Abstract
Objectives:
Chronic cerebrospinal venous insufficiency (CCSVI) has recently been implicated in the pathogenesis of multiple sclerosis (MS). This comprehensive meta-analysis of case–control studies investigates the association of CCSVI with MS.
Methods:
Through Medline, EMBASE and Cochrane database searches, case–control ultrasound studies comparing CCSVI frequency among patients with MS and healthy controls were identified.
Results:
We identified 19 eligible studies including 1250 patients with MS and 899 healthy controls. The pooled analysis showed that CCSVI was associated with MS [odds ratio (OR) 8.35; 95% confidence interval (CI) 3.44–20.31; p < 0.001) with considerable heterogeneity across studies (I2 = 80.1%). This association was substantially attenuated in sensitivity analyses excluding studies that were carried out by the group that originally described CCSVI, included investigators who had also been involved in publications advocating endovascular procedures for CCSVI treatment, or were conducted in Italy. Our most conservative sensitivity analysis combining different exclusion criteria yielded no association of CCSVI with MS (OR 1.35; 95% CI 0.62–2.93; p = 0.453) without any heterogeneity (I2 = 0%).
Conclusion:
There is considerable heterogeneity across different case–control studies evaluating the association of CCSVI and MS. The greatest factor contributing to this heterogeneity appears to be the involvement of investigators in other publications supporting endovascular procedures as a novel MS treatment.
Keywords: chronic cerebrospinal venous insufficiency, meta-analysis, multiple sclerosis, ultrasound
Introduction
A chronic state of impaired venous drainage from the central nervous system (CNS), termed chronic cerebrospinal venous insufficiency (CCSVI), has recently been proposed by Zamboni and colleagues to be causally implicated in the pathogenesis of multiple sclerosis (MS) [Zamboni et al. 2009a, 2009c, 2009d]. More specifically, the hypothesis postulates that multiple extracranial abnormalities (stenoses/obstructions) in the internal jugular vein (IJV) or azygous vein may cause a venous reflux in the cerebrospinal compartment, leading to increased intracranial intravenous pressure, followed by blood–brain barrier breakdown, perivenous iron deposition and inflammation of the CNS [Zamboni, 2006]. After introducing a set of five ultrasound criteria yielding 100% sensitivity and specificity in CCSVI detection in their pivotal study [Zamboni et al. 2009c], the group has recently developed a detailed neurosonology protocol with standard criteria to optimize CCSVI detection. Moreover, based on the assumption that CCSVI plays a causative role in MS, they conducted a small trial performing percutaneous transluminal angioplasty of extracranial veins in a cohort of patients with MS. In this study, which was not randomized and not blinded, the authors concluded that the interventional procedure was safe and potentially effective in improving clinical and quality of life parameters [Zamboni et al. 2009b]. Despite the lack of higher-class evidence, this treatment (‘Liberation procedure’) has gained a considerable amount of attention and emotional comments by patients with MS worldwide [Chafe et al. 2011] and has started to be tested in patients with MS in nonrandomized, uncontrolled and unblinded studies [Ludyga et al. 2010; Hubbard et al. 2012; Denislic et al. 2012]. Only recently, a group of investigators led by Zamboni has embarked in a multicenter, randomized, blinded, parallel-group, sham-controlled trial aiming to assess the safety and efficacy of the Liberation procedure in a sample of 679 patients with MS [Zamboni et al. 2012b].
Other independent ultrasound studies evaluating the association of CCSVI with MS have yielded widely inconsistent findings (see studies in Table 1). In addition, there are scarce data regarding the reproducibility of the proposed ultrasound criteria for CCSVI detection [Zivadinov et al. 2011; Tsivgoulis et al. 2011; Menegatti et al. 2010]. Moreover, the etio-pathogenetic role of CCSVI in MS has not been confirmed by additional reports using other neuroimaging modalities to evaluate abnormal CNS venous drainage, including phase-contrast magnetic resonance imaging (MRI) [Supplemental References s-1, s-2] or selective venography [Supplemental Reference s-3]. Furthermore, endovascular treatments based on the former hypothesis have been complicated by serious adverse events [Denislic et al. 2012; Supplemental References, s-4, s-5, s-6, s-7]. The lack of definitive evidence confirming the causative association of CCSVI with MS in combination with the absence of randomized data to support invasive and potentially hazardous endovascular procedures have led to substantial challenge of the CCSVI hypothesis by position papers and international scientific organizations [Reekers et al. 2011; Baracchini et al. 2012].
Table 1.
Baseline characteristics of patients with multiple sclerosis (MS) and healthy controls investigated in the case–control studies that were included in the present meta-analysis.
| Study | Patients with MS |
Healthy controls |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | CIS |
RRMS |
SPMS/PPMS |
Mean age, years | Women |
Total | Mean age, years | Women |
||||||
| n | % | n | % | n | % | n | % | n | % | |||||
| Zamboni et al. [2009c] | 109 | 0 | 0 | 69 | 63 | 40 | 37 | 40 | 64 | 59 | 132 | 37/58* | 75 | 57 |
| Zamboni et al. [2009d] | 16 | 0 | 0 | 16 | 100 | 0 | 0 | 35 | 10 | 63 | 8 | 32 | 6 | 75 |
| Al-Omari and Rousan [2010] | 25 | 0 | 0 | 21 | 84 | 4 | 16 | 35 | 13 | 52 | 25 | 34 | 13 | 52 |
| Doepp et al. [2010] | 56 | 0 | 0 | 41 | 73 | 15 | 27 | 42 | 36 | 64 | 20 | 41 | 12 | 60 |
| Krogias et al. [2010] | 10 | 0 | 0 | 2 | 20 | 8 | 80 | 42 | 3 | 30 | 2 | 38 | 1 | 50 |
| Baracchini et al. [2011a] | 50 | 50 | 100 | 0 | 0 | 0 | 0 | 33 | 35 | 70 | 110 | 33/63$ | 67 | 61 |
| Centonze et al. [2011] | 84 | 0 | 0 | 69 | 82 | 15 | 18 | 39 | 52 | 62 | 56 | 42 | 36 | 64 |
| Zivadinov et al. [2011] | 304 | 21 | 7 | 191 | 63 | 92 | 30 | 48 | 230 | 76 | 163 | 46 | 88 | 54 |
| Mayer et al. [2011] | 20 | 0 | 0 | 17 | 85 | 3 | 15 | 42 | 13 | 65 | 20 | 34 | 10 | 50 |
| Tsivgoulis et al. [2011] | 42 | 0 | 0 | 38 | 90 | 4 | 10 | 39 | 25 | 60 | 43 | 38 | 27 | 63 |
| Auriel et al. [2011] | 27 | 0 | 0 | 21 | 78 | 6 | 22 | 42 | 17 | 63 | 32 | 36 | 23 | 72 |
| Baracchini et al. [2011a] | 60 | 0 | 0 | 0 | 0 | 60 | 100 | 46 | 33 | 55 | 60 | 46 | 33 | 55 |
| Marder et al. [2011] | 18 | 1 | 6 | 6 | 33 | 11 | 61 | 55 | 3 | 17 | 11 | 55 | 4 | 36 |
| Blinkenberg et al. [2012] | 24 | 0 | 0 | 24 | 100 | 0 | 0 | 37 | 16 | 67 | 15 | 37 | 11 | 73 |
| Zamboni et al. [2012b] | 44 | NA | NA | NA | NA | NA | NA | 41 | 25 | 57 | 40 | 41 | 18 | 45 |
| Kantarci et al. [2012] | 62 | 0 | 0 | 32 | 52 | 30 | 48 | 37 | 40 | 65 | 54 | 37 | 27 | 50 |
| Amato et al. [2012] | 15 | 0 | 0 | 15 | 100 | 0 | 0 | 18 | 9 | 60 | 16 | 18 | 7 | 44 |
| Mancini et al. [2012] | 103 | 0 | 0 | 41 | 40 | 62 | 60 | 42 | 62 | 60 | 42 | 38 | 23 | 55 |
| Zaniewski et al. [2013] | 181 | 0 | 0 | 98 | 54 | 83 | 46 | 41 | 110 | 61 | 50 | 40 | 31 | 62 |
CIS, clinically isolated syndrome; NA, not available; PPMS, primary progressive multiple sclerosis; RRMS, relapsing–remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis.
Two control groups were used in the study. The mean age was 37 and 58 years in the first (n = 60) and second (n = 72) control group, respectively.
Two control groups were used in the study. The mean age was 33 and 63 years in the first (n = 50) and second (n = 60) control group, respectively.
Here we set out to perform a comprehensive meta-analysis of case–control ultrasound studies that have investigated the association of CCSVI with MS. We also attempted to interpret heterogeneity across different trials by conducting a rich compendium of sensitivity analyses in order to investigate potential, clinically and methodologically meaningful sources of heterogeneity.
Methods
Trial identification and data abstraction
This meta-analysis has adopted the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews and meta-analyses [Supplemental Reference, s-9]. Eligible articles reporting the prevalence of CCSVI diagnosed using neurosonology criteria (Supplemental Table 1) among patients with MS and among healthy controls were identified by searching MEDLINE, EMBASE, the CENTRAL Register of Controlled Trials and the Cochrane Database of Systematic Reviews databases. Studies published from January 2006 to June 2012 were sought using the following combination of search strings: ‘multiple sclerosis’ AND ‘(veins OR venous insufficiency)’ AND (‘ultrasound OR sonography’). No language restrictions were imposed. Reference lists of all articles that met the criteria and of relevant review articles were examined to identify studies that may have been missed by the database search. Further details regarding the eligibility and selection of studies included in the present meta-analysis are provided in the online supplemental material.
Standard protocol approvals, registration and consents
The study methods have been reviewed and approved by the institutional review boards of all participating institutions.
Statistical analyses
Odds ratios (ORs) were calculated to express the comparison of patients with MS versus controls; OR values larger than 1 denote a positive association between MS and CCSVI diagnosed by at least two ultrasound criteria. For studies with a zero cell we used a continuity correction of 0.5, as appropriate [Supplemental Reference s-9]. We also investigated the association of each individual ultrasound criterion with the diagnosis of MS. The five ultrasound criteria proposed by Zamboni and colleagues for CCSVI diagnosis are briefly described in Supplemental Table 1.
The fixed-effects model (Mantel–Haenszel method) or the random effects (DerSimonian Laird) model were used to calculate the pooled ORs. The equivalent z test was performed for each pooled OR; p < 0.05 was considered statistically significant. Heterogeneity between studies was assessed by the Cochran Q and I2 statistic [Supplemental Reference s-10]. In case of statistically significant heterogeneity (p value derived from Cochran Q < 0.1, irrespective of the I2 estimation), random effects models were employed to allow for it. For the qualitative interpretation of heterogeneity, I2 values of at least 50% are usually considered to represent substantial heterogeneity, while values of at least 75% indicate considerable heterogeneity according to the Cochrane Handbook [Supplemental Reference s-11].
To test the robustness of the main results and to investigate potential sources of heterogeneity, we conducted a series of predefined sensitivity analyses. First, we removed all studies that included Zamboni as a coauthor or in which the authors have previously participated in original reports by Zamboni’s group introducing the association of CCSVI with MS. Second, to minimize differences in ultrasound techniques we excluded all studies that were unable to assess all five ultrasound criteria. Third, to minimize potential conflicts of interest we excluded all studies whose authors were involved in trials of endovascular treatment for CCSVI or whose authors had endorsed the Liberation procedure in their clinical management of patients with MS [Supplemental References s-12, s-13, s-14, s-15]. Fourth, given the geographical variations in MS prevalence indicating a latitudinal incidence gradient [Supplemental Reference s-16] and after taking into account the large representation of Italian studies in the present meta-analysis, we repeated all analyses after removing studies that had been conducted in Italy. In our final, combined sensitivity analysis we pooled criteria applied to the third and fourth sensitivity analyses. Publication bias was assessed at the overall analysis, to maximize the power of the test; Egger’s statistical test was performed [Supplemental Reference s-17]. All analyses were conducted using STATA 11.1 (STATA Corp., College Station, TX, USA).
Results
Characteristics of eligible studies
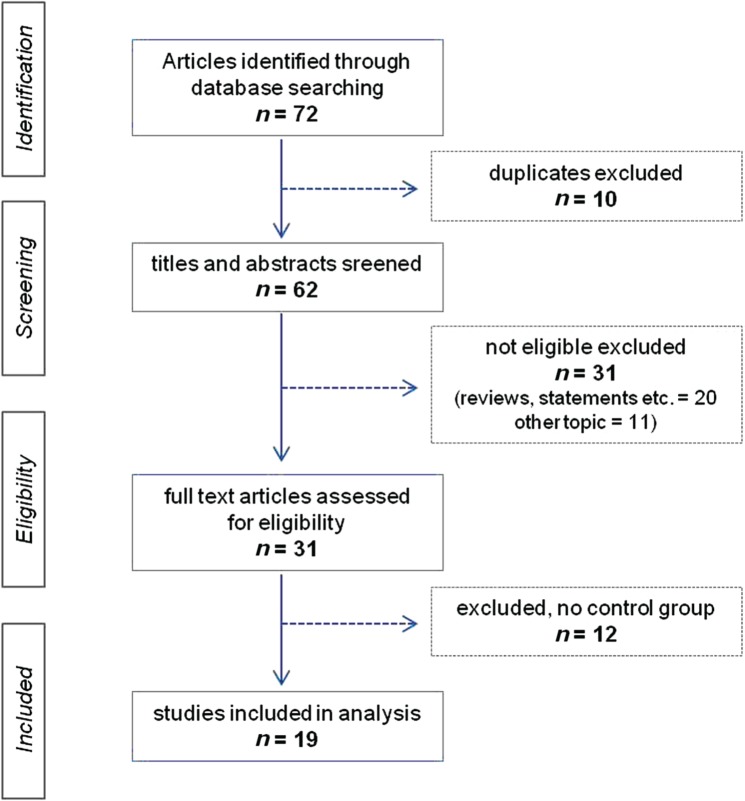
The first step of the database search identified 72 studies; 65 hits were achieved from the EMBASE database. Excluding 10 duplicate studies, the remaining 62 were screened for eligibility criteria. Potentially eligible studies for the meta-analysis (n = 31) were retained; we identified no control group in 12 studies that were consequently excluded. Data were extracted from the full-text version of the remaining 19 studies (see studies in Table 1 and flow diagram in Figure 1).
Figure 1.
Flow chart presenting the selection of eligible studies.
A total of 19 case–control studies meeting our prespecified inclusion criteria were identified. Supplemental Table 2 summarizes the characteristics of the included studies. There were eight studies from Italy, three from the USA and three from Germany. The remaining studies were conducted in Poland (n = 1), Greece (n = 1), Turkey (n = 1), Jordan (n = 1), Israel (n = 1) and Denmark (n = 1). The total number of patients with MS and controls included in the present meta-analysis was 1250 and 899 respectively. The association of all five ultrasound criteria with MS was not reported in four studies [Al-Omari and Rousan, 2010; Centonze et al. 2011; Tsivgoulis et al. 2011; Zaniewski et al. 2013] in which the investigators were unable to perform the complete neurosonology protocol. Finally, the investigators of six ultrasound studies [Zamboni et al. 2009a, 2009c, 2012; Al-Omari and Rousan, 2010; Zivadinov et al. 2011; Amato et al. 2012] were also involved in reports advocating the safety and efficacy of the Liberation procedure [Chafe et al. 2011; Supplemental References, s-7, s-13, s-14]. The lead author of one study was commercially advertising the service of the Liberation procedure for patients with MS with CCSVI [Supplemental References, s-14].
Baseline characteristics of patients with MS and control individuals are shown in Table 1. The limited data available on intra- and inter-rater reproducibility of the ultrasound protocol are summarized in Supplemental Table 3. Only one study [Tsivgoulis et al. 2011] described a run-in period during which intra- and inter-observer reliability was determined to be satisfactory in three out of five neurosonology criteria and poor in the remaining two. The intra-rater reproducibility was assessed in another study and was reported to be good. However, this investigation did not formally investigate the inter-rater reproducibility [Zivadinov et al. 2011].
Pooling of studies
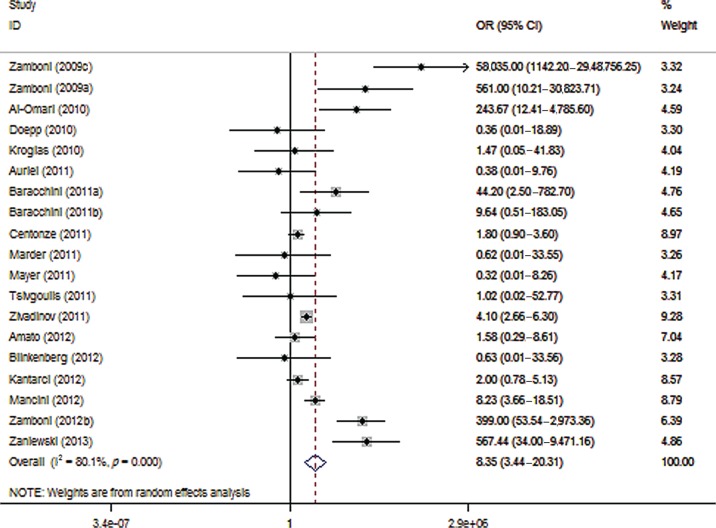
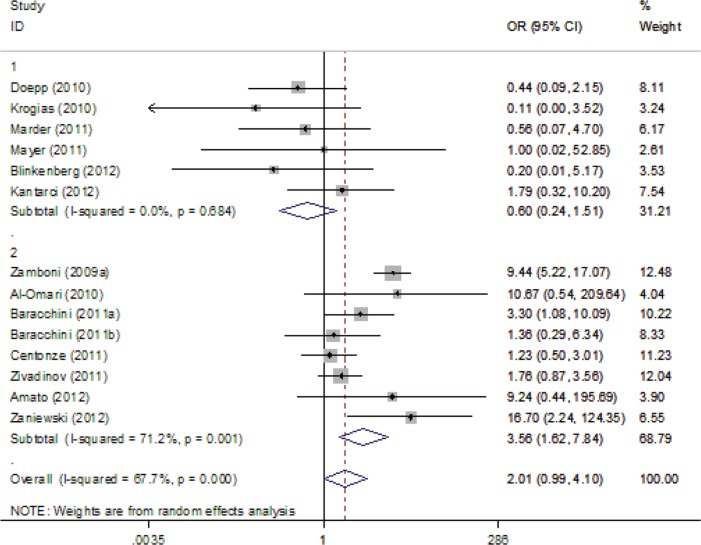
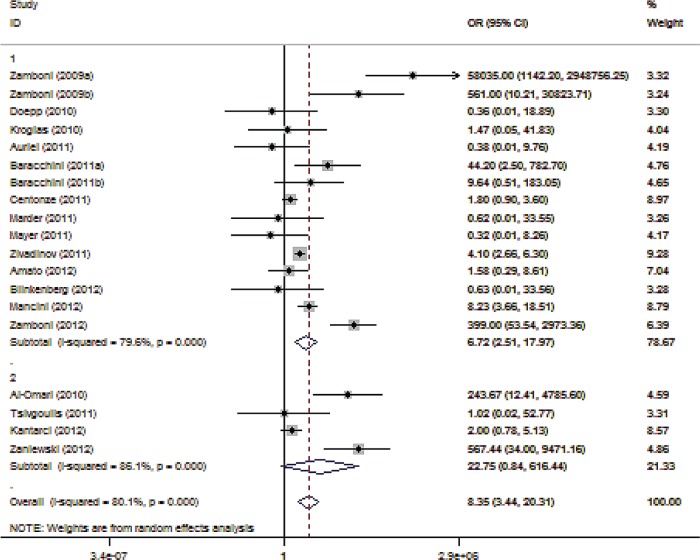
The association of CCSVI with MS varied widely across different studies (Figure 2). More specifically the OR ranged from 58,035 (95% CI 1142–2,948,756) in Zamboni’s pilot study [1] to 0.32 (95% CI 0.01–8.26) in a German study [Mayer et al. 2011]. The pooled analysis (Figure 2) showed that CCSVI was significantly associated with MS (OR 8.35; 95% CI 3.44–20.31; p < 0.001) with considerable heterogeneity (I2 = 80.1%) across studies (p < 0.001, by the Cochran Q statistic). No publication bias was evident (p = 0.916, Egger’s test).
Figure 2.
Forest plot describing the overall association between multiple sclerosis (MS) and chronic cerebrospinal venous insufficiency (CCSVI). CI, confidence interval; OR, odds ratio.
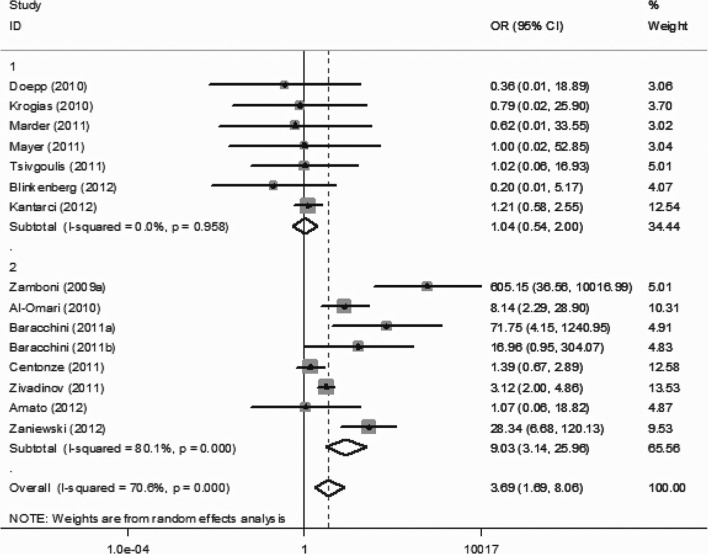
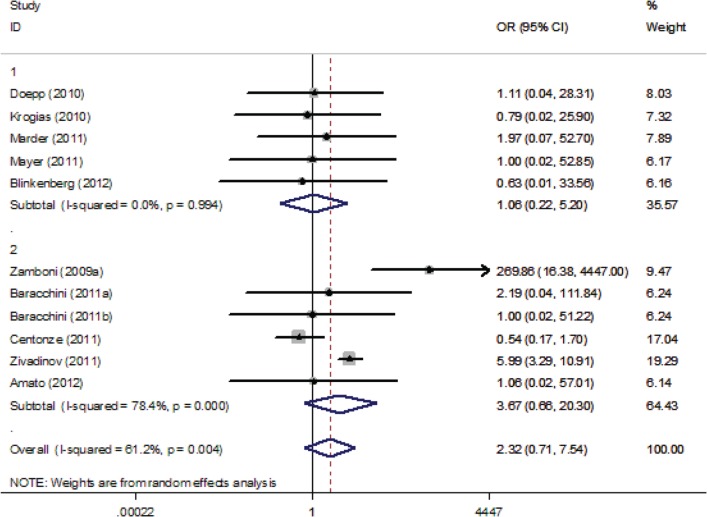
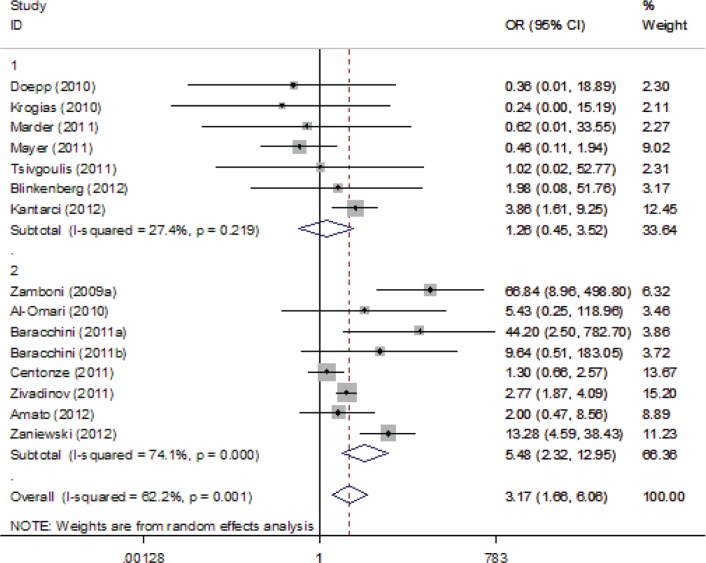
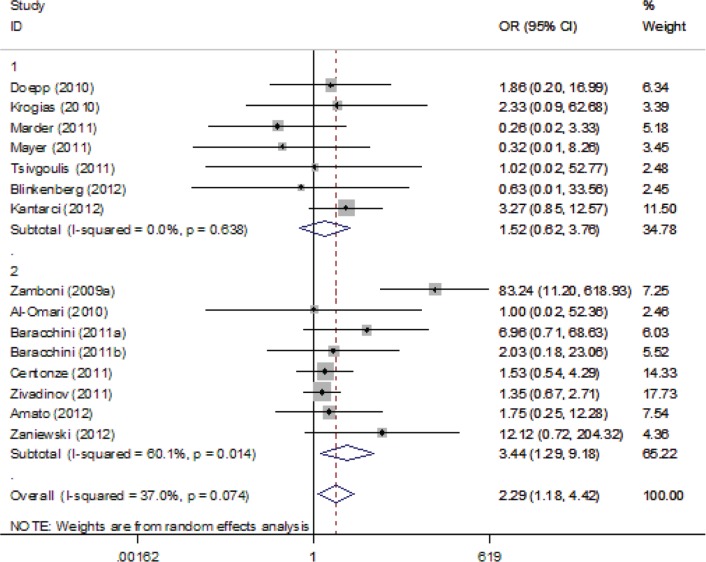
For the overall analyses, ultrasound criterion 1 (OR 3.69, 95% CI 1.69–8.06), criterion 3 (OR 3.17, 95% CI 1.66–6.06) and criterion 4 (OR 2.29, 95% CI 1.18–4.42) were positively associated with CCSVI; however, criterion 2 (OR 2.32, 95% CI 0.71–7.54) and criterion 5 (OR 2.01, 95% CI 0.99–4.10) were not associated with CCSVI. The respective forest plots are presented in Supplemental Figures 1–5.
We decided to introduce several sensitivity levels. Since Zamboni and colleagues have been criticized as being too enthusiastic on the pathogenetic relevance of CCSVI, we decided to formally remove studies (n = 4) that included authors from Zamboni’s group or groups that have previously cooperated with Zamboni. The association of CCSVI with MS was attenuated (OR 3.82; 95% CI 1.56–9.36; p = 0.003; Supplemental Figure 6) with substantial heterogeneity (I2 = 65.8%, p < 0.001) across this subset of studies. In contrast, a stronger association between CCSVI and MS was identified in the four studies (including 473 patients with MS and 343 healthy controls) conducted by the groups of Zamboni and Zivadinov (OR 362.49; 95% CI 5.87–22,397; p = 0.005). However, we documented considerable heterogeneity (I2 = 93.4%, p < 0.001) across these four studies, despite the small number of studies belonging in this subset.
In the second sensitivity analysis we removed all studies with incomplete neurosonology protocol regarding CCSVI screening (four studies involving 310 patients with MS and 172 healthy controls). The association between CCSVI and MS was slightly weakened (OR 6.72; 95% CI 2.51–17.97; p < 0.001; Supplemental Figure 7). Considerable heterogeneity (I2 = 79.6%, p < 0.001) was documented across studies with complete neurosonology protocol.
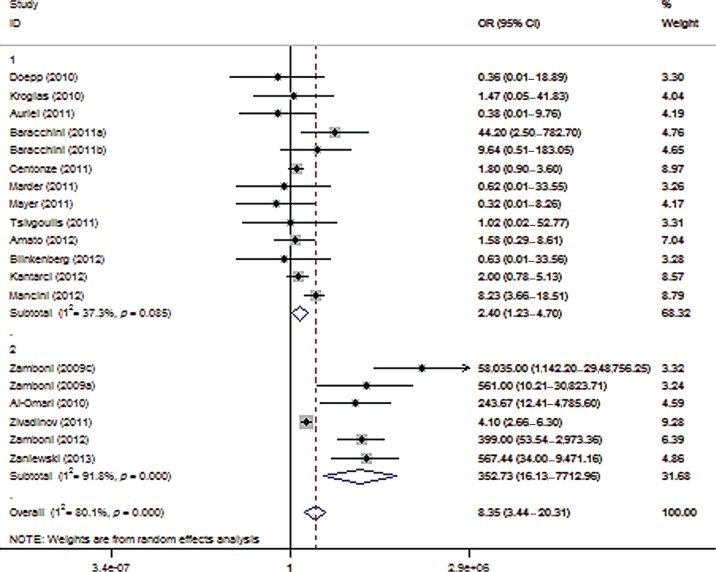
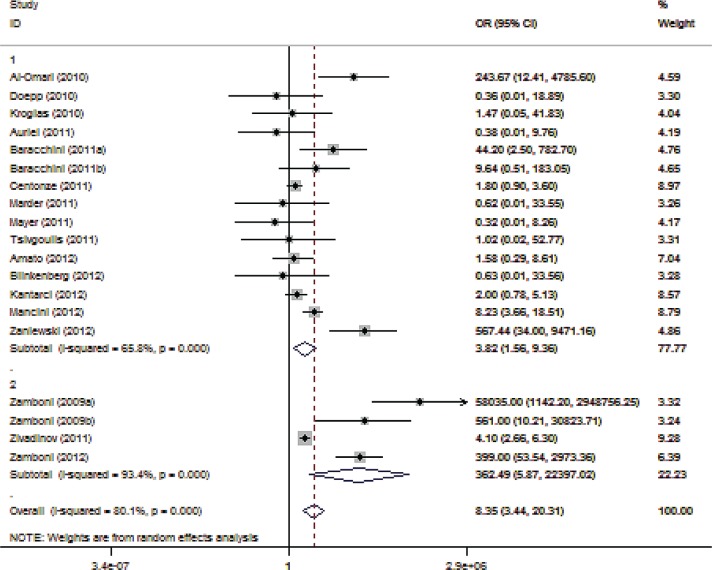
According to the third sensitivity analysis, the exclusion of reports that included coauthors who had also been involved in studies underscoring the safety and efficacy of the Liberation procedure (six studies involving 679 patients with MS and 418 healthy controls) greatly attenuated the reported association between CCSVI and MS (OR 2.40; 95% CI 1.23–4.70; p = 0.010; Figure 3) and reduced heterogeneity (I2 = 37.3%, p = 0.085). In contrast, the evaluation of studies conducted by investigators involved in the Liberation procedure yielded a much stronger relationship (OR 352.73; 95% CI 16.13–7712.96; p < 0.001) with considerable heterogeneity (I2 = 91.8%, p < 0.001) across studies.
Figure 3.
Forest plot describing the results of sensitivity analysis 3 (modifying role mediated by the involvement with the Liberation procedure). Upper panels: studies conducted by investigators not involved in the Liberation procedure (n = 13); Lower panels: studies conducted by investigators involved in the Liberation procedure (n = 6). CI, confidence interval; OR, odds ratio.
In our fourth sensitivity analysis we removed all studies conducted in Italy (eight reports involving 481 patients with MS and 464 healthy controls). This analysis generated a markedly weaker and borderline significant association (OR 3.27; 95% CI 1.06–10.04; p = 0.039; Supplemental Figure 8) with substantial heterogeneity (I2 = 65.9%, p = 0.001). Notably, the heterogeneity was even greater (I2 = 88.2%; p < 0.001) when the Italian studies were separately evaluated (OR 33.06; 95% CI 6.10–179.06; p < 0.001).
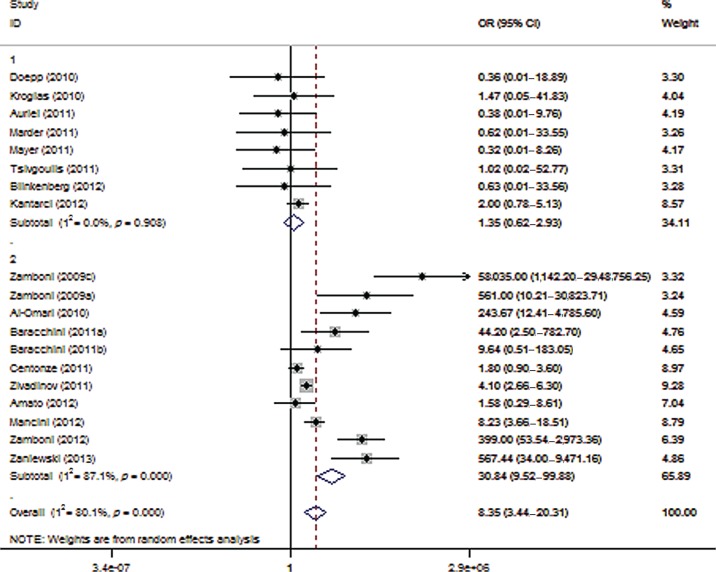
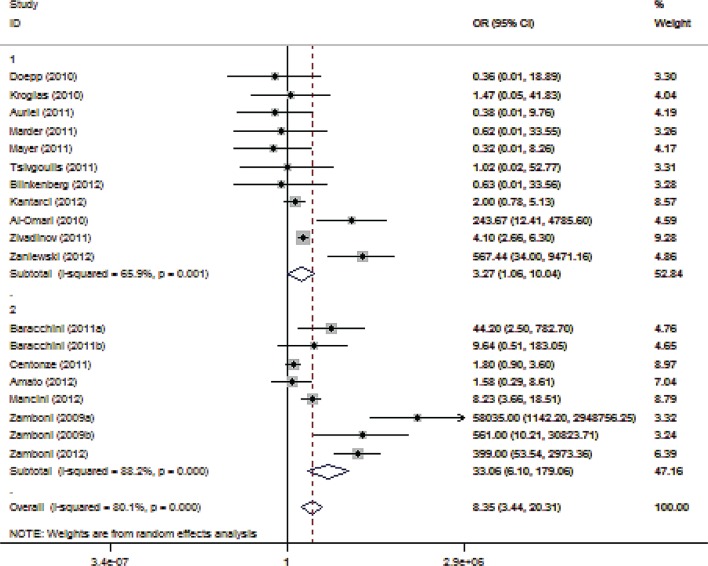
In our final, combined sensitivity analysis, we combined the criteria applied to the third (involvement in the Liberation procedure) and fourth (studies conducted in Italy) sensitivity analyses and evaluated the association of CCSVI with MS in the remaining eight reports, including 259 patients with MS and 197 controls. There was no association between CCSVI and MS (OR 1.35; 95% CI 0.62–2.93; p = 0.453; Figure 4), without any heterogeneity (I2 = 0%, p = 0.908) across the remaining studies included in this final sensitivity analysis. The lack of association between MS and CCSVI in this subset of studies was reproducible upon all five ultrasonographic criteria, as evidenced by the application of the combined sensitivity analysis upon the criteria-specific approaches (Supplemental Figures 1–5).
Figure 4.
Forest plot describing the results of the combined sensitivity analysis (modifying role mediated by recruitment of Italian patients or involvement with the Liberation procedure). Upper panels: studies conducted by investigators not involved in the Liberation procedure and not recruiting Italian patients (n = 8); Lower panels: studies conducted by investigators involved in the Liberation procedure or recruiting Italian patients (n = 11). CI, confidence interval; OR, odds ratio.
Discussion
Our meta-analysis showed a positive association between CCSVI detected using previously proposed ultrasound criteria and MS, however with considerable heterogeneity among included case–control studies. The former association was substantially attenuated in our sensitivity analyses excluding studies that were carried out by the group that first described the potential causative role of CCSVI in MS or removing reports that included investigators who had also been involved in publications advocating endovascular procedures or eliminating investigations that were conducted in Italy. Our most conservative sensitivity analysis combining all exclusion criteria yielded no association between CCSVI and MS with no heterogeneity across the remaining studies.
Our findings contradict a recent review from the original group underscoring a causative association between CCSVI and MS [Morovic and Zamboni, 2012]. More specifically, the two authors have performed an incomplete and partly incorrect listing of different case–control studies investigating the relationship of CCSVI and MS in their systematic review [Morovic and Zamboni, 2012]. First, they include the study by Dolic and colleagues and Zivadinov colleagues in a table summarizing the prevalence of CCSVI in patients with MS and healthy controls without stating that the same population was used for both studies [Dolic et al. 2011; Zivadinov et al. 2011]. Second, they include in the same table another study reporting a positive association between CCSVI and MS without noting that the investigators of this study used brain MRI for CCSVI detection [Dolic et al. 2011] in contrast to the other studies that evaluated CCSVI prevalence with ultrasound. Third, two studies reporting a strikingly high CCSVI prevalence in their MS cohorts (86% and 91%) were erroneously cited in the table summarizing the data of case–control studies despite the fact that no control group was included in these reports [Zaharchuk et al. 2011; Simka et al. 2010]. Fourth and most important, they did not include other studies failing to document any association between CCSVI and MS either by ultrasound [Tsivgoulis et al. 2011; Auriel et al. 2011; Baracchini et al. 2011b] or MRI [Supplemental References s-1, s-2].
Our report expands the findings of an earlier meta-analysis conducted by Laupacis and colleagues in Canada [Laupacis et al. 2011]. They identified a strong and statistically significant relationship (OR 13.5; 95% CI 2.6–71.4) between CCSVI and MS, but there was extensive, unexplained heterogeneity across studies (I2 = 89%). A potential cause of heterogeneity was considered the inclusion of studies that involved both healthy individuals and patients with other neurological disorders as controls. Despite the fact that we evaluated only case–control studies enrolling exclusively healthy individuals in the control group and although the number of eligible studies increased from eight (sample of 632 patients with MS, 510 healthy individuals and 134 controls with other neurological diseases) in the Canadian meta-analysis to 19 (sample of 1250 patients with MS and 899 healthy individuals) in the present report we documented an attenuated association between MS and CCSVI (OR 8.40; 95% CI 3.45–20.44) with considerable heterogeneity (I2 = 80.1%) across included studies.
Interestingly, in the sensitivity analyses of the Canadian study (excluding the studies by Zamboni and colleagues, removing unblinded studies and using a continuity correction of one for studies with zero cells that were originally excluded) the documented extensive heterogeneity was not reduced. In contrast, when we excluded studies whose authors have also advocated endorsement of the Liberation procedure either commercially or with subsequent publications indicating the safety and feasibility of endovascular treatments for patients with MS and CCSVI, the heterogeneity was reduced (from I2 =80.1% to 37.3%) to a nonsignificant level. This observation indicates a potential source of bias and even conflict of interest in ultrasound investigators screening for CCSVI who are also involved in the development of endovascular procedures as potential novel therapy for MS. In view of the considerable difference in the ORs between studies conducted by investigators who were not involved (OR 2.40; 95% CI 1.22–4.70) and the reports whose authors also participated in subsequent publications supporting the Liberation procedure (OR 355.13; 95% CI 16.75–7,528.35) the validity of the reported associations between CCSVI and MS by the second group of researchers may be seriously questioned. Given the numerous reports of adverse events complicating the Liberation procedure (Table 2), including stent thrombosis, stent migration, serious bleeding due to antithrombotic therapy, cardiac arrhythmias, vaso-vagal syncope, cerebral venous sinus thrombosis and even death, our meta-analysis lends support to current recommendations issued by international scientific organizations [Reekers et al. 2011; Baracchini et al. 2012] that strongly discourage all interventional treatments for CCSVI in patients with MS.
Table 2.
Complications reported after endovascular treatments (‘Liberation procedure’) for chronic cerebrospinal venous insufficiency (CCSVI) in patients with multiple sclerosis (MS).
| Study | Country | Patients treated with ‘Liberation procedure’ (n) | Complications |
|---|---|---|---|
| Zamboni et al. [2009c] | Italy | 65 | No major complication reported |
| Mild post-procedural headache with spontaneous resolution (n = 6) | |||
| Minor hemorrhages (hematomas) at the vascular access sites (exact number not reported) | |||
| Samson [2010] | USA | > 35 | Fatal brainstem hemorrhage in a patient treated with coumadin following insertion of two self-regulating stents in the right IJV (n = 1) |
| Migration of stent placed in IJV to the right ventricle. Open heart surgery was performed to remove the device (n = 1) | |||
| Ludyga et al. [2010] | Poland | 331 | Stent thrombosis (n = 2) |
| Surgical removal of angiographic balloon (n = 1) | |||
| Local bleeding from groin (n = 4) | |||
| Two cases with femoral artery pseudoaneurysm treated with thrombin injection (n = 2) | |||
| Gastrointestinal bleeding requiring hospitalization following clopidogrel treatment after stent placement (n = 1) | |||
| Transient atrial fibrillation during the procedure requiring pharmacological treatment (n = 2) | |||
| Migration of stent placed in IJV (n = 4). Second stent placement required to secure the first one (n = 4) | |||
| Thapar et al. [2011] (Supplemental Reference s-4) | UK | Not reported. Endovascular procedure performed outside UK | IJV thrombosis following venoplasty (n = 1). Open thrombectomy performed for symptom relief |
| Burton et al. [2011] (Supplemental Reference s-5) | Canada | Not reported. Endovascular procedures performed outside Canada in Eastern Europe (n = 2), India (n = 1), Mexico (n = 1) and USA (n = 1) | IJV thrombosis following stent placement (n = 1) |
| Cranial nerve palsies (hypoglossal and accessory nerves) caused by bilateral oversized stent placement in IJV (n = 1) | |||
| Migration of stent from azygos to renal vein causing syncope (n = 1) | |||
| Surgical dissection of femoral vein during balloon withdrawal causing large extraperitoneal hematoma within the space of Retzius causing bladder compression (n = 1) | |||
| IJV thrombosis following stent placement complicated by thrombosis of ipsilateral transverse and sigmoid sinuses (n = 1). Anticoagulation was required to treat iatrogenic cerebral venous sinus thrombosis | |||
| Hubbard et al. [2012] | USA | 259 | Deep vein thrombosis at the venous access site (n = 1) |
| Doležal et al. [2012] (Supplemental Reference s-6) | Slovakia | Not reported. Endovascular procedure performed in Poland | Dislocation of right IJV stent to ipsilateral brachiocephalic vein and thrombosis of left IJV stent requiring anticoagulation (n = 1) |
| Zamboni et al. [2012b] | Italy/USA | 8 | Vaso-vagal syncope reported 3 h after procedure (n = 1) |
IJV, internal jugular vein.
Certain limitations of the present meta-analysis also need to be acknowledged. Our strict inclusion criteria limited the number of eligible studies to 19 with substantial variations in sample sizes across different reports. We were unable to investigate whether differences in the success of blinding or in the adopted neurosonology protocol may have contributed to the heterogeneity across different studies, since in most reports there was poor documentation of blinding and limited description of employed ultrasound methodology. Moreover, publication bias cannot be excluded, although it should be kept in mind that the potential association of CCSVI with MS remains a highly controversial topic with both negative and positive studies being likely to be published. Finally, despite the substantial disparities in age (mean age ranging from 35 to 55 years) and gender (proportions of women included ranging from 17% to 76%) in MS populations of different studies (Table 1), we did not have access to individual patient data and we failed to investigate whether the association of CCSVI with MS was affected by these demographic factors.
In conclusion, this meta-analysis highlights the considerable heterogeneity across different ultrasound case–control studies evaluating the association of CCSVI and MS. The major contributing factor to this heterogeneity appears to be the involvement of investigators in other publications supporting endovascular procedures as a novel MS treatment. Moreover, poor reporting of intra- and inter-rater reliability in the proposed neurosonology protocol for CCSVI screening limits the generalizability and validity of the proposed ultrasound criteria. These findings argue against the hypothesis proposing CCSVI as the underlying mechanism of MS. Potentially harmful interventional procedures attempting to improve impaired cerebral venous drainage in patients with MS and CCSVI should be strongly discouraged outside the setting of randomized clinical trials.
Supplemental Material
Methods
Trial Identification and Data Abstraction
Studies were eligible if they met all of the following criteria: (i) report of original data in a peer-reviewed publication; (ii) use of ultrasound to detect venous abnormalities; (iii) diagnosis of CCSVI by fulfilling two of the five previously described ultrasound criteria; (iv) evaluation of patients with MS (chronic progressive and/or relapsing-remitting) in a case-control setting performing comparison to a healthy control group. If the reported control group included patients with other neurological disorders, these subjects were excluded from further analysis.
Titles, abstracts and, whenever appropriate, full texts of all identified studies were screened independently by two reviewers (G.T. and C.K.); disagreements were resolved by consensus of all contributing authors. Duplicate publications were also excluded. In case that the full-text version of the study did not report the frequency of abnormal findings separately for each of the five CCSVI-criteria, the corresponding author of the respective study was asked to provide the missing data, as appropriate in order to maximize the amount of synthesized data.
The two reviewers (G.T. and C.K.) independently abstracted data about the patient and control group characteristic, the methodological quality and the reported ultrasound results. Once again, disagreements were resolved by consensus of all contributing authors. The following data were collected: journal name, year of publication, country, one of authors being involved in reports advocating the safety or efficacy of “Liberation procedure”, frequency of CCSVI diagnosis, frequency of positive findings of each of the five sonographic diagnostic criteria.
Supplemental References
s-1. Sundström P, Wåhlin A, Ambarki K, Birgander R, Eklund A, Malm J (2010). Venous and cerebrospinal fluid flow in multiple sclerosis: a case-control study. Ann Neurol.; 68: 255–259.
s-2. Wattjes MP, van Oosten BW, de Graaf WL, Seewann A, Bot JC, van den Berg R, et al (2011). No association of abnormal cranial venous drainage with multiple sclerosis: a magnetic resonance venography and flow-quantification study. J Neurol Neurosurg Psychiatry; 82: 429–435.
s-3. Yamout B, Herlopian A, Issa Z, Habib RH, Fawaz A, Salame J, et al (2010). Extracranial venous stenosis is an unlikely cause of multiple sclerosis. Mult Scler ; 16: 1341–1348.
s-4. Thapar A, Lane TR, Pandey V, Shalhoub J, Malik O, Ellis M et al (2011). Internal jugular thrombosis post venoplasty for chronic cerebrospinal venous insufficiency. Phlebology; 26: 254–256.
s-5. Burton JM, Alikhani K, Goyal M, Costello F, White C, Patry D, et al (2011). Complications in MS patients after CCSVI procedures abroad (Calgary, AB). Can J Neurol Sci.; 38: 741–746.
s-6. Doležal O, Horáková D, Gdovinová Z, Szilasiová J (2012). Serious Complication of Percutaneous Angioplasty with Stent Implantation in so Called “Chronic Cerebrospinal Venous Insufficiency” in Multiple Sclerosis Patient. Prague Med Rep.; 113: 289–293.
s-7. Zamboni P, Galeotti R, Weinstock-Guttman B, Kennedy C, Salvi F, Zivadinov R (2012). Venous angioplasty in patients with multiple sclerosis: results of a pilot study. Eur J Vasc Endovasc Surg.; 43:116–122.
s-8 Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol.; 62: e1–e34.
s-9. Sweeting MJ, Sutton AJ, Lambert PC (2004). What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med.; 23: 1351–1375.
s-10. Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003). Measuring inconstistency in meta-analyses. BMJ; 327: 560
s-11. http://handbook.cochrane.org/chapter_9/9_analysing_data_and_undertaking_meta_analyses.htm (last accessed December 27, 2012)
s-12. Kostecki J, Zaniewski M, Ziaja K, Urbanek T, Kuczmik W, Krzystanek E, et al (2011). An endovascular treatment of Chronic Cerebro-Spinal Venous Insufficiency in multiple sclerosis patients - 6 month follow-up results. Neuro Endocrinol Lett.; 32: 557–562.
s-13. Al-Omari MH, Al-Bashir A (2012). Internal jugular vein valve morphology in the patients with chronic cerebrospinal venous insufficiency (CCSVI); angiographic findings and schematic demonstrations. Rev Recent Clin Trials; 7: 83–87.
s-14. http://www.ccsvijordan.com/web/en-team (last accessed December 27, 2012)
s-15. Bastianello S, Romani A, Viselner G, Tibaldi EC, Giugni E, Altieri M, et al (2011). Chronic cerebrospinal venous insufficiency in multiple sclerosis: clinical correlates from a multicentre study. BMC Neurol.; 11: 132.
s-16. Koch-Henriksen N, Sørensen PS (2010). The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol.; 9: 520–532.
s-17. Egger M, Davey Smith G, Schneider M, Minder C (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ.; 315(7109): 629–634.
Supplemental Tables
Supplemental Table 1.
Proposed ultrasound criteria for detection of chronic cerebrospinal venous insufficiency.
| Ultrasound Criterion | Description |
|---|---|
| I | Reflux in cervical veins |
| II | Reflux in deep cerebral veins |
| III | High-resolution B-mode evidence of proximal IJV stenosis |
| IV | Flow not-Doppler detectable in IJVs |
| V | Reverted postural control of the main cerebral venous outflow in IJVs |
IJV: internal jugular veinSupplemental.
Table 2.
Baseline characteristics of the case-control studies evaluating the association of chronic cerebrospinal venous insufficiency (CCSVI) with multiple sclerosis (MS) that were included in the present meta-analysis.
| Study | Countries | Zamboni’s group or Zamboni as coauthor | Involved in “Liberation procedure” | Examination performed for all five US criteria | MS, n | HC, n | CCSVI (+) MS, n | CCSVI (+) HC, n |
|---|---|---|---|---|---|---|---|---|
| Zamboni et al., 2009a | Italy | yes | yes | yes | 109 | 132 | 109 | 0 |
| Zamboni et al., 2009c | Italy-USA | yes | yes | yes | 16 | 8 | 16 | 0 |
| Al-Omari & Rousan, 2010 | Jordan | no | yes | no | 25 | 25 | 21 | 0 |
| Doepp et al., 2010 | Germany | no | no | yes | 56 | 20 | 0 | 0 |
| Krogias et al., 2010 | Germany | no | no | yes | 10 | 2 | 2 | 0 |
| Baracchini et al., 2011 | Italy | no | no | yes | 50 | 110 | 8 | 0 |
| Centonze et al., 2011 | Italy | no | no | yes | 84 | 56 | 42 | 20 |
| Zivadinov et al., 2011 | USA | yes | yes | yes | 304 | 163 | 166 | 37 |
| Mayer et al., 2011 | Germany | no | no | yes | 20 | 20 | 0 | 1 |
| Tsivgoulis et al., 2011 | Greece | no | no | no | 42 | 43 | 0 | 0 |
| Auriel et al., 2011 | Israel | no | no | yes | 27 | 32 | 0 | 1 |
| Baracchini et al., 2011 | Italy | no | no | yes | 60 | 60 | 4 | 0 |
| Marder et al., 2011 | USA | no | no | yes | 18 | 11 | 0 | 0 |
| Blinkenberg et al., 2012 | Denmark | no | no | yes | 24 | 15 | 0 | 0 |
| Zamboni et al., 2012 | Italy | yes | yes | yes | 44 | 40 | 42 | 2 |
| Kantarci et al., 2012 | Turkey | no | no | no | 62 | 54 | 16 | 8 |
| Amato et al., 2012 | Italy | no | no | yes | 15 | 16 | 4 | 3 |
| Mancini et al., 2012 | Italy | no | no | yes | 103 | 42 | 79 | 12 |
| Zaniewski et al., 2012 | Poland | no | yes | no | 181 | 50 | 154 | 0 |
MS: multiple sclerosis, HC: healthy control, US: ultrasound.
Supplemental Table 3.
Inter- and intra-rater reliability of chronic cerebrospinal venous insufficiency (CCSVI) diagnosis by ultrasound across different studies.
| Study | Number of patients | Zamboni’s group | Findings |
|---|---|---|---|
| Menegatti et al, 2010 | 36 (12 MS, 12 HC, 12 OND) | Yes |
|
| Tsivgoulis et al, 2011 | 15 (8 MS, 7HC) | No |
|
| Zivadinov et al, 2011 | 36 (11 MS, 14 HC, 3 OND) | Yes |
|
MS: multiple sclerosis, HC: healthy control, OND: other neurological disorders.
Criterion I: reflux in cervical veins, Criterion II: reflux in deep cerebral veins Criterion III: high-resolution B-mode evidence of proximal internal jugular vein stenosis (IJV), Criterion IV: flow not-Doppler detectable in IJVs, Criterion V: reverted postural control of the main cerebral venous outflow in IJVs.
Supplemental Figures
Supplemental Figure 1.
Forest plot describing the association between Ultrasound Criterion 1 and multiple sclerosis. Apart from the overall analysis, the results of the combined sensitivity analysis are shown.
Upper panels: studies conducted by investigators not involved in “Liberation procedure” and conducted outside Italy. Lower panels: studies conducted by investigators involved in “Liberation procedure” or conducted in Italy.
Supplemental Figure 2.
Forest plot describing the association between Ultrasound Criterion 2 and multiple sclerosis. Apart from the overall analysis, the results of the combined sensitivity analysis are shown.
Upper panels: studies conducted by investigators not involved in “Liberation procedure” and conducted outside Italy. Lower panels: studies conducted by investigators involved in “Liberation procedure” or conducted in Italy.
Supplemental Figure 3.
Forest plot describing the association between Ultrasound Criterion 3 and multiple sclerosis. Apart from the overall analysis, the results of the combined sensitivity analysis are shown.
Upper panels: studies conducted by investigators not involved in “Liberation procedure” and conducted outside Italy. Lower panels: studies conducted by investigators involved in “Liberation procedure” or conducted in Italy.
Supplemental Figure 4.
Forest plot describing the association between Ultrasound Criterion 4 and multiple sclerosis. Apart from the overall analysis, the results of the combined sensitivity analysis are shown.
Upper panels: studies conducted by investigators not involved in “Liberation procedure” and conducted outside Italy. Lower panels: studies conducted by investigators involved in “Liberation procedure” or conducted in Italy.
Supplemental Figure 5.
Forest plot describing the association between Ultrasound Criterion 5 and multiple sclerosis. Apart from the overall analysis, the results of the combined sensitivity analysis are shown.
Upper panels: studies conducted by investigators not involved in “Liberation procedure” and conducted outside Italy. Lower panels: studies conducted by investigators involved in “Liberation procedure” or conducted in Italy.
Supplemental Figure 6.
Forest plot describing the results of the sensitivity analysis #1 (modifying role mediated by Zamboni’s group). Upper panels: studies which did not include authors coming from Zamboni’s group or coming from groups that have previously cooperated with Zamboni (n=15); Lower panels: studies related to Zamboni’s group (n=4).
Supplemental Figure 7.
Forest plot describing the results of the sensitivity analysis #2 (modifying role mediated by completeness of the neurosonology protocol). Upper panels: studies with complete neurosonology protocol regarding CCSVI screening (n=15); Lower panels: studies with incomplete protocol (n=4).
Supplemental Figure 8.
Forest plot describing the results of the sensitivity analysis #4 (modifying role mediated by recruitment of studies conducted in Italy). Upper panels: studies conducted outside Italy (n=11); Lower panels: studies conducted in Italy (n=8).
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Dr Georgios Tsivgoulis has been supported by European Regional Development Fund - Project FNUSA-ICRC (No. Z.1.05/1.1.00/02.0123). Theodoros Sergentanis, Konstantinos Voumvourakis, Nikos Triantafyllou, Theodora Psaltopoulou and Christos Krogias have no disclosures. Andrew Chan received personal compensation as a speaker or consultant for Allmirall, Bayer Schering, Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis and Teva Neuroscience. He received research support from the German Ministry for Education and Research (BMBF, ‘German Competence Network Multiple Sclerosis’ (KKNMS), CONTROL MS, 01GI0914), Bayer Schering, Biogen Idec, Merck Serono and Novartis. Ralf Gold has no project-specific disclosures but has received personal compensation and grant support from BiogenIdec, BayerSchering, Novartis, MerckSerono, Sanofi-Genzyme, Roche, TEVA.
Contributor Information
Georgios Tsivgoulis, Second Department of Neurology, ‘Attikon’ Hospital, School of Medicine, University of Athens, Iras 39, Gerakas Attikis, 15344, Athens, Greece and International Clinical Research Center, St Anne’s University Hospital Brno, Brno, Czech Republic.
Theodoros N. Sergentanis, Department of Hygiene, Epidemiology and Medical Statistics, School of Medicine, University of Athens, Athens, Greece
Andrew Chan, Department of Neurology, Ruhr University, St Josef-Hospital, Bochum, Germany.
Konstantinos Voumvourakis, Second Department of Neurology, ‘Attikon’ Hospital, School of Medicine, University of Athens, Athens, Greece.
Nikos Triantafyllou, First Department of Neurology, ‘Eginition’ Hospital, School of Medicine, University of Athens, Athens, Greece.
Theodora Psaltopoulou, Department of Hygiene, Epidemiology and Medical Statistics, School of Medicine, University of Athens, Athens, Greece.
Ralf Gold, Department of Neurology, Ruhr University, St Josef-Hospital, Bochum, Germany.
Christos Krogias, Department of Neurology, Ruhr University, St Josef-Hospital, Bochum, Germany.
References
- Al-Omari M., Rousan L. (2010) Internal jugular vein morphology and hemodynamics in patients with multiple sclerosis. Int Angiol 29: 115–120 [PubMed] [Google Scholar]
- Amato M.P., Saia V., Hakiki B., Giannini M., Pastò L., Zecchino S., et al. (2012) No association between chronic cerebrospinal venous insufficiency and pediatric-onset multiple sclerosis. Mult Scler 18: 1791–1796 [DOI] [PubMed] [Google Scholar]
- Auriel E., Karni A., Bornstein N.M., Nissel T., Gadoth A., Hallevi H. (2011) Extra-cranial venous flow in patients with multiple sclerosis. J Neurol Sci 309: 102–104 [DOI] [PubMed] [Google Scholar]
- Baracchini C., Perini P., Calabrese M., Causin F., Rinaldi F., Gallo P. (2011a) No evidence of chronic cerebrospinal venous insufficiency at multiple sclerosis onset. Ann Neurol 69: 90–99 [DOI] [PubMed] [Google Scholar]
- Baracchini C., Perini P., Causin F., Calabrese M., Rinaldi F., Gallo P. (2011b) Progressive multiple sclerosis is not associated with chronic cerebrospinal venous insufficiency. Neurology 77: 844–850 [DOI] [PubMed] [Google Scholar]
- Baracchini C., Valdueza J.M., Del Sette M., Baltgaile G., Bartels E., Bornstein N.M. (2012) CCSVI and MS: a statement from the European Society of neurosonology and cerebral hemodynamics. J Neurol 259: 2585–2589 [DOI] [PubMed] [Google Scholar]
- Blinkenberg M., Akeson P., Sillesen H., Lövgaard S., Sellebjerg F., Paulson O.B. (2012) Chronic cerebrospinal venous insufficiency and venous stenoses in multiple sclerosis. Acta Neurol Scand 126: 421–427 [DOI] [PubMed] [Google Scholar]
- Centonze D., Floris R., Stefanini M., Rossi S., Fabiano S., Castelli M. (2011) Proposed chronic cerebrospinal venous insufficiency criteria do not predict multiple sclerosis risk or severity. Ann Neurol 70: 51–58 [DOI] [PubMed] [Google Scholar]
- Chafe R., Born K.B., Slutsky A.S., Laupacis A. (2011) The rise of people power. Nature 472: 410–411 [DOI] [PubMed] [Google Scholar]
- Denislic M., Milosevic Z., Zorc M., Ravnik I.Z., Mendiz O. (2012) Disability caused by multiple sclerosis is associated with the number of extra cranial venous stenoses: possible improvement by venous angioplasty. Results of a prospective study. Phlebology 30 November (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Doepp F., Paul F., Valdueza J.M., Schmierer K., Schreiber S.J. (2010) No cerebrocervical venous congestion in patients with multiple sclerosis. Ann Neurol 68: 173–183 [DOI] [PubMed] [Google Scholar]
- Dolic K., Marr K., Valnarov V., Dwyer M.G., Carl E., Hagemeier J. (2011) Sensitivity and specificity for screening of chronic cerebrospinal venous insufficiency using a multimodal non-invasive imaging approach in patients with multiple sclerosis. Funct Neurol 26: 205–214 [PMC free article] [PubMed] [Google Scholar]
- Hubbard D., Ponec D., Gooding J., Saxon R., Sauder H., Haacke M. (2012) Clinical improvement after extracranial venoplasty in multiple sclerosis. J Vasc Interv Radiol 23: 1302–1308 [DOI] [PubMed] [Google Scholar]
- Kantarci F., Albayram S., Demirci N.O., Esenkaya A., Uluduz D., Uysal O. (2012) Chronic cerebrospinal venous insufficiency: does ultrasound really distinguish multiple sclerosis subjects from healthy controls? Eur Radiol 22: 970–979 [DOI] [PubMed] [Google Scholar]
- Krogias C., Schröder A., Wiendl H., Hohlfeld R., Gold R. (2010) [‘Chronic cerebrospinal venous insufficiency’ and multiple sclerosis: critical analysis and first observation in an unselected cohort of MS patients]. Nervenarzt 81: 740–746 [DOI] [PubMed] [Google Scholar]
- Laupacis A., Lillie E., Dueck A., Straus S., Perrier L., Burton J.M., et al. (2011) Association between chronic cerebrospinal venous insufficiency and multiple sclerosis: a meta-analysis. CMAJ 183: E1203–E1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludyga T., Kazibudzki M., Simka M., Hartel M., Swierad M., Piegza J., et al. (2010) Endovascular treatment for chronic cerebrospinal venous insufficiency: is the procedure safe? Phlebology 25: 286–295 [DOI] [PubMed] [Google Scholar]
- Mancini M., Morra V.B., Di Donato O., Maglio V., Lanzillo R., Liuzzi R., et al. (2012) Multiple sclerosis: cerebral circulation time. Radiology 262: 947–955 [DOI] [PubMed] [Google Scholar]
- Marder E., Gupta P., Greenberg B.M., Frohman E.M., Awad A.M., Bagert B., et al. (2011) No cerebral or cervical venous insufficiency in US veterans with multiple sclerosis. Arch Neurol 68: 1521–1525 [DOI] [PubMed] [Google Scholar]
- Mayer C.A., Pfeilschifter W., Lorenz M.W., Nedelmann M., Bechmann I., Steinmetz H., et al. (2011) The perfect crime? CCSVI not leaving a trace in MS. J Neurol Neurosurg Psychiatry 82: 436–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegatti E., Genova V., Tessari M., Malagoni A.M., Bartolomei I., Zuolo M., et al. (2010) The reproducibility of colour Doppler in chronic cerebrospinal venous insufficiency associated with multiple sclerosis. Int Angiol 29: 121–126 [PubMed] [Google Scholar]
- Morovic S., Zamboni P. (2012) CCSVI is associated with multiple sclerosis. Neurol Res 34: 770–779 [DOI] [PubMed] [Google Scholar]
- Reekers J.A., Lee M.J., Belli A.M., Barkhof F. (2011) Cardiovascular and Interventional Radiological Society of Europe commentary on the treatment of chronic cerebrospinal venous insufficiency. Cardiovasc Intervent Radiol 34: 1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson K. (2010) Experimental multiple sclerosis vascular shunting procedure halted at Stanford. Ann Neurol 67: A13–A15 [DOI] [PubMed] [Google Scholar]
- Simka M., Kostecki J., Zaniewski M., Majewski E., Hartel M. (2010) Extracranial Doppler sonographic criteria of chronic cerebrospinal venous insufficiency in the patients with multiple sclerosis. Int Angiol 29: 109–114 [PubMed] [Google Scholar]
- Tsivgoulis G., Mantatzis M., Bogiatzi C., Vadikolias K., Voumvourakis K., Prassopoulos P. (2011) Extracranial venous hemodynamics I multiple sclerosis: a case-control study. Neurology 77: 1241–1245 [DOI] [PubMed] [Google Scholar]
- Zaharchuk G., Fischbein N.J., Rosenberg J., Herfkens R.J., Dake M.D. (2011) Comparison of MR and contrast venography of the cervical venous system in multiple sclerosis. AJNR Am J Neuroradiol 32: 1482–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni P. (2006) The big idea: iron-dependent inflammation in venous disease and proposed parallels in multiple sclerosis. J R Soc Med 99: 589–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni P., Bertolotto A., Boldrini P., Cenni P., D’Alessandro R., D’Amico R. (2012a) Efficacy and safety of venous angioplasty of the extracranial veins for multiple sclerosis. Brave Dreams Study (Brain Venous Drainage Exploited Against Multiple Sclerosis): study protocol for a randomized controlled trial. Trials 13: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni P., Galeotti R., Menegatti E., Malagoni A.M., Tacconi G., Dall’Ara S. (2009a) Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 80: 392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni P., Galeotti R., Menegatti E., Malagoni A.M., Gianesini S., Bartolomei I., et al. (2009b) A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency. J Vasc Surg 50: 1348–1358 [DOI] [PubMed] [Google Scholar]
- Zamboni P., Menegatti E., Conforti P., Shepherd S., Tessari M., Beggs C. (2012b) Assessment of cerebral venous return by a novel plethysmography method. J Vasc Surg 56: 677–685 [DOI] [PubMed] [Google Scholar]
- Zamboni P., Menegatti E., Galeotti R., Malagoni A.M., Tacconi G., Dall’Ara S., et al. (2009c) The value of cerebral Doppler venous haemodynamics in the assessment of multiple sclerosis. J Neurol Sci 282: 21–27 [DOI] [PubMed] [Google Scholar]
- Zamboni P., Menegatti E., Weinstock-Guttman B., Schirda C., Cox J.L., Malagoni A.M., et al. (2009d) The severity of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis is related to altered cerebrospinal fluid dynamics. Funct Neurol 24: 133–138 [PubMed] [Google Scholar]
- Zaniewski M., Kostecki J., Kuczmik W., Ziaja D., Opala G., Świat M., et al. (2013) Neck duplex Doppler ultrasound evaluation for assessing chronic cerebrospinal venous insufficiency in multiple sclerosis patients. Phlebology 28: 24–31 [DOI] [PubMed] [Google Scholar]
- Zivadinov R., Marr K., Cutter G., Ramanathan M., Benedict R.H., Kennedy C., et al. (2011) Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS. Neurology 77: 138–144 [DOI] [PubMed] [Google Scholar]