinson JL, Kirkpatrick K, Grywalski R. Hospital-based case management for medically fragile infants: results of a randomized trial. Patient Educ Couns. 1991;17(1):59–70. doi: 10.1016/0738-3991(91)90051-6. [DOI] [PubMed] [Google Scholar]
- 44.Naar-King S, Siegel PT, Smyth M, Simpson P. An evaluation of an integrated health care program for children with special needs. Child Health Care. 2003;32(3):233–243. [Google Scholar]
- 45.Berman S, Rannie M, Moore L, Elias E, Dryer LJ, Jones MD., Jr Utilization and costs for children who have special health care needs and are enrolled in a hospital-based comprehensive primary care clinic. [Accessed March 11, 2011];Pediatrics. 2005 115(6):e637–e642. doi: 10.1542/peds.2004-2084. http://pediatrics.aappublications.org/cgi/content/full/115/6/e637. [DOI] [PubMed] [Google Scholar]
- 46.Palfrey JS, Sofis LA, Davidson EJ, Liu J, Freeman L, Ganz ML. Pediatric Alliance for Coordinated Care. The Pediatric Alliance for Coordinated Care: evaluation of a medical home model. Pediatrics. 2004;113(suppl)(5):1507–1516. [PubMed] [Google Scholar]
- 47.Sutton D, Stanley P, Babl FE, Phillips F. Preventing or accelerating emergency care for children with complex healthcare needs. Arch Dis Child. 2008;93(1):17–22. doi: 10.1136/adc.2007.117960. [DOI] [PubMed] [Google Scholar]
- 48.Grossman LK, Rich LN, Michelson S, Hagerty G. Managed care of children with special health care needs: the ABC Program. Clin Pediatr (Phila) 1999;38(3):153–160. doi: 10.1177/000992289903800305. [DOI] [PubMed] [Google Scholar]
- 49.Hawkins MR, Diehl-Svrjcek B, Dunbar LJ. Caring for children with special health-care needs in the managed care environment. Lippincotts Case Manag. 2006;11(4):216–223. doi: 10.1097/00129234-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Gordon JB, Colby HH, Bartelt T, Jablonski D, Krauthoefer ML, Havens P. A tertiary care–primary care partnership model for medically complex and fragile children and youth with special health care needs. Arch Pediatr Adolesc Med. 2007;161(10):937–944. doi: 10.1001/archpedi.161.10.937. [DOI] [PubMed] [Google Scholar]
- 51.Olsen R. Efficiency and cost of a hospital-based medical home: children with special healthcare needs. Fla Public Health Rev. 2009;6:85–92. [Google Scholar]
- 52.Cohen E, Friedman JN, Mahant S, Adams S, Jovcevska V, Rosenbaum P. The impact of a complex care clinic in a children’s hospital. Child Care Health Dev. 2010;36(4):574–582. doi: 10.1111/j.1365-2214.2009.01069.x. [DOI] [PubMed] [Google Scholar]
- 53.Cady R, Finkelstein S, Kelly A. A telehealth nursing intervention reduces hospitalizations in children with complex health conditions. J Telemed Telecare. 2009;15(6):317–320. doi: 10.1258/jtt.2009.090105. [DOI] [PubMed] [Google Scholar]
- 54.Klitzner TS, Rabbitt LA, Chang RK. Benefits of care coordination for children with complex disease: a pilot medical home project in a resident teaching clinic. J Pediatr. 2010;156(6):1006–1010. doi: 10.1016/j.jpeds.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 55.Jackson B, Finkler D, Robinson C. A case management system for infants with chronic illnesses and developmental disabilities. Child Health Care. 1992;21(4):224–232. doi: 10.1207/s15326888chc2104_5. [DOI] [PubMed] [Google Scholar]
- 56.Bergius H, Eng A, Fagerberg M, et al. Hospital-managed advanced care of children in their homes. J Telemed Telecare. 2001;7(suppl 1):32–34. doi: 10.1177/1357633X010070S113. [DOI] [PubMed] [Google Scholar]
- 57.Liptak GS, Burns CM, Davidson PW, McAnarney ER. Effects of providing comprehensive ambulatory services to children with chronic conditions. Arch Pediatr Adolesc Med. 1998;152(10):1003–1008. doi: 10.1001/archpedi.152.10.1003. [DOI] [PubMed] [Google Scholar]
- 58.Stein RE, Jessop DJ. A noncategorical approach to chronic childhood illness. Public Health Rep. 1982;97(4):354–362. [PMC free article] [PubMed] [Google Scholar]
- 59.Pless IB, Pinkerton P. Chronic Childhood Disorder: Promoting Patterns of Adjustment. London, England: Henry Kimpton; 1975. [Google Scholar]
- 60.American Academy of Pediatrics. American Academy of Pediatrics Ad Hoc Task Force on Definition of the Medical Home: the medical home. Pediatrics. 1992;90(5):774. [PubMed] [Google Scholar]
- 61.Homer CJ, Cooley WC, Strickland B. Medical home 2009: what it is, where we were, and where we are today. Pediatr Ann. 2009;38(9):483–490. doi: 10.3928/00904481-20090820-06. [DOI] [PubMed] [Google Scholar]
- 62.Vest JR, Bolin JN, Miller TR, Gamm LD, Siegrist TE, Martinez LE. Medical homes:“where you stand on definitions depends on where you sit”. Med Care Res Rev. 2010;67(4):393–411. doi: 10.1177/1077558710367794. [DOI] [PubMed] [Google Scholar]
- 63.Mayer ML, Skinner AC, Freed GL. Interspecialty differences in the care of children with chronic or serious acute conditions: a review of the literature. J Pediatr. 2009;154(2):164–168. doi: 10.1016/j.jpeds.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.