Abstract
Objective
To report the design and implementation of the Right Drug, Right Dose, Right Time: Using Genomic Data to Individualize Treatment Protocol that was developed to test the concept that prescribers can deliver genome guided therapy at the point-of-care by using preemptive pharmacogenomics (PGx) data and clinical decision support (CDS) integrated in the electronic medical record (EMR).
Patients and Methods
We used a multivariable prediction model to identify patients with a high risk of initiating statin therapy within 3 years. The model was used to target a study cohort most likely to benefit from preemptive PGx testing among Mayo Clinic Biobank participants with a recruitment goal of 1000 patients. Cox proportional hazards model was utilized using the variables selected through the Lasso shrinkage method. An operational CDS model was adapted to implement PGx rules within the EMR.
Results
The prediction model included age, sex, race, and 6 chronic diseases categorized by the Clinical Classifications Software for ICD-9 codes (dyslipidemia, diabetes, peripheral atherosclerosis, disease of the blood-forming organs, coronary atherosclerosis and other heart diseases, and hypertension). Of the 2000 Biobank participants invited, 50% provided blood samples, 13% refused, 28% did not respond, and 9% consented but did not provide a blood sample within the recruitment window (October 4, 2012 – March 20, 2013). Preemptive PGx testing included CYP2D6 genotyping and targeted sequencing of 84 PGx genes. Synchronous real-time CDS is integrated in the EMR and flags potential patient-specific drug-gene interactions and provides therapeutic guidance.
Conclusion
These interventions will improve understanding and implementation of genomic data in clinical practice.
Pharmacogenomics (PGx) is the study of the role of genetic variation in drug response phenotypes.1–4 An individual’s drug response phenotype can range from serious, potentially life-threatening adverse drug reactions at one end of the spectrum, to lack of therapeutic efficacy at the other. As a result, the clinical implementation of PGx at the bedside could make it possible to avoid adverse drug reactions, maximize drug efficacy, and select medications to optimize effect for specific indications based on the genetic profile of individual patients. Over the past decade, a large number of PGx variants with demonstrated clinical utility have been identified and incorporated into drug labels by the United States Food and Drug Administration (FDA).5
Widespread incorporation of PGx into clinical practice, despite its potential clinical implications that could, ultimately, affect virtually every patient, has proved to be challenging due to (1) delay in the initiation of therapy when traditional reactive ordering of PGx testing at point-of-care is used, (2) lack of support for commercial electronic medical record (EMR) systems to integrate large-scale genomic data linked to automated clinical decision support (CDS), (3) development of quality CDS, (4) prescriber uncertainty about benefits, both clinical and economical, for genome-guided therapy, and (5) ethical, legal, social, and financial concerns with regard to genomic medicine by patients and their families.6 Changing the clinical paradigm to preemptively sequencing patients at high risk of needing specific medications and provide parallel CDS around results interpretation and actions could minimize some of these challenges by cost-effectively interrogating a large panel of PGx genes and integrating clinically actionable results into the patients EMR that can be used by clinicians at the point-of-care. A distinct advantage to this approach is the ability to review the available sequence data, and based on new PGx discoveries; update the patient’s record without the need for additional specimen collection and testing provided that the variant was included in the PGx panel. Furthermore, CDS integrated in the EMR may increase awareness of drug-gene interactions, facilitate knowledge and acceptance of PGx testing, and guide the individualization of drug/dose selection.
Few aspects of genomic medicine have the potential to immediately impact the care of patients in a clinically meaningful fashion like PGx. Accordingly, the National Institutes of Health facilitated a collaboration between the Pharmacogenomics Research Network (PGRN) (http://www.pgrn.org) and the Electronic Medical Records and Genomics (eMERGE) Network7 (http://emerge.mc.vanderbilt.edu) to support pilot preemptive PGx DNA sequencing projects. The Right Drug, Right Dose, Right Time –Using Genomic Data to Individualize Treatment (RIGHT Protocol) is an outcome of this collaboration in concert with the Mayo Clinic Center for Individualized Medicine.6 The RIGHT Protocol is tasked with extending PGx implementation beyond “reactive genotyping”, which may in some instances have less than optimal turn-around times and cost, to include “preemptive sequencing”, with integration of the clinically actionable PGx variants in the EMR to drive point-of-care CDS. Herein we report the design and implementation of the RIGHT Protocol.
MATERIALS AND METHODS
Study Objectives
The goal of this project is to develop best practices for the implementation of genetic sequence data into clinical systems to improve patient outcomes. Specifically, the RIGHT Protocol pilot has three main objectives. First, identify 1,000 Mayo Clinic Biobank8 participants who have a high likelihood that PGx information will be useful to their care within a 1–3 year window. This approach is justified given the relatively small sample size for this preemptive genotyping project and the need to optimize the number of “events” (i.e. drug-gene pairing) during a limited follow-up period. Second, deploy a PGx panel test that includes the Next-Generation Sequencing (NGS) reagent developed by the PGRN (PGRN-Seq)9 that captures 84 pharmacogenes (Supplemental Table 1) and CYP2D6 genotyping in a Clinical Laboratory Improvement Amendments (CLIA) and College of American Pathologists (CAP) certified environment and integrate clinically actionable variants with existing clinical data in the patient EMR. Third, develop and implement CDS at the point-of-care for clinically actionable PGx variants. Overarching these aims is the evaluation of objective specific metrics to begin to form a narrative for best-practices of genomic medicine implementation.
Prediction Model Development
Given the limited sample size for this pilot project, a prediction model was developed to target a population of patients with a high likelihood of being prescribed a commonly used drug and, thus who might benefit in the near term from preemptive genotyping. Statin medication was chosen for the model because these are commonly prescribed and have an actionable PGx variant.11 Complete details of the model development and performance are included in the supplementary materials.
Community Advisory Board (CAB) Review of the Recruitment and Consenting Process
The Mayo Clinic Biobank CAB, formed in 2010 as the result of recommendations from an earlier deliberative community engagement event, is one mechanism that the Mayo Clinic Center for Individualized Medicine has in place to obtain input and feedback from members of the local public. CAB members, have overtime, acquired awareness and appreciation of the ethical, legal, social implications associated with biobanking, data sharing, and genetic and genomic research. Because of their experiences in providing recommendations to the Mayo Clinic Biobank leadership, we sought input from CAB members on recruitment and consenting materials for the RIGHT Protocol. Members were sent the materials prior to a regularly scheduled meeting and asked to critically review them. At the meeting, the group was split in two and a focus group approach was used by two trained moderators to assess CAB members understanding of the materials and solicit feedback on ways to improve them. In addition, questions were designed to explore CAB members’ hopes, concerns, and expectations about the RIGHT study specifically, and more generally, about the use of PGx in clinical practice. Categories of discussion raised in the moderator’s guide included participants’ response to receiving the RIGHT protocol materials, their concerns and questions regarding the materials, how informative the materials were, who they would want more information from, what would motivate them to participate, expectations for the study, and what method of recruitment they would prefer. All discussions were audio-recorded, transcribed, and de-identified. Recommendations from the CAB were incorporated into the final version of the RIGHT Protocol study materials.
Recruitment
This prediction model developed and validated using data from the REP was applied to the Mayo Clinic Biobank Cohort, details have been previously described.8 In brief, Mayo Clinic Biobank started participant recruitment on April 1, 2009 with an enrollment goal of 50,000 participants with health data (e.g. self-reported questionnaire and EMR data) and biological sample (e.g. serum, plasma, DNA) collections. Biobank participants who were eligible for the study met the following criteria: a predicted risk for statin use of 0.35 or higher, less than 70 years of age, empanelled in within the Mayo Clinic primary care practice, and had no previous prescription history of warfarin, statin, or clopidogrel. Biobank staff mailed an invitation to participate, a brochure outlining study specifics, and a consent form to 2000 participants meeting these criteria. If no response was received after a period of 4 weeks, one additional attempt was made with a second identical mailing. The Center for Individualized Medicine created an information call center to triage questions pertaining to the study. Subjects who chose to participate in the study returned a signed consent form and agreed to a blood draw with no remuneration offered. A venipuncture order was then placed by a study coordinator and subjects were mailed a request asking that they complete the blood draw at one of several local clinical venipuncture sites within 30 days.
PGx Sequencing and Genotyping Approach
Preemptive PGx testing in the RIGHT protocol is conducted using clinically validated methods performed jointly by the CLIA-certified and CAP accredited Mayo Clinic Clinical Genome Sequencing Laboratory (CGSL) and Personalized Genomics Laboratory (PGL). PGx testing requires a combination of NGS and allele specific PCR methods due to the genomic complexity of the CYP2D6 locus, the latter of which cannot be reliably determined by current short read NGS platforms. The PGRN-Seq capture reagent was designed to capture 84 PGx relevant genes using the NimbleGen in-solution custom capture method and CYP2D6 was genotyped using the Luminex CYP2D6 ASPE kit v2. The CYP2D6 phenotype is predicted based upon the number of functional, partially functional, and nonfunctional alleles present in a sample. Supplemental Table 2 summarizes the phenotyping algorithm used by the PGL. There are instances in which a phenotype prediction is not categorical and, in these cases, a range of possible phenotypes will be given. It should be noted that other laboratories might use different phenotype prediction methods as there is no consensus at this time. Clinical validation of this combined method was performed jointly by the Mayo CGSL and PGL. Complete details of the lab methods are included in the supplemental materials.
Clinical and Research Sequence Analyses
The bioinformatics analysis is carried out using parallel methods, with the data passing through Mayo’s CLIA compliant bioinformatics analysis pipeline for interpretation of the clinically actionable variants approved for EMR deposition and through Mayo’s research bioinformatics analysis pipeline for further investigation of the entire PGRN panel. The clinical sequencing pipeline accepts data directly from the Illumina sequencers and passes it to CASAVA, an Illumina supplied tool, to generate the raw FASTQ sequence reads (see supplemental methods for further details). CASAVA is also used for sample de-multiplexing. The subsequent FASTQ read files are processed by CLC Bio’s Server software, v4.1, which carries out read realignment and sequence mutation detection (single-nucleotide variants, SNVs; small insertions and deletions, INDELs). SNV calling is carried out using a neighborhood quality standard algorithm with a neighborhood minimum quality score of 15, maximum gap penalty of 2, a neighborhood radius of 5, and minimum variant base quality of 20. FastQC is used to interrogate the quality of individual sample reads, with results passed into our interpretative environment, the NGS Workbench. The NGS Workbench is an internally Mayo Clinic developed program that facilitates results interpretation. It runs a series of commands to annotate variants, and stores both the automatic annotation and expertuser annotations within a relational database for subsequent recall. It also presents a summary of all QC metrics, target region coverage data, and facilitates the generation of a summary report of actionable variants. All variants that pass the bioinformatics pipeline QC thresholds are then loaded into the clinical sequence database, Oracle’s Translation Research Platform (see Supplemental Materials for complete details). These variants include those approved for clinical use and those that may, in the future, be approved by the Task Force for use in clinical care.
Return of Clinical Results
Consented participants agreed to have clinically actionable PGx results placed into their EMR and individual results are available to patients online at their Mayo Clinic Online Patient Services account. Genetic counseling services will be made available if required.
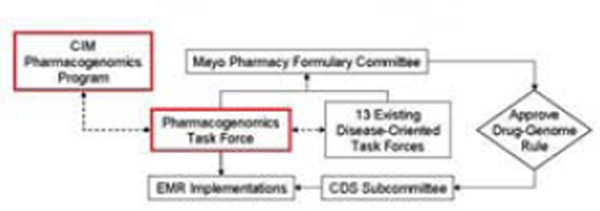
Pharmacogenomics Task Force Process
The Mayo Clinic Center for Individualized Medicine Pharmacogenomics Task Force was established to provide institutional oversight for the selection and clinical implementation of PGx “drug-gene” pairs and for the development of CDS. This Task Force considers drug-gene pairs from the FDA list of PGx biomarkers, PharmGKB12 listing of genes and drugs, Indiana University Drug Interactions website,13 articles published on the subject of PGx, and current PGx tests offered through the Mayo Clinic’s Department of Laboratory Medicine and Pathology. Candidate drug-gene pairs are stratified as follows: 1) drug toxicity or drug non-response risk to patient, 2) strength of support in the literature (i.e. quality and quantity of articles, number of subjects, presence of prospective studies, and presence of studies involving medical and economic benefit), 3) range of use among medical specialties (i.e. number of specialties using a medication), 4) volume of drug use, and 5) existence of protocol/practice guidelines (i.e. PGRN Clinical Pharmacogenetics Implementation Consortium14 and other medical society guidelines). Supporting documentation for CDS rules is reviewed by the Task Force, and if approved, reviewed by the appropriate Disease Oriented Task Force under the Mayo Pharmacy Formulary Committee (Figure 1). Implementation of the rules is considered by the Mayo Clinical Decision Support Subcommittee and the Pharmacy and Therapeutics Committee. Educational content for prescribers is generated to provide “just in time” education about drug gene pairs through the “AskMayoExpert” system—a home-grown Mayo knowledge content system for delivering context-specific clinical informational content to providers.
Figure 1.
Mayo Clinic Center for Individualized Medicine (CIM) Process for Clinical Implementation of Pharmacogenomic Rules
Clinical Decision Support (CDS) Development
The CDS Program at Mayo Clinic is charged to promote and coordinate common development, implementation, evaluation, and maintenance of computer-based CDS across the continuum of medical care. A multidisciplinary team (clinical experts, informatics, IT) defines the functional and technical specifications of the rules that will execute in real-time to provide active CDS. The specifications comprise all the necessary information to develop, test, implement, and maintain the CDS rules, including knowledge translation, workflow analysis, data mapping and log specifications. PGx alerts are developed in the computerized physician order entry (CPOE) applications for inpatient and outpatient settings. Actionable PGx variants are converted into a standard notation and interpretation (e.g., HLA-B*1502 test positive), and stored in the EMR as a molecular diagnostic laboratory result. This result is used to trigger the CDS rules. The alerts and reminders are designed to deliver the necessary information and facilitate further actions including a link to additional related information in “AskMayoExpert”. PGx CDS rules are implemented in the laboratory review context (e.g., support for interpreting laboratory results) and the medication order entry context (e.g., support for prescribing decisions), and provide actionable alert messages, based on the post-condition(s) for the rule using recommendations from the FDA, CPIC, and Mayo Clinic PGx Task Force. “Alert fatigue” is also considered in the design and exclusion criteria are included in the rules to avoid unnecessary repetitive alerts. Transactional data associated to the alerts is collected to assess performance and clinical impact of the rules.
Patient and Prescriber Education
Though primary care physicians have a major part in offering and educating patients about PGx testing, lack of formal education and experience are key barriers to its adoption in routine clinical practice.15 In our study approach, information on drug-gene pairs is available and linked to CDS rules to provide “just-in-time” support at the point-of-care. Prescribers are alerted by the ordering system when prescribing a medication for which a PGx test result is available. This presents a challenge to quickly educate hundreds of providers and patients alike. To address this issue, we are conducting focus groups with clinicians to better understand the types of education resources and modes they prefer and would find most beneficial in their integration of pre-emptive PGx into their clinical practice. RIGHT participants will receive education materials describing specific drug-gene pairs, information about the PGx testing, result interpretation, and test limitations. This information will be accessible via their Online Patient Services account. Patient education materials are developed in accordance with institutional plain language and readability standards, and submitted to the CAB for review and comments. A link to patient education materials is also available to the ordering provider via AskMayoExpert for immediate printing and dissemination to patients.
RIGHT Pilot Project Evaluation
The ultimate goal of clinical implementation of PGx at the bedside is to avoid adverse drug reactions, maximize drug efficacy, and select medications, which based on the genetic profile of individual patients, produce optimal effects for specific indications. However, well powered investigations to study these goals are not feasible within the relatively small sample size of the RIGHT Study. Therefore, outcome measures to evaluate the success of this initial PGx translation effort will focus on assessing the impact on clinical practice and patient care process and the impact on institutional health care structure (Table 5).
Table 5.
RIGHT Protocol Evaluation: Clinical Implementation Outcome Measures
| Impact on clinical practice and patient care process |
|
| Impact on institutional healthcare structure |
|
RESULTS
A total of 2000 Biobank participants were invited to the study. Fifty percent consented and provided blood samples, while 13% refused (Table 1). We observed that more females responded to the study invitation. Participants who did not consent had more baseline medical conditions related to high lipid levels. While median predicted risk of initiating statin therapy was similar among consented, refused, and non-responders, non-responders had a higher proportion of subjects with a predicted risk of 50% or higher (196 (35%) for non-responders vs. 420 (29%) for consented and refused subjects). Frequent prior use of drugs for which the FDA has incorporated genomic information into drug labels illustrates the extent to which preemptive information, had it been available at the time of prescription, could have been used for clinical care. Table 2 lists the top 20 most frequent drugs, for which PGx information is included in the FDA drug label that were prescribed to the RIGHT participants from August 1994 – April 2013.
TABLE 1.
Baseline Characteristics of Those Invited to Participate in the RIGHT Protocol
| Consented (with sample) |
Consented (without sample) a |
Refused | Non-Responders | |
|---|---|---|---|---|
| n | 1013 (51%) | 176 (9%) | 256 (13%) | 555 (28%) |
| Sex, % male | 475 (47%) | 76 (43%) | 114 (44%) | 304 (55%) |
| Race, % White | 867 (86%) | 155 (88%) | 217 (85%) | 466 (84%) |
| Median Age, years | 56 | 53 | 55 | 54 |
| (25th and 75th percentiles) | (52, 59) | (50, 57) | (52, 59) | (50, 58) |
| Presence of Clinical Classification Code | ||||
| Dyslipidemia, % | 482 (48%) | 74 (42%) | 142 (55%) | 295 (53%) |
| Diabetes, % | 441 (44%) | 79 (45%) | 110 (43%) | 267 (48%) |
| Peripheral atherosclerosis, % | 95 (9%) | 13 (7%) | 29 (11%) | 51 (9%) |
| Diseases of the blood-forming organs, % | 231 (23%) | 37 (21%) | 55 (21%) | 106 (19%) |
| Coronary atherosclerosis and other heart diseases, % | 124 (12%) | 9 (5%) | 27 (10%) | 69 (12%) |
| Hypertension, % | 418 (41%) | 65 (37%) | 117 (45%) | 243 (44%) |
| Predicted risk, median | 0.44 | 0.42 | 0.44 | 0.45 |
| (25th 75th percentiles) | (0.38, 0.52) | (0.37, 0.50) | (0.37, 0.52) | (0.40, 0.54) |
Those that did not provide a DNA sample within the recruitment period were contacted and informed that they were not included in the study.
TABLE 2.
Prescription History for Drugs with Pharmacogenomic Information from the FDA from August 1994 – April 2013 for the 1013 RIGHT Participants
| Rank | Drug Name | N | % |
|---|---|---|---|
| 1 | Tramadol and Acetaminophen | 758 | 75 |
| 2 | Omeprazole | 689 | 68 |
| 3 | Codeine | 491 | 49 |
| 4 | Pantoprazole | 294 | 29 |
| 5 | Citalopram | 232 | 23 |
| 6 | Venlafaxine | 162 | 16 |
| 7 | Warfarin | 162 | 16 |
| 8 | Nortriptyline | 160 | 16 |
| 9 | Metoprolol | 154 | 15 |
| 10 | Chlordiazepoxide and Amitriptyline | 144 | 14 |
| 11 | Fluoxetine | 135 | 13 |
| 12 | Celecoxib | 126 | 12 |
| 13 | Terbinafine | 119 | 12 |
| 14 | Clozapine | 117 | 12 |
| 15 | Diazepam | 117 | 12 |
| 16 | Atorvastatin | 105 | 10 |
| 17 | Paroxetine | 101 | 10 |
| 18 | Thioguanine | 94 | 9 |
| 19 | Rabeprazole | 71 | 7 |
| 20 | Tamoxifen | 70 | 7 |
As of July 2013, the Task Force approved and CDS rules have been developed and implemented for the following drug-gene pairs; carbamazepine/HLA-B*1502, abacavir/HLA-B*5701, thiopurines/TPMT, and interferon/IL28B. On-going expansion of PGx-CDS efforts include CYP2D6 (codeine, tamoxifen, and tramadol), CYP2C19 (clopidogrel), and SLCO1B1 (simvastatin).
Preemptive sequencing is underway for all participants in our CLIA-certified and CAP accredited clinical laboratories. Genotyping of the CYP2D6 locus has been completed for all 1013 participants and Table 3 list the allele frequencies and Table 4 summarizes the phenotype interpretation based on those genetic results. For NGS, we conducted clinical validation of the PGRN-Seq reagent by confirming a minimum of 10 patient samples for each variant using a previously validated genotyping method. For CYP2C19, CYP2C9 and VKORC1 confirmation, Sanger sequencing was used. For SLCO1B1 confirmation, a TaqMan Qualitative Assays on the ABI StepOnePlus and 7500 Fast was used.
TABLE 3.
CYP2D6 Counts and Allele Frequencies
| Allele | N | Allele Frequency (%) |
|---|---|---|
| *1 | 748 | 40 |
| *2 | 13 | <1 |
| *2A | 465 | 23 |
| *3 | 29 | 1.4 |
| *4 | 398 | 20 |
| *5 | 79 | 3.9 |
| *6 | 19 | 1 |
| *7 | 0 | 0 |
| *8 | 1 | <1 |
| *9 | 51 | 2.5 |
| *10 | 35 | 1.7 |
| *11 | 0 | 0 |
| *12 | 0 | 0 |
| *14A | 0 | 0 |
| *14B | 2 | <1 |
| *15 | 1 | <1 |
| *17 | 3 | <1 |
| *41 | 180 | 9 |
| Duplications | 39 | 3.8 |
TABLE 4.
CYP2D6 Phenotypes for RIGHT Protocol Participants
| CYP2D6 Metabolizer Phenotype | N (%) |
|---|---|
| Ultra-rapid | 83 (8%) |
| Extensive to Ultra-rapid | 162 (16%) |
| Extensive | 203 (20%) |
| Intermediate to Ultra-rapid | 1 (<1%) |
| Intermediate to Extensive | 199 (20%) |
| Intermediate | 217 (21%) |
| Poor to Intermediate | 71 (7%) |
| Poor | 77 (8%) |
DISCUSSION
Numerous PGx variants with demonstrated clinical utility have been identified, opening the way for the application of this genomic information to help individualize drug use for optimal outcomes. As a result, the routine integration of PGx data into drug therapy decision-making has the potential to reduce healthcare costs and improve patient outcomes, safety, satisfaction, and quality. However, even though the U.S. Food and Drug Administration (FDA) has provided extensive information on pharmacogenomics, including “black box warnings” linking genetic variants to clinically important variation in drug response, the implementation and integration of PGx into routine clinical care has been slow. The RIGHT Protocol pilot has recruited 1013 participants for PGx genomic testing with the goal of developing best practices for wider implementation of PGx.
The current clinical paradigm of ordering specific point-of-care PGx testing during the initiation of a treatment regimen can be expensive and often leads to delays in therapeutic decision-making thus contributing to the lack of acceptance by prescribers. The RIGHT Protocol is designed to address these challenges by deploying preemptive genomic testing. A key advantage to this approach is that we can accurately genotype the regions of known clinical utility and thousands of other regions that might become relevant in the future. In this preemptive paradigm, additional variants approved by the Task Force that can be validated from the existing genomic data from the PGx Panel test can be updated in the patient’s EMR without the need for additional specimen collection and testing. CDS would fire at the time of prescription order for each patient with genetic data available. Furthermore, our approach in adopting the recommendation from the New York Department of Health allows us to eliminate costly Sanger verification once each variant found is confirmed in 10 unique samples (http://www.wadsworth.org/labcert/TestApproval/forms/NextGenSeq_ONCO_Guidelines.pdf). Thus, significant time and expense are saved by adopting this conventionally accepted verification approach.
CONCLUSION
This translational project provides an opportunity to begin to evaluate the impact of preemptive sequencing and EMR-driven genome-guided therapy. PGx CDS tools implemented and validated for use in the point-of-care setting as part of this effort will lay the foundation of future work to other gene variants to examine their potential for the development of further CDS. Furthermore, expansion of the study sample that is more representative of the patient population (i.e. not specific to statin risk) is warranted given the frequent use of drugs with PGx indications that are used throughout adulthood. We hypothesize that the knowledge and tools gained from this pilot study will enhance our ability to do comparative effectiveness studies with improved design specificity built into overall less costly study protocols. The results of this and future studies in this area, including a broader dissemination of our findings and experience in community based healthcare settings, could lead to more cost-efficient use of national health care dollars and result in improved health outcomes for patients and a decrease in the overall cost of delivery health care in key areas of disease management.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the support and efforts of our staff including study coordinators Ellen Koepsell and laboratory personnel Mary Beth Karow, Brenda Moore, Laura Train, Susan Lagerstedt, Kimberley Harris, Paul Heimgartner, Jessica Vander Pol, Ann Wimmer, Kathleen Wingate, Alexander Reberg, and Brian A. Dukek.
This work was supported in part by Mayo Clinic Center for Individualized Medicine, National Institutes of Health grants U19 GM61388 (The Pharmacogenomics Research Network), R01 GM28157, U01 HG005137, R01 CA138461, R01 AG034676 (The Rochester Epidemiology Project), and U01 HG06379 and U01 HG06379 Supplement (The Electronic Medical Record and Genomics (eMERGE) Network).
Abbreviations and Acronyms
- CAB
Community Advisory Board
- CAP
College of American Pathologists
- CCS
Clinical Classifications Software
- CDS
clinical decision support
- CLIA
Clinical Laboratory Improvement Amendments
- EMR
electronic medical record
- EMERGE
Electronic Medical Record and Genomics Network
- FDA
Food and Drug Administration
- NGS
Next-Generation Sequencing
- PGL
Personalized Genomics Laboratory
- PGRN
Pharmacogenomics Research Network
- PGRN-Seq
Pharmacogenomics Research Network Sequencing
- PGx
Pharmacogenomics
- RIGHT
Right Drug, Right Dose, Right Time – Pharmacogenomics to Individualize Treatment
- SNV
single-nucleotide variants
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Weinshilboum R. Inheritance and drug response. N Engl J Med. 2003;348(6):529–537. doi: 10.1056/NEJMra020021. [DOI] [PubMed] [Google Scholar]
- 2.Weinshilboum R, Wang L. Pharmacogenomics: bench to bedside. Nat Rev Drug Disc. 2004;3(9):739–748. doi: 10.1038/nrd1497. [DOI] [PubMed] [Google Scholar]
- 3.Weinshilboum RM, Wang L. Pharmacogenetics and pharmacogenomics: development, science, and translation. Annu Rev of Genomics Hum Genet. 2006;7:223–245. doi: 10.1146/annurev.genom.6.080604.162315. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med. 2011;364(12):1144–1153. doi: 10.1056/NEJMra1010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration. Table of pharmacogenomic biomarkers in drug labels. http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.Htm.
- 6.Farrugia G, Weinshilboum RM. Challenges in implementing genomic medicine: The Mayo Clinic Center for Individualized Medicine. Clin Pharmacol Ther. 2013;94(2):204–206. doi: 10.1038/clpt.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kho AN, Pacheco JA, Peissig PL, et al. Electronic medical records for genetic research: results of the eMERGE consortium. Sci Transl Med. 2011;3(79) doi: 10.1126/scitranslmed.3001807. 79re71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson JE, Ryu E, Johnson KJ, et al. The Mayo Clinic Biobank: a building block for individualized medicine. Mayo Clin Proc. 2013;88(9):952–962. doi: 10.1016/j.mayocp.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon AS, Smith JD, Xiang Q, et al. New sequencing-based platform for high-throughput pharmacogenomic implementation and discovery. Presented at the 62nd Annual Meeting of The American Society of Human Genetics; November, 7,2012; San Francisco, California. (Program #244) http://www.ashg.org/2012meeting/abstracts/fulltext/f120122669.htm. [Google Scholar]
- 10.Rocca WA, Yawn BP, St. Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilke RA, Ramsey LB, Johnson SG, et al. The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastat-ininduced myopathy. Clin Pharmacol Ther. 2012;92(1):112–117. doi: 10.1038/clpt.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altman RB. PharmGKB: a logical home for knowledge relating genotype to drug response phenotype. Nat Genet. 2007;39(4):426. doi: 10.1038/ng0407-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flockhart DA. Drug interactions: Cytochrome p450 drug interaction table. Indiana University School of Medicine; Http://medicine.Iupui.Edu/clinpharm/ddis/clinical-table/2007. [Google Scholar]
- 14.Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther. 2011;89(3):464–467. doi: 10.1038/clpt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haga SB, Burke W, Ginsburg GS, Mills R, Agans R. Primary care physicians' knowledge of and experience with pharmacogenetic testing. Clin Genet. 2012;82(4):388–394. doi: 10.1111/j.1399-0004.2012.01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.