Abstract
Contents release from redox-responsive liposomes is anion specific. Liposomal contents release is initiated by contact of apposed liposome bilayers having in their outer leaflet 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), whose presence is due to redox-stimulated removal of a quinone propionic acid protecting group (Q) from Q-DOPE lipids. Contents release occurs upon the phase transition of DOPE from its lamellar liquid crystalline (Lα) to hexagonal-II inverted micelle (HII) phase. Contents release is slower in the presence of weakly hydrated chaotropic anions versus highly hydrated kosmotropic anions and is attributed to ion accumulation near the zwitterionic DOPE head groups, in turn altering head group hydration, as indicated by the Lα→HII phase transition temperature, TH, for DOPE. The results are significant, not only for mechanistic aspects of liposome contents release in DOPE-based systems, but also for drug delivery applications wherein exist at drug targeting sites variations in type and concentration of ions and neutral species.
INTRODUCTION
Lyotropic (Hofmeister)1, 2 cations and anions, as well as osmolytes, are present in time- and location-dependent concentrations within, and in the vicinity of, cancerous tumors and cells. For example, lactate concentrations in tumors are 2–4 times and bicarbonate values 4–6 mM higher that in plasma.3 In addition, intracellular/extracellular Cl−, CO32−, and glucose concentrations vary with tumor type,4 and elevated Na+ concentrations are found in brain and breast tumors.5
Stimuli-responsive drug delivery vesicles based on phospholipids have been identified for the treatment of a variety of diseases, and have received great attention for improving cancer therapy, due to the ability of 70–200-nm diameter liposomes to accumulate at diseased sites, thereby decreasing the side effects and improving the efficacy of the drug contained within.6 During in vitro assessment of the capabilities of various responsive liposome systems, the solution conditions used for their evaluation—particularly salt and buffer identity and concentration—vary widely between individual studies. Comparison of contents release characteristics for liposome systems in different laboratories is challenging, especially those systems whose success hinges on interliposomal or membrane–liposome, lipid–lipid events.
Phosphoethanolamine (PE) lipids are highly targeted for use in stimuli-responsive liposomes, due to their ability to promote liposome contents release at a selected site, typically via liposome bilayer-bilayer (aggregation/contents dispensing) or liposome bilayer-cell membrane (hemifusion/fusion) interactions that are predicated on the PE liquid crystalline lamellar to inverted hexagonal phase transition (Lα→HII).6 However, to date, the possible influence of any salt or osmolyte (Hofmeister species) on the kinetics of PE-based liposome contents delivery has not been addressed. Thus, it is of great value to gain fundamental knowledge about the influence of salt identity and concentration on the contents release process that is based on the PE Lα→HII phase transition.
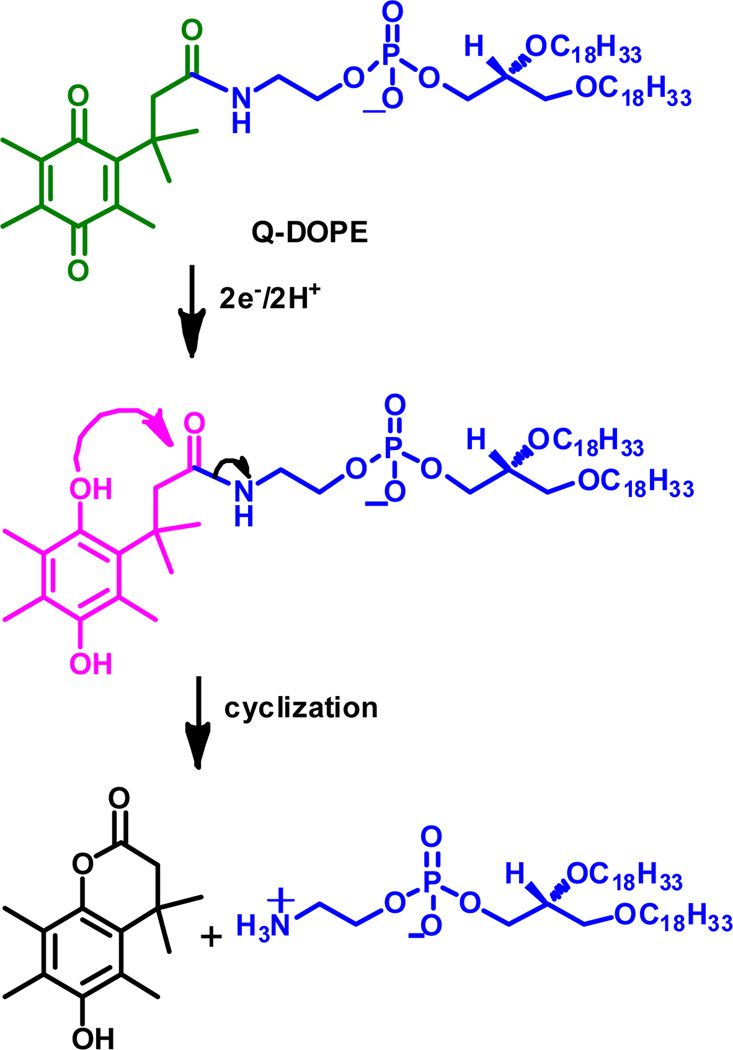
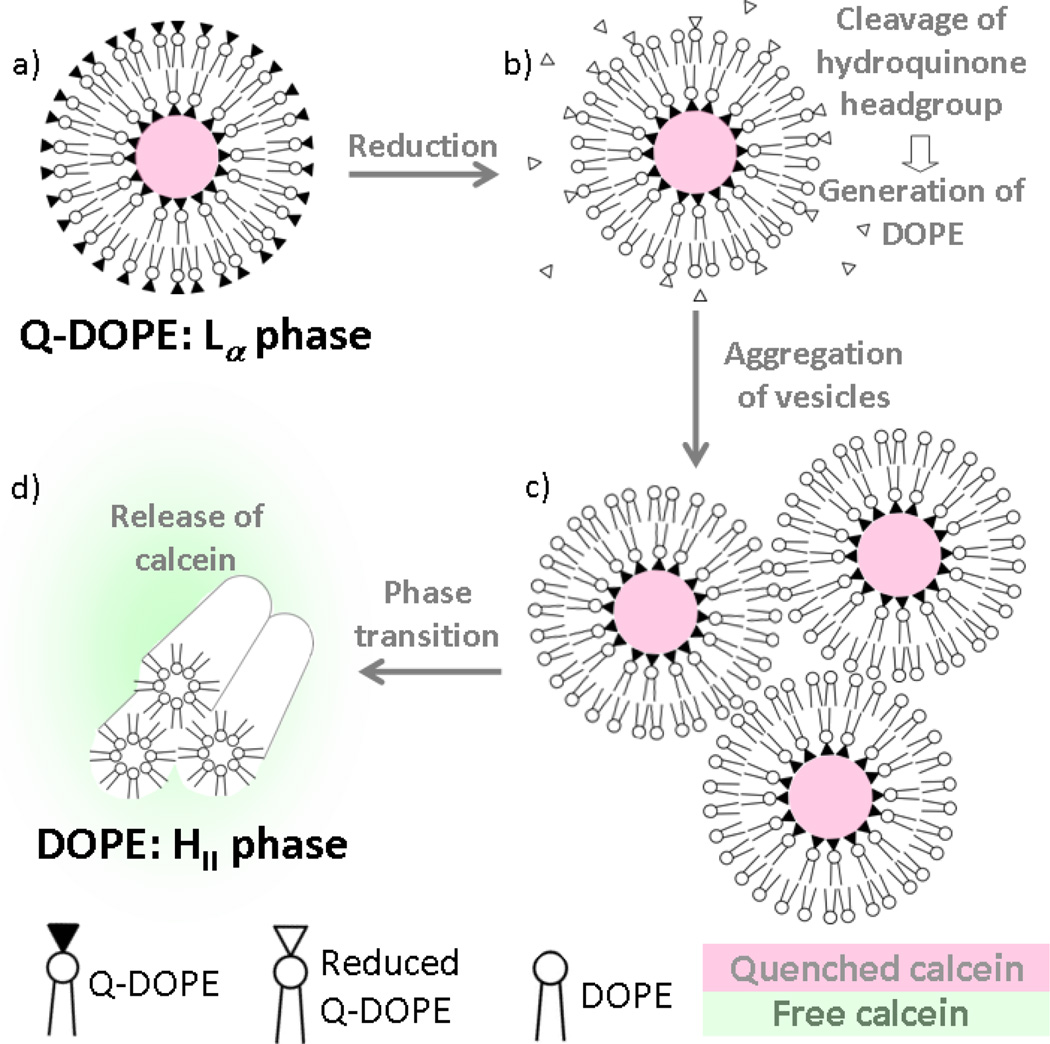
We have chosen to evaluate the impact of salt nature on liposome contents release using the Q-DOPE lipid-based liposome system we have developed, Scheme 1, because initiation of release is anticipated to occur at a number of cancer tumors that over express NAD(P)H:quinine oxidoreductase isozyme 1 (NQO1), known to specifically reduce quinones.7 In addition, contents release from reduced liposomes composed of Q-DOPE occurs by contact-induced lysis of liposomes possessing in their outer leaflet a critical amount of the HII phase-prone DOPE, which is generated upon cleavage of the reduced quinone moiety, Scheme 2.8, 9 Upon formation of the critical number of PE lipids to allow for liposome aggregation10 and bilayer apposition at experimental temperatures Texp ≥ TH, the Lα→HII transition of the PE occurs,11 leading to burst-phase contents release.8, 9
Scheme 1.
Q-DOPE lipid, and its reduction and subsequent products
Scheme 2.
Q-DOPE liposome contents release
Based on observations by Petrache12 and Leontidis13 with multilamellar phosphatidylcholine (PC) bilayer assemblies and those by Seddon14 and Quinn15 for PE bilayer assemblies, we anticipated the presence of anions having varying polarizabilities/hydration levels would influence the aggregation and contents release from reduced Q-DOPE liposomes in a manner dictated by the Hofmeister series.1 This hypothesis is founded on conclusions, drawn from experimental12, 13 and theoretical12, 16 outcomes for the PC layers above, that point to the accumulation of ions near the head group region of the lipids, as a result of ion partitioning events or non-electrostatic dispersion forces17 acting between the ions and the lipids. Theoretical studies indicate the existence of anion concentrations as high as seven times that of the bulk value,18 which can in turn result in anion adsorption at the lipid head groups for more polarizable anions,13, 19 a change in interfacial water order/density,18, 20–22 and dehydration of lipid head groups.12, 13, 16
RESULTS AND DISCUSSION
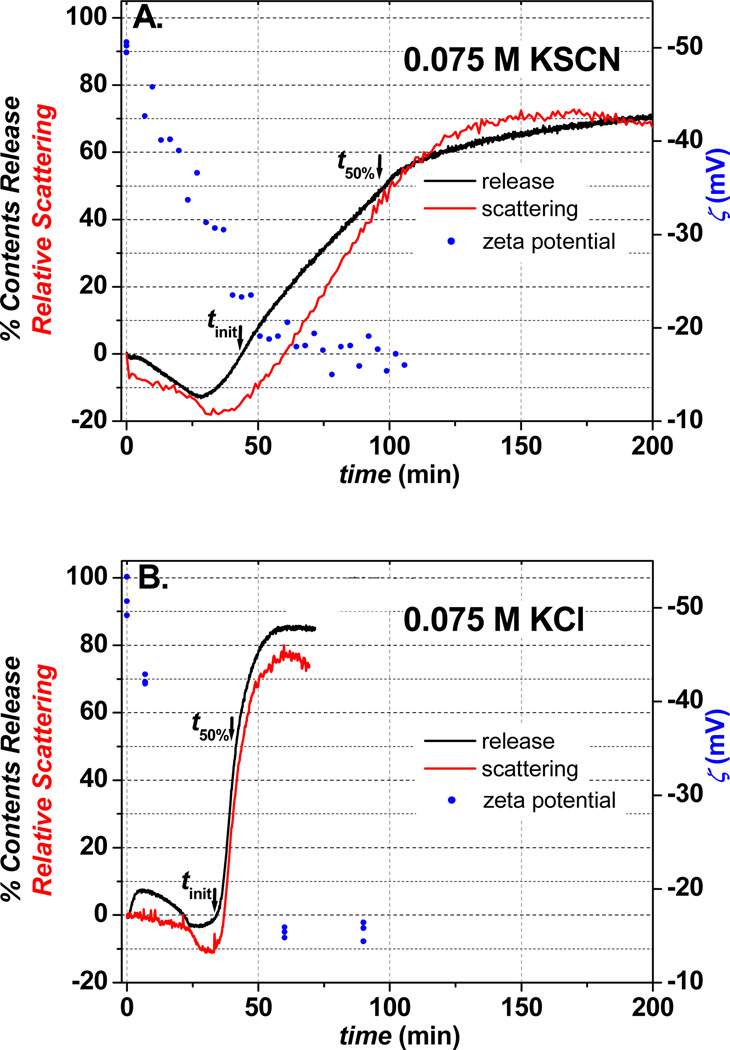
Displayed in Figure 1 are the time-dependent contents release, aggregation (light scattering), and zeta (ζ) potential measurements for ~120-nm Q-DOPE liposomes reduced by dithionite.23 The ion specificity of the contents release process is borne out when comparing the three aforementioned observables for reduced Q-DOPE liposomes in the presence of 0.075 M KSCN (Figure 1A) and 0.075 M KCl (Figure 1B).24 The time required for initial aggregation/contents release25 tinit and that for 50% maximum contents release8 t50% are significantly larger for the Q-DOPE liposomes in the presence of the more polarizable/less hydrated SCN− (44 min, 98 min) in comparison to those for the less polarizable/more hydrated Cl− (34 min, 42 min).26 The amount of DOPE in the outer leaflet for Q-DOPE in the presence of KCl at tinit = 34 min is 53% of the maximum possible, while it is 62% for the KSCN at tinit = 44 min,23 pointing to the requirement of more DOPE exposed to achieve aggregation and subsequent contents release in the presence of the more polarizable thiocyanate. For both anions, the zeta potential ζ is approximately −20 mV at the point of initial aggregation/contents release, having become more positive than the statistically equivalent initial ζ of −50 ± 1 mV (SCN−) and −51 ± 2 mV (Cl−) at t = 0. The similarity in ζ values for the two anions at tinit points to the following possibilities: no appreciable anion binding or poor experimental precision/low frequency of ζ measurement during the lengthy in situ experiment. We suggest the latter need to be improved; the difference in ζ for 100% PC liposomes in 0.5 M KBr and KNO3, or KI and KBr is roughly 3 mV,12 while it is 4 mV for 80% PC:20% phosphatidic acid liposomes in 0.15 M NaBr versus NaCl.27
Figure 1.
Impact of anion identity (0.075 M) on liposome contents release process: (A) KSCN and (B) KCl. Release of self-quenched calcein, the relative light scattering response at 90°, and the zeta potential for ~120-nm diameter, 0.0975 mg mL−1 (100 µM) 100% Q-DOPE liposomes after their reduction by 5 equivalents of the membrane impermeable dithionite, S2O42−, in pH 7.40, 0.050 M phosphate (potassium salt) buffer. T = 25 °C in all cases.
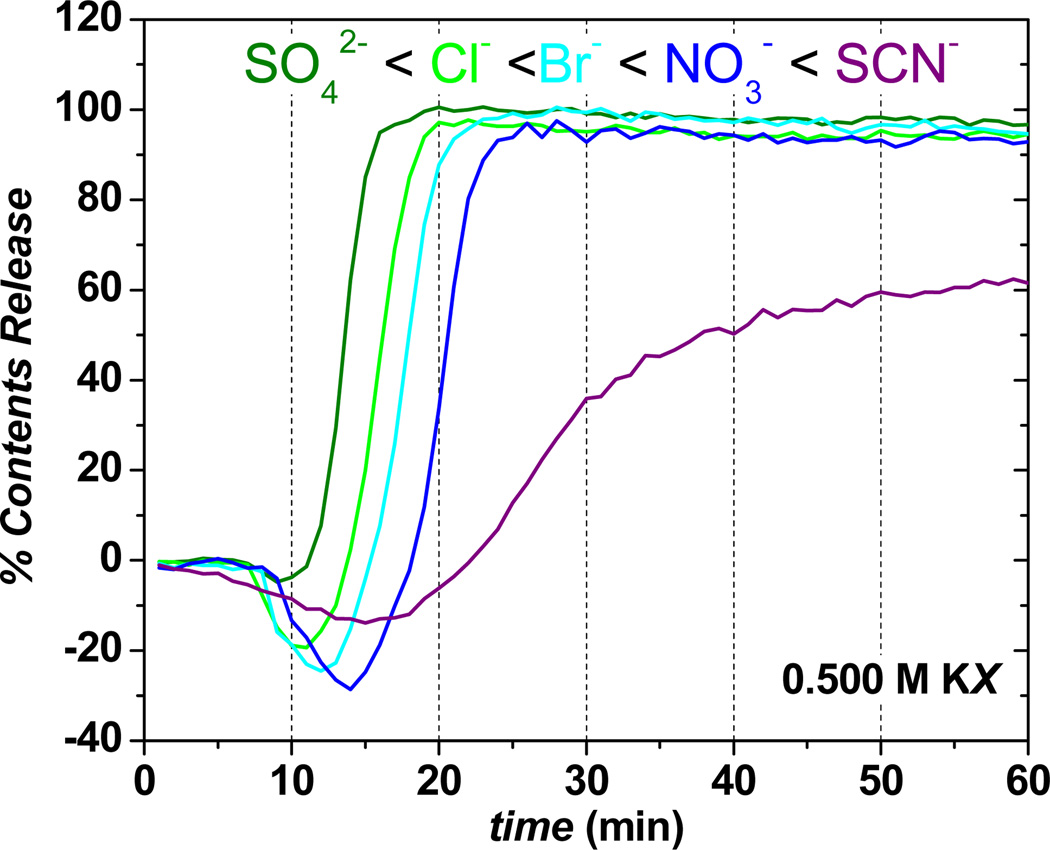
To explore further the ion specificity of the contents release process, a select group of Hofmeister anions with a common cation (K+) were used, Figure 2. The tinit values increase with increasing polarizability (chaotropicity) of the anion in solution, while there is a decrease in the initial release rate (slope of the release curves,28 which directly correlates with the rate of aggregation, as noted in Figure 1) during the burst-phase process, Table 1. Times for initial liposome aggregation/contents release follow the inverse Hofmeister series (SCN− > NO3− > Br− > Cl− > SO42−), with the initial release rate tracking the direct Hofmeister series, under salt concentrations where electrostatic interactions between approaching HII-prone lipid layers are minimal (double layer ~0.4 nm). Furthermore, Q-DOPE liposomes that have not been reduced are stable indefinitely in the presence of 0.500 M salts. Thus, the observed ion-specific events are due to characteristic properties of outer leaflet DOPE under the influence of aqueous anions.
Figure 2.
Effect of anion (0.500 M) identity on contents release from 100% Q-DOPE liposomes in pH 7.40, 0.050 M phosphate (potassium salt) buffer after their reduction via addition of one molar equivalent of dithionite, S2O42−. T = 25 °C in all cases.
Table 1.
Effect of Anion on Time Required for Initial Aggregation/Contents Release tinit and Initial Release Rate for Reduced Q-DOPE Liposomes in Figure 2 at 25 °C
| Salt (0.500 M) | tinit (min)a | initial release rate (% min−1)b |
|---|---|---|
| K2SO4 | 12 | 26.5 ± 1.5 |
| KCl | 14 | 21.2 ± 2.1 |
| KBr | 16 | 15.0 ± 1.9 |
| KNO3 | 19 | 11.0 ± 1.8 |
| KSCN | 2 | 4.8 ± 0.1 |
Uncertainties are ± 1 min.
Average with ± 1 standard deviation.
The HII phase of PE lipids is energetically favored29 with low amounts of interfacial water at the phosphoethanolamine moiety, because of the decreased lateral pressure in the head group region (smaller head group/increased hydrogen bonding/reduced spontaneous radius of curvature); while at higher water values, the larger PE head group prefers the expanded Lα phase.15 Thus, the TH of the Lα→HII transition of PEs increases with increased head group hydration, and decreases with decreased hydration; the sensitivity of this effect can be as large as 7 °C per water of hydration.14
We posited that well-hydrated, less polarizable anions (kosmotropes) will accumulate near the DOPE head group region and dehydrate the DOPE so as to cause a decrease in the TH of DOPE, while accumulation of weakly hydrated, more polarizable anions (chaotropes) near the head group will tend to increase TH. This hypothesis is based on experimental work with PC bilayers wherein head group hydration level is anion specific.13, 19 Surprisingly, no calorimetric data exist for DOPE in the presence of different Hofmeister series salts. The measured TH of DOPE follows the inverse Hofmeister series (SCN− > NO3− > Br− > Cl− > SO42−), with the more polarizable/less hydrated anions increasing TH, and those that are less polarizable/more hydrated lowering TH, in comparison to the case of no added KX salt, Table 2. These data parallel that for 1-palmitoyl-2-oleoyl-phosphoethanolamine (POPE) in the presence of kosmotropic and chaotropic agents and that of PEs at different levels of head group hydration.14, 15
Table 2.
Calorimetrically determined TH values for DOPE (1.88 × 10−2 M) in pH 7.40, 0.050 M phosphate buffer with added 0.500 M salt
| Salt | TH (°C)a |
|---|---|
| K2SO4 | 8.8 ± 0.4 |
| KCl | 9.8 ± 0.5 |
| KBr | 13.3 ± 0.4 |
| None | 13.1 ± 0.5 |
| KNO3 | 15.6 ± 0.6 |
| KSCN | 36.7 ± 0.8 |
Average with 1 standard deviation for three replicates.
Based on the work of Aroti and Leontidis concerning ion accumulation near PC head groups that leads to their ion-specific dehydration,13, 16 a similar event for DOPE in the reduced Q-DOPE liposomes would result in increased intermolecular hydrogen bonding between neighboring phosphate and protonated ethanolamine groups when in the presence of less polarizable anions. Larger intermolecular hydrogen bonding exists in the much less hydrated HII phase of PEs versus the disordered Lα phase.14, 30 A salt-induced, dehydration-driven increase in DOPE inter-lipid association would be in accord with the larger inter-lipid interactions found for POPE bilayers in the presence of NaCl,31 and the anion-specific lipid head group hydration of DOPC bilayers that follows the inverse Hofmeister series.19 Furthermore, it has been shown that the ability of apposed lipid bilayers to approach each other and make initial bilayer-bilayer contact (stalk formation) at a critical separation distance is dictated by only the work required to dehydrate the apposed lipid head groups.32 Thus, it is expected that hydration of DOPE in the reduced Q-DOPE liposomes will be decreased in the presence of salt, and the hydration level will follow the inverse Hofmeister series. Such a scenario will result in increased time to initial liposome aggregation (tinit) for increasing DOPE hydration caused by accumulation of more polarizable/less hydrated anions, while the initial rate of contents release will correlate with the direct Hofmeister series, because the HII phase will be favored in the presence of less polarizable kosmotropes that dehydrate DOPE. This model is in agreement with our observations.
The results from the work at hand may also be explained, in part, by the impact of salt identity on the interbilayer water thickness between apposed bilayers, a characteristic designated as a measure of interbilayer repulsion in multilamellar PC stacks that follows the inverse Hofmeister series.12, 13 Our tinit data agree with the inverse Hofmeister trend for this model, and also the unusual behavior for the thiocyanate case, which could be attributed to direct interaction of this special chaotropic anion with the DOPE head group region. Anion binding that gives rise to head group expansion that favors the Lα phase of DOPE should result in an increased tinit due to electrostatic repulsion between approaching liposomes, and also a decreased rate of contents release. However, the rate of release for 0.5 M KSCN (4.8 ±0.1 % min−1) is larger than in the absence of added KX salt (3.5 ±0.1 % min−1). In addition, we find that tinit is decreased when any salt is present (tinit= 59 min for no added salt). Our observations are counter to the increase in repulsion found upon salt addition for the PC systems.12, 13
OUTLOOK
The outcomes from the work here point to its importance and that of future work aimed at gaining additional fundamental knowledge about the influence of salt anion/cation and osmolyte identity and their concentration on contents release from this and other PE- or diacylglycerol-based liposome systems targeted for drug delivery applications. As stalk formation is the key step in the Lα→HII transition32 for contents release from stimuli-responsive Q-DOPE liposomes8, 9 and others,6 it may be possible to gain additional knowledge about ion-specific effects using strategies that lead to stabilization of aggregated liposomes, stalk structures, and hemifused liposomes.9, 32 In addition, it has been shown that the Lα→HII transition of di-dodecylphosphatidylethanolamine is exceedingly sensitive to the presence of short-chain carboxylate anions, even becoming inaccessible at sufficiently high carboxylate concentrations.14 Thus, the elevated lactate/lactic acid concentrations in the interstitial fluid of cancer tumors3, 4 may have a profound influence on the way in which therapeutic liposomes release their drug contents. In addition, clathrin-mediated endocytosis of drug delivery vehicles33 has the potential be influenced by the presence of Hofmeister salts, noted by their impact on clathrin-vesicle stability.34 Furthermore, sugars have a significant effect on the TH of lipids,35 which can also lead to the stabilization of cell membranes. Thus, the dynamic glucose concentration levels associated with tumors is anticipated to impact the pharmacokinetics of liposomal drug delivery. Finally, it will be valuable to learn if the presence of various ions and osmolytes leads to a change in the mechanism of liposomal contents release (burst release vs. leakage)9 when the experimental temperature is below that of TH of the lipid bilayer, as for SCN− in Figure 2.
Supplementary Material
ACKNOWLEDGMENT
This work was made possible by financial support from the US National Science Foundation (CHE-0910845) and the US National Institutes of Health (5R21CA135585). We thank Professor Vincent LiCata for use of the calorimeter.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Materials and methods, 31P NMR spectra of Q-DOPE liposomes before and after reduction, and electrochemical demonstration of independence of quinine propionic acid head group cyclization rate in the presence of different ions. This material is available free of charge via the Internet at http://pubs.acs.org
The authors declare no competing financial interest.
REFERENCES
- 1.Hofmeister F, Zur Lehre von der Wirkung der Salze Archiv für experimentelle Pathologie und Pharmakologie. 1887;24(1):247–260. [Google Scholar]
- 2.Zhang Y, Cremer PS. Chemistry of Hofmeister Anions and Osmolytes. Annu. Rev. Phys. Chem. 2010;61:63–83. doi: 10.1146/annurev.physchem.59.032607.093635. [DOI] [PubMed] [Google Scholar]
- 3.Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiological Reviews. 2012;92(3):1005–1060. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL, Artemov D, Bedi A, Bhujwalla Z, Chiles K, Feldser D, Laughner E, Ravi R, Simons J, Taghavi P, Zhong H. 'The metabolism of tumours': 70 years later. Novartis Foundation Symposium. 2001;Vol. 240:251–260. 2001/12/01 ed. [PubMed] [Google Scholar]
- 5.Ouwerkerk R, Bleich KB, Gillen JS, Pomper MG, Bottomley PA. Tissue Sodium Concentration in Human Brain Tumors as Measured with 23Na MR Imaging. Radiology. 2003;227(2):529–537. doi: 10.1148/radiol.2272020483. [DOI] [PubMed] [Google Scholar]
- 6.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 7.Ross D. Quinone reductases multitasking in the metabolic world. Drug Metab. Rev. 2004;36(3–4):639–654. doi: 10.1081/dmr-200033465. [DOI] [PubMed] [Google Scholar]
- 8.Ong W, Yang Y, Cruciano AC, McCarley RL. Redox-triggered contents release from liposomes. J. Am. Chem. Soc. 2008;130(44):14739–14744. doi: 10.1021/ja8050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loew M, Forsythe JF, McCarley RL. Lipid nature and their influence on opening of redox-active liposomes. Langmuir. 2013;29(22):6615–6623. doi: 10.1021/la304340e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolber M, Haynes D. Evidence for a role of phosphatidyl ethanolamine as a modulator of membrane-membrane contact. J. Membr. Biol. 1979;48(1):95–114. doi: 10.1007/BF01869258. [DOI] [PubMed] [Google Scholar]
- 11.See Figure S-1 of Supporting Information.
- 12.Petrache HI, Zemb T, Belloni L, Parsegian VA. Salt screening and specific ion adsorption determine neutral-lipid membrane interactions. Proc. Natl. Acad. Sci. U.S.A. 2006;103(21):7982–7987. doi: 10.1073/pnas.0509967103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aroti A, Leontidis E, Dubois M, Zemb T. Effects of monovalent anions of the hofmeister series on DPPC lipid bilayers Part I: swelling and in-plane equations of state. Biophys. J. 2007;93(5):1580–1590. doi: 10.1529/biophysj.106.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seddon JM, Cevc G, Marsh D. Calorimetric studies of the gel-fluid (Lβ-Lα) and lamellar-inverted hexagonal (Lα-HII) phase transitions in dialkyl- and diacylphosphatidylethanolamines. Biochemistry. 1983;22(5):1280–1289. doi: 10.1021/bi00274a045. [DOI] [PubMed] [Google Scholar]
- 15.Sanderson PW, Lis LJ, Quinn PJ, Williams WP. The Hofmeister effect in relation to membrane lipid phase stability. Biochim. Biophys. Acta. 1991;1067(1):43–50. doi: 10.1016/0005-2736(91)90024-3. [DOI] [PubMed] [Google Scholar]
- 16.Leontidis E. Phospholipid Aggregates as Model Systems to Understand Ion-Specific Effects: Experiments and Models. In: Kunz W, editor. Specific Ion Effects. World Scientific Publishing; 2010. pp. 55–84. [Google Scholar]
- 17.Boström M, Deniz V, Ninham BW. Ion specific surface forces between membrane surfaces. J. Phys. Chem. B. 2006;110(19):9645–9649. doi: 10.1021/jp0606560. [DOI] [PubMed] [Google Scholar]
- 18.Lima ERA, Boström M, Horinek D, Biscaia EC, Kunz W, Tavares FW. Co-Ion and Ion Competition Effects: Ion Distributions Close to a Hydrophobic Solid Surface in Mixed Electrolyte Solutions. Langmuir. 2008;24(8):3944–3948. doi: 10.1021/la7037069. [DOI] [PubMed] [Google Scholar]
- 19.Vacha R, Jurkiewicz P, Petrov M, Berkowitz ML, Bockmann RA, Barucha-Kraszewska J, Hof M, Jungwirth P. Mechanism of Interaction of Monovalent Ions with Phosphatidylcholine Lipid Membranes. The Journal of Physical Chemistry B. 2010;114(29):9504–9509. doi: 10.1021/jp102389k. [DOI] [PubMed] [Google Scholar]
- 20.Chandler D. Interfaces and the driving force of hydrophobic assembly. Nature. 2005;437(7059):640–647. doi: 10.1038/nature04162. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Flores SC, Lim S-M, Zhang Y, Yang T, Kherb J, Cremer PS. Specific Anion Effects on Water Structure Adjacent to Protein Monolayers. Langmuir. 2010;26(21):16447–16454. doi: 10.1021/la1015862. [DOI] [PubMed] [Google Scholar]
- 22.Parsons DF, Ninham BW. Surface charge reversal and hydration forces explained by ionic dispersion forces and surface hydration. Colloids Surf. A. 2011;383(1–3):2–9. [Google Scholar]
- 23.The extent of reduction at 0.28 M or 0.1 M salt is such that 50% of the outer leaflet lipids are reduced within 180+/−20 s, and it is independent of salt concentration. The previously reported rate constant of 0.022 min−1 for the cyclization reaction on the surface of the Q-DOPE liposomes was used.
- 24.The "dip" in the release and scattering curves has been attributed to liposome contraction and deformation that results in inward budding, as we have recently discussed in Loew M, Forsythe JC, McCarley RL. Langmuir. 2013;29:6615–6623. doi: 10.1021/la304340e. and as previously observed during sphingomyelinase-induced lipid head group removal in phospholipid vesicles, Holopainen JM, Angelova MI, Kinnunen PKJ. Biophys. J. 2000;78:830–838. doi: 10.1016/S0006-3495(00)76640-9.
- 25.This value was determined by examining when the contents release increased above zero and the slope of the scattering data became positive these two events were coincidental at tinit.
- 26.Cleavage of the reduced quinone is unaffected by anion identity and anion concentration. See Supporting Information Figure S-2 and associated text.
- 27.Carrión FJ, De La Maza A, Parra JL. The Influence of Ionic Strength and Lipid Bilayer Charge on the Stability of Liposomes. J. Colloid Interface Sci. 1994;164(1):78–87. [Google Scholar]
- 28.Slopes of initial release were obtained from linear-least-squares analysis of the initial data (contents release greater than zero) which produced a correlation coefficient of greater than 0.99.
- 29.Kirk GL, Gruner SM, Stein DL. A thermodynamic model of the lamellar to inverse hexagonal phase transition of lipid membrane-water systems. Biochemistry. 1984;23(6):1093–1102. [Google Scholar]
- 30.Boggs JM. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochim. Biophys. Acta. 1987;906(3):353–404. doi: 10.1016/0304-4157(87)90017-7. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Manyes S, Oncins G, Sanz F. Effect of Ion-Binding and Chemical Phospholipid Structure on the Nanomechanics of Lipid Bilayers Studied by Force Spectroscopy. Biophys. J. 2005;89(3):1812–1826. doi: 10.1529/biophysj.105.064030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aeffner S, Reusch T, Weinhausen B, Salditt T. Energetics of stalk intermediates in membrane fusion are controlled by lipid composition. Proc. Natl. Acad. Sci. U.S.A. 2012;109(25):E1609–E1618. doi: 10.1073/pnas.1119442109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hillaireau H, Couvreur P. Nanocarriers' entry into the cell: relevance to drug delivery. Cell. Mol. Life Sci. 2009;66(17):2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nandi PK, Edelhoch H. The effects of lyotropic (hofmeister) salts on the stability of clathrin coat structure in coated vesicles and baskets. J. Biol. Chem. 1984;259(18):1290–1296. [PubMed] [Google Scholar]
- 35.Bryszewska M, Epand RM. Effects of Sugar Alcohols and Disaccharides in Inducing the Hexagonal Phase and Altering Membrane-Properties - Implications for Diabetes-Mellitus. Biochim. Biophys. Acta. 1988;943(3):485–492. doi: 10.1016/0005-2736(88)90381-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.