Abstract
Aims:
Ischemic heart disease is a leading worldwide cause of death. The Seattle Post Myocardial Infarction Model (SPIM) was developed to predict survival 6 months to 2 years after an acute myocardial infarction with evidence of left ventricular dysfunction.
Methods and Results:
A total of 6632 subjects from the EPHESUS trial were used to derive the predictive model, while 5477 subjects from the OPTIMAAL trial were used to validate the model. Cox proportional hazards modeling was used to develop a multivariate risk score predictive of all-cause mortality. The SPIM risk score integrated lab and vital parameters, Killip class, reperfusion or revascularization, the number of cardiac evidence-based medicines (aspirin, statin, β blocker, ACEI/ARB, aldosterone blocker), and the number of cardiac risk factors. The model was predictive of all-cause mortality after myocardial infarction, with an AUC of 0.75 at 6 months and 0.75 at 2 years in the derivation cohort and 0.77 and 0.78 for the same time points in the validation cohort. Model predicted versus Kaplan-Meier observed survival was excellent in the derivation cohort. It remained so in the validation cohort—84.9% versus 85.0% at 2 years. The 10% of subjects with the highest predicted risk had approximately 25 times higher mortality at 2 years than the 10% of subjects with the lowest predicted risk.
Conclusion:
The SPIM score was a powerful predictor of outcomes after myocardial infarction with left ventricular dysfunction. Its highly accurate predictions should improve patient and physician understanding of survival and may prove a useful tool in post-infarct risk stratification.
Keywords: Myocardial infarction, risk model, survival
Introduction
In-hospital and 30-day mortality after an acute myocardial infarction decreased by approximately 25% between the 1990s and 2000s,1,2 with especially large reductions noted in several European registries of patients with ST segment elevation myocardial infarction (STEMI).3,4 A significant portion of these gains can be attributed to appropriate selection for and application of reperfusion therapy and evidence based medicines.5 Although many scores have been developed to assist in short-term risk stratification in the immediate aftermath of an acute coronary syndrome,6–9 coronary heart disease (CHD) remains a major cause of mortality after the acute period of stabilization. Approximately 20% of patients experiencing an acute myocardial infarction die within 1 year with over half the first-year mortality occurring greater than 30 days after presentation.10–12 Less attention has been paid to longer-term stratification and prediction of outcomes after an acute myocardial infarction.
The PREDICT score estimated survival up to 6 years after a myocardial infarction, but was derived and validated in a patient cohort that preceded the development of many modern interventions for CHD.13 Longer-term survival predictions based on risk scores developed for prediction of shorter-term outcomes have been notable for significant errors in longer term accuracy. Extending risk predictions from the PURSUIT score for 1-year outcomes showed its predicted rates of mortality to be less than half of observed mortality,14 while application of the TIMI risk index to predict 1 and 3 year outcomes showed progressive attenuation in discriminative ability for longer-term event prediction.15 An attempt to extend the angiography-derived SYNTAX score with the clinically derived ACEF score showed low calibration in predicting long-term outcomes after percutaneous coronary intervention (PCI).16 The Grace discharge score was developed for prediction of 6-month survival after discharge from hospital,17 but has had considerably less validation and follow-up work than the GRACE in-hospital mortality model.
Assessment of longer-term survival after stabilization for an acute myocardial infarction has received much less attention than immediate risk stratification. We developed the Seattle Post Myocardial Infarction Model (SPIM) to provide a well-calibrated and highly discriminative tool that could accurately predict survival 6 months, 1 year, and 2 years after a myocardial infarction in patients with evidence of left ventricular dysfunction.
Methods
Patient population and individual variables
EPHESUS (the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) was a clinical trial of eplerenone versus placebo in 6632 patients.18 Subjects were enrolled up to 14 days (average of 7 days) after an index myocardial infarction. This was defined as abnormal cardiac enzymes or an evolving electrocardiogram consistent with myocardial infarction, associated with evidence of left ventricular dysfunction.19 OPTIMAAL (Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan) was a clinical trial of 5477 patients randomized to Losartan or Captopril.20 Subjects with a sign or symptom of heart failure or a history of new or established q waves in an anterior distribution on an electrocardiogram (EKG) were enrolled up to 10 days (median of 3 days) following a myocardial infarction-- defined as two or more of the following symptoms: typical chest pain for >20 minutes, positive cardiac enzymes, or a diagnostic EKG.21 EPHESUS and OPTIMAAL included the spectrum of acute myocardial infarction – both STEMI and Non ST segment elevation myocardial infarction (NSTEMI).
Fifty two candidate variables were considered for inclusion in the predictive model. Potential variables included basic clinical features (age, region of the participant, race, gender, alcohol and tobacco use), features of the presenting myocardial infarction (new q waves, left bundle branch block, ST segment elevation or depression >0.5 mm, extent of elevation of cardiac enzymes, typical chest pain, presence of a third heart sound, evidence of pulmonary congestion, Killip class), laboratory features (alanine transaminase (ALT), aspartate transaminase (AST), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, blood urea nitrogen (BUN), creatinine, fasting glucose, white blood cell count, hemoglobin, potassium, sodium, uric acid), vital signs (systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse, height, weight), reperfusion or revascularization therapy and the ejection fraction during the index hospitalization, presence of pre-existing comorbidities (diabetes, chronic obstructive pulmonary disease, hypertension, heart failure, cardiovascular disease (prior myocardial infarction, peripheral vascular disease, or cerebrovascular accident, (CVA))), and the medications the patient was on at the time of enrollment (aspirin, beta-blockers, digoxin, diuretics, calcium channel blockers, anti-arrhythmics, statin, angiotensin converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB) and aldosterone blockers). Laboratory values were collected at enrollment in the study, with the exception of cardiac enzyme elevation, which was the peak observed elevation. Several derived variables, such as body mass index and glomerular filtration estimated by the Cockroft-Gault and Modification of diet in renal disease (MDRD) equations, were also considered.
Statistical analysis
SPSS 19 (Chicago, IL, USA) and Stata 11.2 (College Station, TX, USA) were used for all statistical analyses. Variables were initially evaluated for univariate associations with mortality. Continuous variables were evaluated graphically with Kaplan-Meier observed survival plotted against the medians of 10 equally populated strata of laboratory value. In the case of variables where 10 divisions was not sufficient to determine if there might be an upper or lower limit of association with risk or linearity of hazard was not clear, up to 40 equally populated strata were created.
EPHESUS was used as the derivation cohort for the model, which was then prospectively validated with no changes to the baseline survival function or the beta coefficients in the OPTIMAAL cohort. Cox proportional hazards modeling with forward conditional entry was used to generate the model. A p<0.005 was used as the cut-point for entry and 0.01 for removal from the Cox model. Variables and transformations included in the final model were selected to achieve high predictive power while also being routinely obtained for patients during hospitalization for acute coronary syndrome. For patients with missing values for individual variables, the cohort median was assigned. Ninety one percent of the patients in EPHESUS and 90% of the patients in OPTIMAAL had complete data for all values that entered the final model, with 99.7% of the EPHESUS patients and 97% of the OPTIMAAL patients missing no more than two values. The SPIM score was calculated by summing the products of each individual variable entering the final model multiplied by the beta coefficient for that variable. Predicted survival for a particular patient at a time point, t, was calculated using the following equation:
The term BaselineSurvival(t) (baseline survival function for a patient with a SPIM score of 0 at time point t) was determined in SPSS at 6 months, 1 year, and 2 years in the derivation cohort and then used to calculate predicted survival for each individual patient.
Survival curves were compared using the log-rank test. Assessment of the discrimination of the model was done via calculation of the area under the receiver-operator characteristic curve (AUC) at multiple time points. Comparison of AUCs was made using the method suggested by Hanley and McNeil.22 The accuracy of the model was assessed using the Hosmer-Lemeshow goodness of fit test23 and calculation of correlation coefficients between predicted and observed survival by deciles of risk score.
Results
Comparison of the derivation and validation cohorts
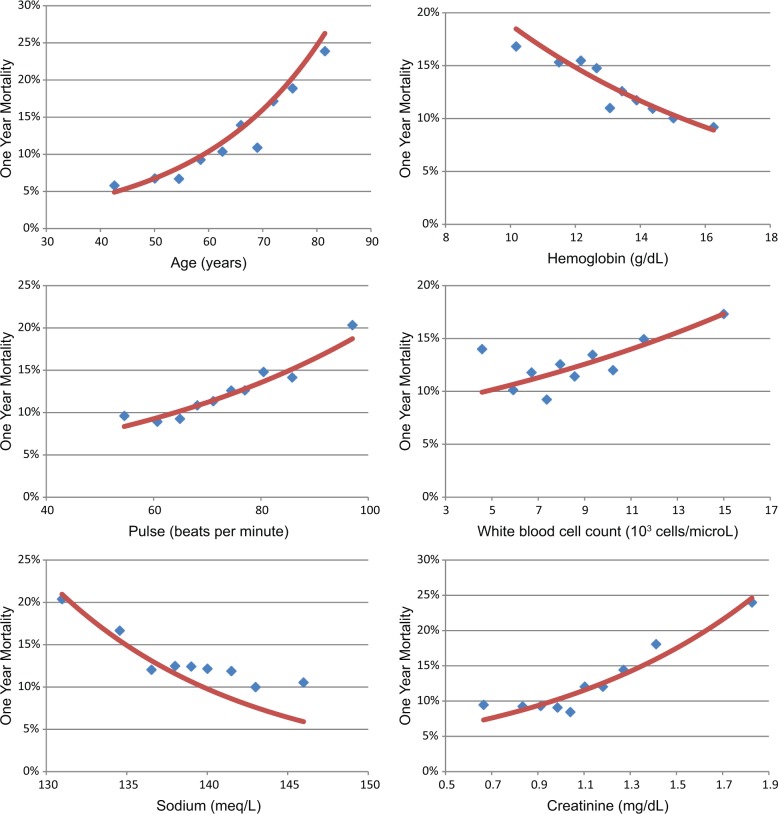
Full details of the patient populations of OPTIMAAL and EPHESUS have been published.18,20 A selected summary of the cohorts included in each trial is listed in Table 1. The EPHESUS population was younger than the OPTIMAAL population, but had a greater comorbidity burden and lower rate of receiving reperfusion therapy. The EPHESUS population was a worldwide cohort, while all the patients in OPTIMAAL were from Western Europe. Age, creatinine, reperfusion or revascularization, and the number of cardiac evidence-based medicines at trial enrollment appeared to be especially powerful univariate predictors of survival. One-year Kaplan-Meier observed mortality by deciles of several predictors included in the final model, along with smoothed univariate hazard functions, are plotted in Figure 1.
Table 1.
Differences in selected baseline characteristics of the patient populations in EPHESUS and OPTIMAAL.
| Study characteristics | EPHESUS (Derivation) |
OPTIMAAL (Validation) |
|---|---|---|
| Number of patients | 6632 | 5477 |
| Age, years | 64±12 | 67±10 |
| Gender, % male | 71.1 | 71.2 |
| % in Latin America | 8.6 | 0 |
| Alcohol, none | 57.2 | 39.6 |
| Alcohol, 1 serving/day | 35.6 | 50.4 |
| Alcohol, >1 serving/day | 7.2 | 10.0 |
| Tobacco use, current | 30.8 | 33.5 |
| Tobacco use, former | 30.1 | 34.1 |
| Tobacco use, never | 39.0 | 32.4 |
| History of diabetes, % | 32.3 | 17.2 |
| History of hypertension, % | 60.4 | 36.0 |
| History of cardiovascular disease, % | 73.1 | 42.2 |
| History of chronic obstructive pulmonary disease, % | 9.4 | 5.3 |
| History of heart failure, % | 14.7 | 6.2 |
| Killip class, mean | 2.1±0.7 | 1.8±0.7 |
| I, % | 15.3 | 31.7 |
| II, % | 64.5 | 57.2 |
| III, % | 16.5 | 9.5 |
| IV, % | 3.1 | 1.6 |
| Heart rate, beats per minute | 75±12 | 75±14 |
| SBP, mmHg | 119±17 | 123±17 |
| Serum total cholesterol, mg/dL | 194±49 | 212±43 |
| Serum HDL cholesterol, mg/dL | 40±17 | 45±12 |
| Serum LDL cholesterol, mg/dL | 121±52 | 130±42 |
| Serum sodium, mEq/L | 139±4 | 139±3 |
| Serum creatinine, mg/dL | 1.1±0.3 | 1.1±0.3 |
| Serum white blood cell count, 103 cells/microL | 8.8±3.0 | 9.2±3.2 |
| Serum hemoglobin, g/dL | 13.3±1.7 | 13.4±1.4 |
| Received reperfusion therapy, % | 45.3 | 63.4 |
| Number of cardiac evidence-based medicines | 3.5±1.0 | 3.1±0.8 |
| Aspirin, % | 88.5 | 88.5 |
| Statin, % | 46.7 | 45.9 |
| Beta-blocker, % | 74.8 | 74.5 |
| ACEI/ARB, % | 86.7 | 100 |
| Aldosterone blocker | 50 | 1.1 |
Values are reported as the number, percentage, or the mean ± standard deviation, as appropriate.
ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin II receptor blocker; HDL: high-density lipoprotein; LDL: low-density lipoprotein; SBP: systolic blood pressure.
Figure 1.
Equally populated deciles of several clinical variables that showed strong univariate associations with 1 year Kaplan-Meier mortality in the derivation cohort plotted with the univariate hazard function.
Derivation of the model
Predictors of survival included in the risk score, expressed as both univariate and multivariate contributors, are listed in Table 2. Several predictors, including ejection fraction (EF), liver function, and uric acid, would have entered the model, but were excluded as they were not universally collected and recorded as part of a hospitalization for myocardial infarction. Uric acid would have been the most powerful single laboratory variable if allowed to enter the model. Digoxin, calcium channel blockers, and anti-arrhythmic medicines were associated with increased mortality, but were excluded from the final model due to lack of documentation on comorbidities (for example, atrial fibrillation or ventricular tachycardia) that may have been the actual mediators of increased risk. The improvement in AUC for a model including all six of these predictors was modest (<0.01 in the derivation cohort when compared to the final SPIM model). An adjusted risk score adding EF and uric acid to the existing variables is available with the online calculator for the SPIM risk score and is attached in a data supplement, but is not used further in this analysis.
Table 2.
Univariate and multivariate predictors of survival in the derivation cohort.
| Predictor | Univariate HR (95% CI) |
Wald χ2 | Multivariate HR (95% CI) |
Wald χ2 |
|---|---|---|---|---|
| Age, each 10 years | 1.540 (1.453–1.631) | 214.3 | 1.430 (1.345–1.521) | 129.0 |
| Number of risk factors, 0–4a | 1.422 (1.325–1.526) | 94.9 | 1.384 (1.289–1.486) | 80.3 |
| Reperfusion therapy | 0.457 (0.399–0.522) | 130.6 | 0.571 (0.496–0.658) | 59.9 |
| Heart rate, each 10 bpm changeb | 1.209 (1.150–1.271) | 55.2 | 1.194 (1.134–1.257) | 45.2 |
| Creatinine, mg/dLc | 2.838 (2.411–3.339) | 157.6 | 1.675 (1.414–1.985) | 35.5 |
| Systolic blood pressure as a polynomiald | 1.034 (1.014–1.054) | 11.3 | 1.061 (1.040–1.082) | 34.2 |
| Killip class | 1.491 (1.370–1.623) | 85.3 | 1.279 (1.175–1.393) | 32.1 |
| Latin American | 1.609 (1.337–1.937) | 25.4 | 1.709 (1.415–2.064) | 30.9 |
| Cardiac evidence-based medicines, 0–5e | 0.753 (0.711–0.796) | 97.3 | 0.850 (0.802–0.902) | 29.5 |
| White blood cell count, 103 cells/microLf | 1.055 (1.033–1.078) | 24.1 | 1.045 (1.022–1.068) | 15.5 |
| Sodium, mEq/L g | 0.919 (0.896–0.941) | 45.5 | 0.962 (0.938–0.987) | 8.8 |
| Hemoglobin, g/dLh | 0.887 (0.855–0.919) | 42.4 | 0.950 (0.916-0.985) | 7.8 |
Current smoker, history of heart failure, history of diabetes mellitus, and history of cardiovascular disease (any of previous myocardial infarction, peripheral vascular disease, cerebrovascular accident, or transient ischemic attack).
Pulse truncated to a range between 60 and 140. (When calculating the risk score, the contribution to predicted risk is capped at maximal/minimal levels – a pulse of 50 is treated as a pulse of 60, while a pulse of 150 is treated as a pulse of 140.)
Creatinine truncated to range between 0.9 and 3.0.
Systolic blood pressure in mmHg expressed as the polynomial ((160-SBP)/20)2.
Aspirin, beta-blocker, statin, ACEI/ARB, aldosterone blocker.
White blood cell count truncated to range between 7 and 25.
Sodium truncated to range between 125 and 138.
Hemoglobin truncated to range between 6 and 16.
ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin II receptor blocker; HR: heart rate.
The baseline survival for a SPIM score of 0 (median score of the EPHESUS cohort) was 93.4% at 6 months, 90.3% at 1 year, and 85.0% at 2 years. Age was a powerful risk factor with a 43% increase in risk for each decade. Laboratory values entering the final model included sodium, hemoglobin, creatinine, and white blood cell count, with levels closer to the normal range associated with reduced risk. SBP was transformed as a polynomial with a nadir of risk at 160 mm Hg. An elevated resting heart rate was associated with a 19% increased risk for each 10 bpm above 60. Killip class was a powerful predictor with a 28% increase for each increase in class. The number of evidence-based medicines was entered into the model as an integer from 0 to 5 for a number of medicines in the aspirin, beta-blocker, statin, ACEI/ARB, and aldosterone blocker classes, with 15% lower mortality for each additional medication (Figure 2(a)). The presence of three pre-existing comorbidities (heart failure, cardiovascular disease, and diabetes mellitus) contributed to the model, as did active tobacco use—with a 38% increase in risk for each additional risk factor (Figure 2(b)). The occurrence of reperfusion therapy (coronary artery bypass graft, percutaneous coronary intervention, or thrombolytics) during therapy for the index infarction was associated with a 43% lower mortality. Subjects from Latin America, including Mexico, had significantly increased mortality (71% higher) compared to those from other regions (Eastern Europe, Western Europe, North America, Oceania, East Asia, and South Africa).
Figure 2.
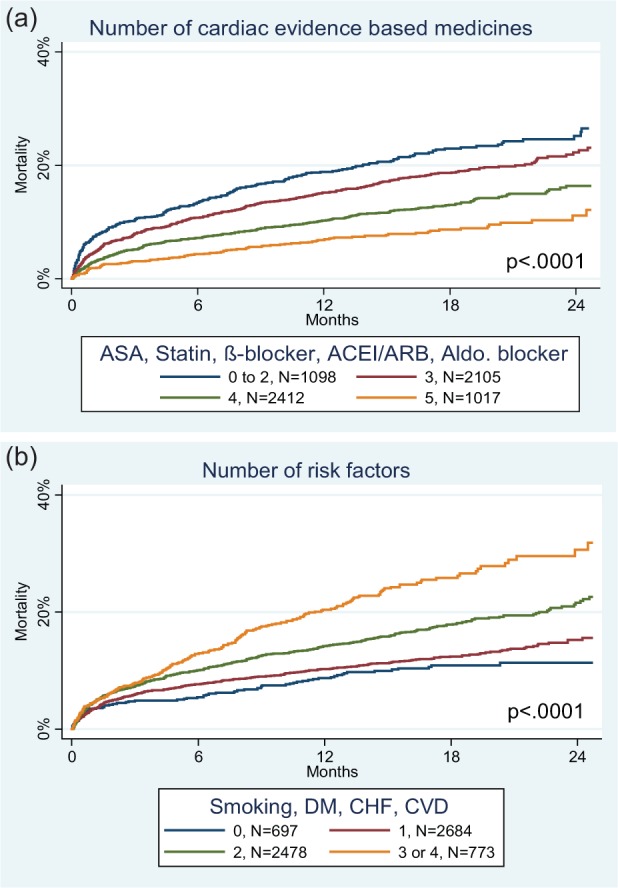
Mortality curves for the derivation cohort based on number of evidence based medicines at baseline (a) and the number of risk factors (b). The number of patients is listed for each grouping.
ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin II receptor blocker; ASA: aspirin; CHF: congestive heart failure; CVD: cerebrovascular disease; DM: diabetes mellitus.
A web-based application was created to allow easy determination of the risk score. The calculator is available at http://www.seattleinfarctionmodel.com and shows both graphical and text depiction of survival at time points between 6 months and 3 years.
Testing the model
The model performed well in both the derivation and validation cohorts. Figure 3 depicts Kaplan-Meier curves of mortality for each decile of SPIM risk score. Predicted survival versus observed survival for the derivation cohort was 91.1% versus 91.2% at 6 months, 87.3% versus 87.3% at 1 year, and 81.2% versus 81.1% at 2 years. For the validation cohort, predicted versus observed survival was 93.0% versus 91.7% at 6 months, 89.9% versus 88.7% at 1 year, and 84.9% versus 85.0% at 2 years. Figure 4 depicts predicted versus observed survival for deciles of SPIM risk score at 1 year and 2 years in the derivation and up to 3 years in the validation cohort. The correlation coefficients between predicted and observed survival were very high in both derivation (r2=0.979, p<0.0001, SEM ±2%) and validation (r2=0.973, p<0.0001, SEM ±2%) cohorts. The Hosmer-Lemeshow goodness of fit statistic confirmed model accuracy in the derivation (p=0.84) and the validation cohorts (p=0.58) at 1 year.
Figure 3.

Kaplan-Meier observed mortality for each decile of SPIM predicted mortality in the derivation (a) and validation (b) cohorts.
Figure 4.
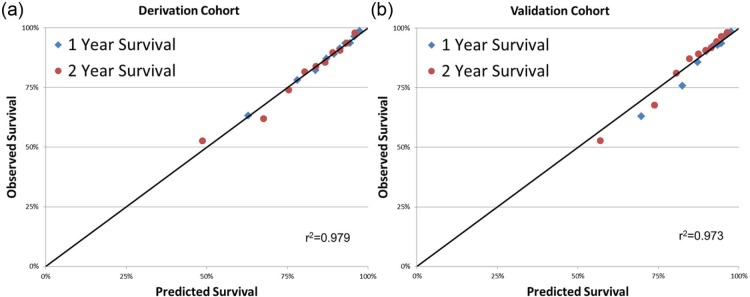
Comparison of deciles of SPIM predicted versus Kaplan-Meier observed survival at 1 and 2 years in the derivation (a) and validation (b) cohorts.
The AUC for the derivation cohort was 0.748 (0.728–0.768) at 6 months, 0.742 (0.724–0.760) at 1 year, and 0.748 (0.725–0.772) at 2 years. For the validation cohort, the AUC was 0.769 (0.747–0.791) at 6 months, 0.774 (0.754–0.794) at 1 year, and 0.778 (0.760–0.796) at 2 years.
Discussion
Components of the SPIM and comparison to previous work
Risk stratification has historically focused on simplicity. Many models have been developed with the explicit goal of being calculated by hand at the bedside. Computers and portable computing devices have become ubiquitous in medical settings. As such, an information age model does not need to compromise its prognostic power by using an overly small dataset of variables or simplifying its calculation by bucketing included parameters into a small number of arbitrary ranges. The length of time required to input into a computer a reasonable number of laboratory and clinical variables, which the computer can then convert to a survival prediction, is comparable to the time it takes to look up a set of laboratory and clinical markers on a piece of paper, manually compute a point score, and reference a nomogram for an actual survival prediction.
The most accurate prediction of survival after an acute myocardial infarction can be achieved with attention to the multiple organ systems and comorbidities that interact with heart disease to affect outcomes. The range of variables included in the SPIM has shown significant prognostic value in previous studies. Age was the dominant predictor of survival in equations developed from the Framingham study of cardiovascular disease risk in the general population; the addition of all the other variables to age and gender increased the AUC by 0.05.24,25 Age was the most powerful single predictor of outcomes in the SPIM, though it accounted for less of the overall predictive power of the multivariate model (approximately 20% based on Wald chi-squared contribution) with the 2-year AUC increasing from 0.64 with age only to 0.75 for the complete SPIM score. Gender was not a multivariate predictor (p=0.48, Wald chi-squared=0.5).
The number of cardiac risk factors was the second-most powerful predictor of mortality in the SPIM. Ischemic risk factors, including diabetes, pre-existing ischemic disease, and smoking, have been shown to have a powerful adverse effect on mortality.26–28 The presence or emergence of advanced heart failure is a stronger predictor of adverse outcome than almost any other, even advanced age,29,30 and its role is captured through several variables in the model: its presence as an included risk factor if present prior to the myocardial infarction and its severity as measured by the Killip class at trial entry. Residence in Latin American was associated with an even greater hazard ratio than with presence of one of these cardiac risk factors, a trend that has been noted to varying extents in other international studies.31–34
Several laboratory and basic clinical markers entered the model. Renal disease has been proven to be an important contributor to the development of CHD,35 with a single measurement of renal function shown to predict increased mortality for at least 10 years after an acute myocardial infarction.36 Elevated creatinine proved to be the single greatest laboratory contributor to survival predictions in the final model. Elevated white blood cell count (WBC) and low hemoglobin are associated with inflammation and increased risk,37,38 which is greatest early after infarction and decreases over time. Hyponatremia is associated with inflammation and deleterious activation of several neuroendocrine pathways, including the renin-angiotensin-aldosterone system, vasopressin release, and the sympathetic nervous system.39 The association between elevated resting heart rate and increased short-term adverse events40 appears to persist in prediction of longer-term adverse outcomes, with a recent study showing a 24% increase in annual mortality for each five beat per minute increase seen 4 years after myocardial infarction.41
Risk modeling and therapeutics
The SPIM provides highly accurate predictions of intermediate-term survival after myocardial infarction and is very effective in risk stratification; the 10% of subjects with the highest predicted risk had approximately 25 times higher mortality at 2 years than the 10% of subjects with the lowest predicted risk. SPIM is different from most previous models in its focus on assessment of survival after the initial treatment for an acute presentation with an acute coronary syndrome. Given the important changes in survival that occur with therapeutics administered during the presenting hospitalization, it is limiting to predict long-term survival without including information about management during the hospitalization for the acute infarction. Reperfusion therapy, in particular, has a powerful effect on survival in appropriately selected patients after myocardial infarction with the mortality benefit persisting for up to 20 years.42–44
Several classes of cardiac medicines have consistently been shown in randomized trials to improve outcomes after acute myocardial infarction and are guideline-recommended for most patients presenting with an acute myocardial infarction.45 It is notable that a significant proportion of patients being discharged from the hospital after myocardial infarction are not on an optimal medical regimen.46 Part of the reason for lower than optimal rates of compliance with guidelines is likely due to errors in physician classification of risk.47 Evidence-based cardiac medicines demonstrated a powerful survival effect in the SPIM—a patient on four evidence-based cardiac medicines has an annual mortality 48% lower than a patient on no evidence-based cardiac medicines. By including the effects of evidence-based cardiac medicines, the SPIM may be able to provide a more concrete numerical understanding of how survival can be altered with the addition of a full regimen of cardiac medicines, promote increased adherence to guidelines, and potentially improve calibration in validation cohorts.
Limitations
The SPIM was developed and validated in two clinical trial populations and will need to be validated in a more generalized community population. Benefits of evidence-based medicines were not calculated individually and were not adjusted for the medication dose. The benefit of being on a medicine was calculated based on whether the medicine was or was not prescribed at enrollment. However, the benefit of being started on an evidence based medicine de novo may be greater than the effect calculated by the model given that improvement in laboratory or clinical properties, such as heart rate in the case of beta blockers, may have already occurred (and medication hazard ratios muted). Being derived in a clinical trial population, it is alternately possible that the dosing of medicines was titrated higher and consistency of patients taking medicines was greater than would be achieved in a real-world cohort and that the survival benefit of medicines in a real-world cohort may not be as great as that calculated by the model. It is unknown if the model will provide accurate estimates in patients without left ventricular dysfunction by signs, symptoms, or measured EF (i.e. inferior myocardial infarction with EF of 55%). The validation cohort, did, however, include a generous definition of left ventricular dysfunction.
Conclusion
The SPIM score was designed to be a powerful predictor of survival after an acute presentation with a myocardial infarction with evidence of left ventricular dysfunction. Its highly accurate predictions and powerful discrimination should improve patient and physician understanding of survival after myocardial infarction and may prove a useful tool in risk stratification in patients with CHD.
Data appendix
The Seattle Post Infarction Model Plus (SPIM+) was developed for patients whose EF and uric acid data were available. These variables were not included in the primary SPIM as they were not universally available in the general myocardial infarction population. The online calculator uses the default SPIM score when EF and uric acid are not entered and the SPIM+ calculation when they are. Within the derivation cohort, EF was available for 6617 patients (99.8%) and uric acid for 6107 (92.1%).
Calculation of the SPIM+ risk score.
| Predictor | Univariate HR (95% CI) |
Wald χ2 | Multivariate HR (95% CI) |
Wald χ2 |
|---|---|---|---|---|
| SPIM score | 2.492 (2.304–2.695) | 521.241 | ||
| EFa | 0.950 (0.943–0.958) | 151.4 | 0.973 (0.964–0.981) | 36.216 |
| Uric acidb | 1.131 (1.108–1.155) | 137.0 | 1.055 (1.024–1.086) | 12.372 |
Ejection fraction truncated to a range between 10 and 40%.
Uric acid truncated to a range between 5 and 14.
EF: ejection fraction; HR: heart rate; SPIM: Seattle Post Infarction Model.
Footnotes
Conflict of interest: EPHESUS was funded by Pfizer and OPTIMAAL by Merck.
Wayne Levy has received funding from GlaxoSmithKline, Boehringer Ingelheim, and Amgen. Bertram Pitt has received funding from Pfizer, Merck, Novartis, Takeda, Bayer, AstraZeneca, Lilly, BMS, GE Healthcare, Relypsa, BG Medicine, Amorcyte, Cytopherx, Aura Sense, Ardelyx, Forrest Laboratories, and Medtronic.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Krumholz HM, Wang Y, Chen J, et al. Reduction in acute myocardial infarction mortality in the United States: Risk-standardized mortality rates from 1995–2006. JAMA 2009; 302: 767–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010; 362: 2155–2165 [DOI] [PubMed] [Google Scholar]
- 3. Puymirat E, Simon T, Steg PG, et al. Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA 2012; 308: 998–1006 [DOI] [PubMed] [Google Scholar]
- 4. Jernberg T, Johanson P, Held C, et al. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA 2011; 305: 1677–1684 [DOI] [PubMed] [Google Scholar]
- 5. Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med 2007; 356: 2388–2398 [DOI] [PubMed] [Google Scholar]
- 6. Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med 2003; 163: 2345–2353 [DOI] [PubMed] [Google Scholar]
- 7. Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 2000; 284: 835–842 [DOI] [PubMed] [Google Scholar]
- 8. Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000; 102: 2031–2037 [DOI] [PubMed] [Google Scholar]
- 9. Boersma E, Pieper KS, Steyerberg EW, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation 2000; 101: 2557–2567 [DOI] [PubMed] [Google Scholar]
- 10. McManus DD, Gore J, Yarzebski J, et al. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med 2011; 124: 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2012 update: A report From the American Heart Association. Circulation 2012; 125: e2–e220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dickstein K, Gleim GW, Snapinn S, et al. The impact of morbid events on survival following hospitalization for complicated myocardial infarction. Eur J Heart Fail 2006; 8: 74–80 [DOI] [PubMed] [Google Scholar]
- 13. Jacobs DR, Jr, Kroenke C, Crow R, et al. PREDICT: A simple risk score for clinical severity and long-term prognosis after hospitalization for acute myocardial infarction or unstable angina: The Minnesota Heart Survey. Circulation 1999; 100: 599–607 [DOI] [PubMed] [Google Scholar]
- 14. Brilakis ES, Wright RS, Kopecky SL, et al. Association of the PURSUIT risk score with predischarge ejection fraction, angiographic severity of coronary artery disease, and mortality in a nonselected, community-based population with non-ST-elevation acute myocardial infarction. Am Heart J 2003; 146: 811–818 [DOI] [PubMed] [Google Scholar]
- 15. Truong QA, Cannon CP, Zakai NA, et al. Thrombolysis in Myocardial Infarction (TIMI) Risk Index predicts long-term mortality and heart failure in patients with ST-elevation myocardial infarction in the TIMI 2 clinical trial. Am Heart J 2009; 157: 673–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garg S, Sarno G, Garcia-Garcia HM, et al. A new tool for the risk stratification of patients with complex coronary artery disease: The Clinical SYNTAX Score. Circ Cardiovasc Interv 2010; 3: 317–326 [DOI] [PubMed] [Google Scholar]
- 17. Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: Estimating the risk of 6-month postdischarge death in an international registry. JAMA 2004; 291: 2727–2733 [DOI] [PubMed] [Google Scholar]
- 18. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348: 1309–1321 [DOI] [PubMed] [Google Scholar]
- 19. Pitt B, Williams G, Remme W, et al. The EPHESUS trial: Eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction. Eplerenone Post-AMI Heart Failure Efficacy and Survival Study. Cardiovasc Drugs Ther 2001; 15: 79–87 [DOI] [PubMed] [Google Scholar]
- 20. Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: The OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet 2002; 360: 752–760 [DOI] [PubMed] [Google Scholar]
- 21. Dickstein K, Kjekshus J. Comparison of baseline data, initial course, and management: Losartan versus captopril following acute myocardial infarction (The OPTIMAAL Trial). OPTIMAAL Trial Steering Committee and Investigators. Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan. Am J Cardiol 2001; 87: 766–771, A7 [DOI] [PubMed] [Google Scholar]
- 22. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143: 29–36 [DOI] [PubMed] [Google Scholar]
- 23. Lemeshow S, Hosmer DW., Jr. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol 1982; 115: 92–106 [DOI] [PubMed] [Google Scholar]
- 24. Anderson KM, Wilson PW, Odell PM, et al. An updated coronary risk profile. A statement for health professionals. Circulation 1991; 83: 356–362 [DOI] [PubMed] [Google Scholar]
- 25. Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998; 97: 1837–1847 [DOI] [PubMed] [Google Scholar]
- 26. Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010; 304: 1350–1357 [DOI] [PubMed] [Google Scholar]
- 27. Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA 2007; 298: 765–775 [DOI] [PubMed] [Google Scholar]
- 28. Pepine CJ, Kowey PR, Kupfer S, et al. Predictors of adverse outcome among patients with hypertension and coronary artery disease. J Am Coll Cardiol 2006; 47: 547–551 [DOI] [PubMed] [Google Scholar]
- 29. Levy WC, Mozaffarian D, Linker DT, et al. The Seattle heart failure model: Prediction of survival in heart failure. Circulation 2006; 113: 1424–1433 [DOI] [PubMed] [Google Scholar]
- 30. Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med 2002; 347: 1397–1402 [DOI] [PubMed] [Google Scholar]
- 31. Giugliano RP, Llevadot J, Wilcox RG, et al. Geographic variation in patient and hospital characteristics, management, and clinical outcomes in ST-elevation myocardial infarction treated with fibrinolysis. Results from InTIME-II. Eur Heart J 2001; 22: 1702–1715 [DOI] [PubMed] [Google Scholar]
- 32. Alberts MJ, Bhatt DL, Mas JL, et al. Three-year follow-up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur Heart J 2009; 30: 2318–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297: 1197–1206 [DOI] [PubMed] [Google Scholar]
- 34. Cohen MG, Pacchiana CM, Corbalan R, et al. Variation in patient management and outcomes for acute coronary syndromes in Latin America and North America: Results from the Platelet IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) trial. Am Heart J 2001; 141: 391–401 [DOI] [PubMed] [Google Scholar]
- 35. Gibson CM, Pinto DS, Murphy SA, et al. Association of creatinine and creatinine clearance on presentation in acute myocardial infarction with subsequent mortality. J Am Coll Cardiol 2003; 42: 1535–1543 [DOI] [PubMed] [Google Scholar]
- 36. Kumler T, Gislason GH, Kober L, et al. Renal function at the time of a myocardial infarction maintains prognostic value for more than 10 years. BMC Cardiovasc Disord 2011; 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Giraldez RR, Sabatine MS, Morrow DA, et al. Baseline hemoglobin concentration and creatinine clearance composite laboratory index improves risk stratification in ST-elevation myocardial infarction. Am Heart J 2009; 157: 517–524 [DOI] [PubMed] [Google Scholar]
- 38. Anker SD, Voors A, Okonko D, et al. Prevalence, incidence, and prognostic value of anaemia in patients after an acute myocardial infarction: Data from the OPTIMAAL trial. Eur Heart J 2009; 30: 1331–1339 [DOI] [PubMed] [Google Scholar]
- 39. Klein L, O'Connor CM, Leimberger JD, et al. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: Results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation 2005; 111: 2454–2460 [DOI] [PubMed] [Google Scholar]
- 40. Bangalore S, Messerli FH, Ou FS, et al. The association of admission heart rate and in-hospital cardiovascular events in patients with non-ST-segment elevation acute coronary syndromes: Results from 135 164 patients in the CRUSADE quality improvement initiative. Eur Heart J 2010; 31: 552–560 [DOI] [PubMed] [Google Scholar]
- 41. Antoni ML, Boden H, Delgado V, et al. Relationship between discharge heart rate and mortality in patients after acute myocardial infarction treated with primary percutaneous coronary intervention. Eur Heart J 2012; 33: 96–102 [DOI] [PubMed] [Google Scholar]
- 42. Wiviott SD, Morrow DA, Frederick PD, et al. Application of the thrombolysis in myocardial infarction risk index in non-ST-segment elevation myocardial infarction: Evaluation of patients in the National Registry of Myocardial Infarction. J Am Coll Cardiol 2006; 47: 1553–1558 [DOI] [PubMed] [Google Scholar]
- 43. Yan AT, Yan RT, Cantor WJ, et al. Relationship between risk stratification at admission and treatment effects of early invasive management following fibrinolysis: Insights from the Trial of Routine ANgioplasty and Stenting After Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction (TRANSFER-AMI). Eur Heart J 2011; 32: 1994–2002 [DOI] [PubMed] [Google Scholar]
- 44. Van Domburg RT, Sonnenschein K, Nieuwlaat R, et al. Sustained benefit 20 years after reperfusion therapy in acute myocardial infarction. J Am Coll Cardiol 2005; 46: 15–20 [DOI] [PubMed] [Google Scholar]
- 45. Wright RS, Anderson JL, Adams CD, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol 2011; 57: e215–367 [DOI] [PubMed] [Google Scholar]
- 46. Bagnall AJ, Yan AT, Yan RT, et al. Optimal medical therapy for non-ST-segment-elevation acute coronary syndromes: Exploring why physicians do not prescribe evidence-based treatment and why patients discontinue medications after discharge. Circ Cardiovasc Qual Outcomes 2010; 3: 530–537 [DOI] [PubMed] [Google Scholar]
- 47. Yan AT, Yan RT, Huynh T, et al. Understanding physicians’ risk stratification of acute coronary syndromes: Insights from the Canadian ACS 2 Registry. Arch Intern Med 2009; 169: 372–338 [DOI] [PubMed] [Google Scholar]